Abstract
Purpose of Review
Telemedicine use in dermatology, termed “teledermatology”, offers a cost-effective model to improve healthcare efficiency and access. Only a minority of dermatology practices has integrated teledermatology into their practice prior to COVID-19. A thorough understanding of the barriers and facilitators may promote teledermatology adoption. Implementation science frameworks offer theoretically driven ways to assess factors affecting teledermatology implementation. This review uses a comprehensive implementation science framework to summarize barriers and facilitators of teledermatology implementation and appraises the quality of existing research.
Recent Findings
Technological characteristics of teledermatology (e.g., user-friendliness) and factors within the outer setting (e.g., reimbursement and legal considerations) were the most commonly reported barriers. No existing studies use a comprehensive implementation framework to identify factors influencing teledermatology implementation. Many included studies have a risk of bias in at least two of the five study quality indices evaluated.
Summary
This systematic review is the first study to summarize the existing teledermatology implementation literature into well-defined constructs from a comprehensive implementation science framework. Findings suggest future studies would benefit from the use of an implementation framework to reduce study bias, improve result comprehensiveness, facilitate comparisons across studies, and produce evidence-based resolutions to implementation barriers. Tools, resources, and recommendations to facilitate the use of an implementation framework in future studies are provided.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13671-020-00323-0.
Keywords: COVID-19, Teledermatology, Telemedicine, Dermatology
Introduction
Compared to in-person clinic visits, telemedicine reduces geographic and financial barriers to healthcare access, increases convenience, lowers visit costs, and facilitates case triaging and scheduling [1–9]. Telemedicine has become particularly important during the recent COVID-19 pandemic because it facilitates patient monitoring while reducing transmission and exposure risks [10]. For these reasons, many institutions in the USA are urging for expanding telehealth implementation [11–13].
Systematic reviews have found comparable health outcomes, feasibility, and stakeholder satisfaction between teledermatology and in-person services [9, 14, 15] providing support for the adoption of teledermatology. In practice however, even though dermatologists are amongst the most frequent and most studied users of telemedicine, only a minority of dermatology practices and residency training programs have integrated telemedicine in patient care [16–19]. This suggests that there are challenges in the adoption of teledermatology in real-world practices. A thorough understanding of the facilitators and barriers to teledermatology implementation may explain why teledermatology is successfully adopted in some settings but not others [20].
This study aims to review barriers and facilitators to teledermatology implementation using the Consolidated Framework for Implementation Research (CFIR) [21]. The CFIR was developed by unifying key implementation constructs across 19 theories to build a comprehensive, “meta-framework” that examines various aspects of implementation, including technology features, characteristics of individuals, and features of the implementation settings. The CFIR was chosen to guide this review because of its comprehensiveness, allowing for the categorization of diverse implementation factors across a variety of studies and healthcare settings [21–24]. One advantage of the CFIR over other comprehensive implementation frameworks [e.g., [22], is that its constructs have been matched to a list of 73 evidence-based implementation strategies based on expert experience and consensus [25–27]. As such, using the CFIR to categorize implementation barriers will inform the selection of evidence-based strategies to resolve these barriers, offering great potential to move the field of teledermatology forward.
The use of an evidence-informed implementation framework is crucial to understanding the barriers and facilitators of teledermatology implementation because it enables the systematic and comprehensive aggregation of findings across studies using standardized terminology [28–30]. Adopting theory or framework in teledermatology research has implications beyond scientific understanding; it has implications for real-world clinical practice. Without empirical foundation, attempts to adopt evidence-based practice continue to be a slow [31], “expensive version of trial-and-error” [30], where successful implementation at one institution cannot be replicated in another. To our knowledge, no existing work employed a theoretical approach to review primary studies of teledermatology implementation.
The objectives of this review are to: (1) examine the use of theory/framework in primary studies of teledermatology implementation, and (2) characterize the reported barriers and facilitators using a standardized taxonomy of definitions outlined by the CFIR.
Methods
A systematic search was conducted across four databases: MEDLINE, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature Complete, and SCOPUS. The search strategy was developed with a research librarian and through consulting search terms used in relevant published review studies. Search terms were identified for three concepts: dermatology, telemedicine, and implementation facilitators and barriers. Given the rapidly evolving nature of telemedicine, only articles published on or after 2010 were searched (see search details in the “Appendix 1” section). We focus our search on the past decade because data from both national (i.e., within the USA) and global surveys suggest that teledermatology was only offered in a very limited number of settings prior to 2010, but a steady increase in use has been noted in the last decade [19, 32, 33].
Inclusion/Exclusion Criteria
After removing duplicates, title and abstract screening followed by full-text review were completed for all articles. Studies included in this review met the following criteria: primary research studies; explored technologies designed to facilitate dermatologist-provided care; reported implementation barriers and/or facilitators in results; published in English. Articles were excluded if they were commentaries, review articles, or study protocols that did no report primary data; described only technology features (e.g., algorithm) or technologies primarily used for education, prevention, or patient reminders/monitoring; reported satisfaction, feasibility, evidence of teledermatology services but did not explore whether these were facilitators or barriers to teledermatology practice (these factors have also been thoroughly reviewed in other studies) [14, 15].
Data Extraction
The Consolidated Framework for Implementation Research was used to guide data extraction. The CFIR describes 37 constructs of implementation, which are categorized into five major domains: intervention characteristics, outer setting, inner setting, characteristics of the individuals involved, and the implementation process [21]. A detailed coding manual with operationalized definitions of CFIR domains and constructs was developed to support data extraction (see the “Appendix 2” section).
The following information was extracted from each article: authors, year of publication, teledermatology description and practice format (store-and-forward or live interaction), study design, use of implementation theory, region, number of teledermatology consultations, and number of participants. Number of studies that reported the use of an implementation theory was quantified. Additionally, reported barriers and facilitators were categorized into the CFIR constructs through discussion until consensus by two reviewers (ED and EK). The number of studies that reported barriers or facilitators for each CFIR construct was calculated in order to identify major factors influencing teledermatology implementation.
Studies were examined for bias using the Risk of Bias Instrument for Cross-Sectional Surveys of Attitudes and Practices [34]. To assess study quality, included studies were evaluated on five quality indices, including the following: participant sample selection, response rate, missing data, clinical relevance of the questionnaire, and reliability and validity of questionnaire (see the “Appendix 3” section).
Results
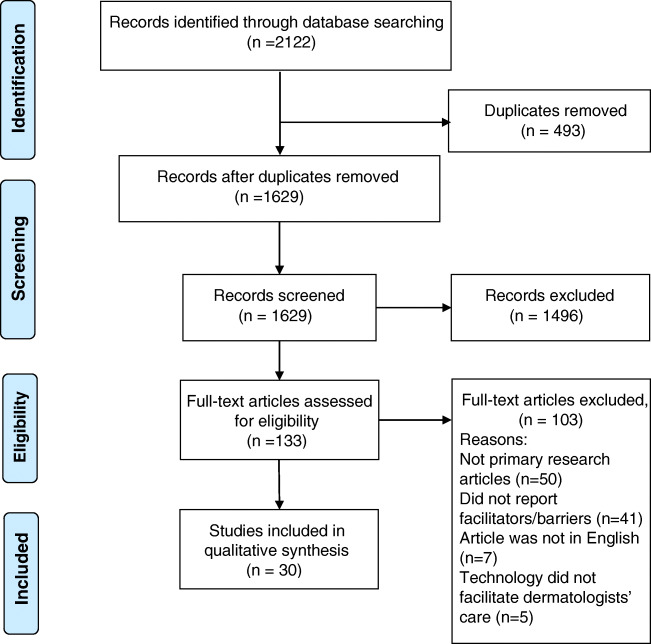
The study selection process is outlined in the Preferred Reporting Items for Systematic Reviews (PRISMA) diagram [35] (Fig. 1). Database search resulted in 1629 unique abstracts. Full-text review was completed on 133 articles that were included from abstract screening. Finally, 30 articles that met inclusion criteria were included in bias evaluation and data extraction (see the “Appendix 4” section). In total, these studies involved at least 384 dermatologists, 509 patients, and 527 referring healthcare providers (e.g., primary care physicians, pediatricians). Most included studies were conducted in the USA (9 studies, 30%), followed by Australia and the Netherlands (3 studies each). The majority of included studies (29 studies) examined implementation of store-and-forward teledermatology. The majority of studies were found to have risk of bias in at least two study quality indices. Characteristics of included studies are presented in Table 1.
Fig. 1.
PRISMA flowchart for study inclusion
Table 1.
Characteristics of included studies
| First author (year) | Front-end user of teledermatology | Telehealth practice format | Reported use of implementation theory? | Country | Number of teleconsults | Number and type of survey participants | Number of study quality indices with risk of bias |
|---|---|---|---|---|---|---|---|
| Armstrong (2010) [36] | Healthcare provider | SAF | No | USA | NA | 8 dermatologists; interview | 2 |
| van der Heijden (2014) [37] | Healthcare provider | SAF | No | Netherlands | 85 | 13 dermatologists; questionnaire and interview | 4 |
| van der Heijden (2010) [38] | Healthcare provider | SAF | No | Netherlands | 28 | 20 dermatologists; questionnaire | 2 |
| Kips (2019) [39] | Healthcare provider | SAF | No | Belgium | 54 | 15 mixed (12 primary care providers; 3 dermatologists); questionnaire | 3 |
| Delaigue (2014) [40] | Healthcare provider | SAF | No | 24 different countries | Not reported | 22 mixed (13 dermatologists and 9 non-dermatologist healthcare providers); questionnaire | 3 |
| Lasierra (2012) [41] | Healthcare provider | SAF | No | Spain | 17 | 14 mixed (13 primary care providers, 1 dermatologist); questionnaire | 3 |
| Orruño (2011) [42] | Healthcare provider | SAF | Yesa | Spain | 254 | 161 mixed (16 dermatologists, 126 family medicine physicians, 19 pediatricians); questionnaire | 2 |
| Janda (2019) [43] | Healthcare provider | SAF | Yesa | Australia (85%), Europe (7%) | Not reported | 52 mixed (16 primary care providers, 22 dermatologists, 14 dermatologist registrars); survey | 1 |
| Ford (2019) [44] | Healthcare provider | SAF | No | USA | Not reported | 29 mixed (17 patients, 8 primary care providers and 4 dermatologists); interview | 2 |
| Manahan (2011) [45] | Healthcare provider | SAF | No | Australia | 2 | 21 mixed (19 pharmacists, 2 patients); questionnaire | 2 |
| Kaliyadan (2013) [46] | Healthcare provider | SAF | No | Saudi Arabia | 166 | 25 mixed (23 patients, 2 dermatologists); questionnaire | 3 |
| Ludwick (2010) [47] | Healthcare provider | SAF | No | Canada | 28 | 10 mixed (9 physicians, 1 dermatologist); interview | 2 |
| von Wangenheim (2019) [48] | Healthcare provider | SAF | No | Brazil | 83,100 | NA; NA | Retrospective chart review, not assessed |
| Nakayama (2012) [49] | Healthcare provider | SAF | No | Japan | 36 | 23 pathologists; questionnaire | 4 |
| Spinks (2016) [50] | Healthcare provider | SAF | No | Australia | Not reported | 35 patients; direct choice experiment | 3 |
| Armstrong (2012) [51] | Healthcare provider | SAF and LI | No | USA | 2760 | 10 primary care providers; interview | 1 |
| Barbieri (2015) [52] | Healthcare provider | Not reported | No | USA | Not reported | 18 primary care providers; survey | 3 |
| O’Toole (2017) [53] | Healthcare provider | SAF | No | Canada | 965 | 217 primary care providers; survey | 3 |
| Costello (2019) [3] | Healthcare provider | SAF | No | USA | 38 | unknown number of primary care providers; survey | 3 |
| Eber (2019) [54] | Mix (healthcare provider and patient) | Not reported | No | Austria | Not reported | 243 dermatologists; questionnaire | 2 |
| Ariens (2017) [55] | Patients | SAF and LI | No | Netherlands | Not reported | 39 dermatologists; survey | 2 |
| Wu (2015) [56] | Patients | SAF | No | USA | Not reported | 31 mixed (2 dermatologists, 29 patients); survey | 4 |
| Fiks (2018) [57] | Patients | SAF | No | USA | Not reported | 135 patients; survey | 3 |
| Kong (2020) [58] | Patients | SAF | No | Australia | Not reported | 28 patients; interview | 3 |
| Pathipati (2016) [59] | Patients | SAF | No | USA | 38 | 38 patients; interview | 4 |
| Horsham (2016) [60] | Patients | SAF | Yesa | Australia | 49 | 49 patients; survey | 2 |
| Al Quran (2015) [61] | Patients | LI | No | Jordan | 90 | 88 patients; interview | 2 |
| Armstrong (2011) [62] | Unclear | SAF and LI | No | USA | Not reported | 17 dermatologists; interview | 2 |
| Walters (2018) [63] | Unclear | Not reported | No | South Africa | NA | NA; NA | 2 |
| Romero (2018) [64] | Unclear | SAF and LI | No | Spain | Not reported | Not reported (providers drawn from 42 dermatology centers); NA | 2 |
SAF store and forward, LI live interactive
aTechnology acceptance model
Theory/Framework Use
Questionnaires (20 studies, 67% of included studies) and interviews (9 studies, 30% of included studies) were the most common methods of data collection. Only three of the thirty studies used a theoretical implementation framework to guide their investigation [42–60]. Each of the three studies used the technology acceptance model, a model designed to assess individuals’ level of acceptance of technology (i.e., teledermatology). Correspondingly, these three studies had the lowest risk of bias in the instrument reliability and validity index of the bias analysis. In five studies, although the questionnaire used for data collection did not appear to be created based on a theoretical model, a formal assessment of the comprehensiveness, clarity, and/or face validity of the questionnaire was conducted, which lowered these studies’ risk of bias [42–63].
Barriers and Facilitators to Teledermatology Implementation
The barriers and facilitators reported across the 30 included studies were summarized into the domains and constructs of the CFIR model. Table 2 summarizes the major themes identified within each CFIR domain.
Table 2.
Major barriers and facilitators to teledermatology across studies
| CFIR domains | Barriers | Facilitators |
|---|---|---|
| Domain 1: intervention characteristics | ||
| Relative advantage |
Teledermatology offers less clinical information than in person clinic visits (e.g., history, outcome, images) Communication challenges, including: providing diagnoses, offering emotional support and advice, having a dialog |
Improved efficiency and convenience (e.g., reduced wait and travel time) Improved access to care Teledermatology improves patients’ and primary care providers’ knowledge of skin disorders |
| Design quality and packaging | Technological limitations of teledermatology | Easy to use and intuitive technology |
| Cost | Time required to learn to use the technology | |
| Domain 2: outer setting | ||
| Patient needs and resources |
Teledermatology cannot address some skin conditions Patients do not wish to be photographed due to social and religious reasons |
Teledermatology meets the needs of patients |
| External policy and incentives |
Reimbursement concerns Providers also reported increased medical liability and risk of privacy breach |
|
| Domain 3: inner setting | ||
| Compatibility | Teledermatology not compatible with existing workflow, health record system, and organizational policy | Teledermatology aligns with existing workflow and clinical needs |
| Available resources | Lack of time, personnel, equipment to adopt teledermatology | Availability of trainings, personnel and infrastructures |
| Domain 4: characteristics of individual | ||
| Self-efficacy | Lack of confidence in taking images of lesion, following recommendations from dermatologists. | Improved primary care provider and patient self-confidence in managing skin diseases |
| Knowledge and beliefs | Belief that teledermatology is not as accurate as in-person consultation | Beliefs in the usefulness of teledermatology |
| Domain 5: process | ||
| External change agents | No studies reported barriers in this domain | Involving experts with experience in teledermatology implementation |
Domain 1: Intervention Characteristics
This domain describes key attributes of the teledermatology technology and was the most commonly reported factor influencing implementation across the included studies.21 Nineteen studies reported barriers in this domain (63%) and 25 studies reported facilitators (80%).
With regard to barriers, relative to in-person care, store-and-forward teledermatology was reported to offer less clinical information to dermatologists, primary care providers, and patients (e.g., case history, available images, outcomes) [37, 39, 40, 58]. Additionally, the lack of in-person contact restricted dialog between patients and dermatologists, and created communication difficulties for providing patients with diagnoses, advice, and emotional support [43–44, 55, 57]. Studies also reported several technological limitations associated with teledermatology; these include slow image uploading and downloading speed [36, 43] and cumbersome technology user interface [37, 38, 46, 48, 57, 59]. Primary care providers reported additional barriers related to the time and money required to set up teledermatology technology [39, 41, 47].
In terms of facilitators, relative to in-person care, teledermatology consultations were more efficient and convenient and involved less travel and wait-time for patients [3, 36, 37, 39, 41, 43, 44, 47, 48, 53, 56–58, 60, 61]. Teledermatology also offers better access to care [41, 44, 51, 52, 55] at a lower cost [52, 60]. Teledermatology technologies were reported to be useful ways to improve both patients’ and primary care providers’ knowledge and management of skin disorders [3, 36, 39, 41, 52, 60]. Technology user friendliness was also reported to facilitate teledermatology implementation in some studies [37, 41, 42, 44, 56, 57, 59, 60].
Domain 2: Outer Setting
This domain includes considerations of the economic, political, and social context in which teledermatology takes place [21]. Barriers within this domain were reported in 14 studies (47%, the second most commonly reported category of barriers). There were three major barriers identified within this domain. First, teledermatology consultation was not able to meet some patients’ needs, particularly for certain skin conditions (e.g., allergic disorders) and management procedures (e.g., corticosteroid injection) [43, 44, 54, 60]. Also, patients who did not want to be photographed were unable to pursue store-and-forward teledermatology consultation [41, 45, 46]. Secondly, service reimbursement concerns were reported as barriers to teledermatology implementation for all stakeholders in several studies. Patients, primary care providers, and dermatologists raised concerns and reported challenges about obtaining reimbursement for teledermatology consultations [3, 37, 43–45, 47, 54, 56, 62]. Lastly, primary care providers and dermatologists reported concerns with liability and privacy issues (e.g., data security) associated with teledermatology [43, 45, 47, 52, 54, 55]. In contrast, only 3 studies (13%) reported facilitators within this domain, making this domain the least commonly reported facilitator to teledermatology implementation. These studies found that teledermatology was able to meet the needs of patients. [42, 52, 63] One study reported that the availability of healthcare professionals to assist patients with mobile teledermoscopy was a facilitator [60].
Domain 3: Inner Setting
This domain describes the impact of an organization’s structure, politics, and culture on the implementation of teledermatology practice [21]. Barriers within this domain were found in 9 studies (30%). Teledermatology was reported to be incompatible with the existing workflow [51], electronic health record system [36, 41, 43], and organizational security policy [55]. Additionally, the lack of internal resources (e.g., personnel, money for equipment, and time) was a commonly reported barrier [41, 43, 45, 55, 63]. Primary care providers reported that the time needed to upload images and complete associated paperwork for teleconsultation competed with their other clinical duties [41, 45]. Factors in the inner setting domain were reported as facilitators to teledermatology implementation in 6 studies. A common theme across these studies was that teledermatology was viewed as compatible with existing workflow and clinical needs [36, 41, 44, 55, 60]. Three studies additionally reported that the availability of training [48, 60], personnel, and infrastructure support [42] were facilitators to teledermatology implementation.
Domain 4: Characteristics of Individual
This domain includes factors related to individuals who are adopting teledermatology practice [21]. Barriers within this domain were reported in 7 studies (23%). Lack of self-efficacy was a commonly reported barrier. Primary care providers reported a lack of confidence in implementing dermatologists’ recommendations and taking images of skin lesions [41, 45, 51]. Patients reported a lack of confidence in performing a self-skin exam [58, 60]. The belief that teledermatology was not as accurate as in-person consultation was reported as a barrier in two studies [47, 58] Facilitators in this domain were reported in 6 studies (20%). Teledermatology improved primary care providers’ [52, 55] and patients’ [60] confidence in managing skin diseases. Two studies reported that teledermatology was perceived as useful and worthy of the additional effort [42, 52].
Domain 5: Process
This domain concerns the steps involved in implementation [21] and was the least reported domain across included studies. Factors within this domain were reported to be a facilitator in 4 studies (13%) and no studies reported barriers within this domain. The implementation of teledermatology was positively influenced by involving individuals with teledermatology experience from outside of the organization [3, 63], the presence of supportive opinion leaders [55], careful planning of the implementation procedure and technology [63], and the inclusion of steps to solicit users’ feedback and monitoring implementation [63].
Discussion
This systematic review identified 30 primary studies that explored the facilitators and barriers to teledermatology implementation. Using the CFIR, a comprehensive implementation science framework, this review summarized the facilitators and barriers to teledermatology implementation reported over the past 10 years. Results indicate that none of the included studies used a comprehensive implementation science theory or framework to explore facilitators and barriers to implementation. Three studies reported using a theoretical approach to guide the design of data collection; however, the model chosen examined only one aspect of teledermatology implementation (i.e., user acceptance). Many of the included studies had a risk of bias in at least two of the five study quality indices evaluated.
In the included studies, the most commonly reported barriers and facilitators to teledermatology implementation were found in the intervention characteristics domain of the CFIR. Communication barriers, the lack of thorough clinical information, and technological difficulties were found to hinder teledermatology implementation, whereas convenience, efficiency, and ease of use facilitated implementation. These findings, particularly the reported barriers, provide a foundation from which to improve teledermatology technology. For example, communication barriers associated with store-and-forward technologies, may be resolved by using a live interactive format or hybrid teledermatology (a combination of asynchronous and synchronous), which suggests a possible need to triage patients for different modes of teledermatology delivery. Additionally, the technological difficulties associated with some teledermatology platforms may point to a need to establish operating system standards of use (e.g., minimum network bandwidth to prevent slow image upload times).
The outer setting domain of the CIFR framework contained the second most commonly reported barriers to teledermatology implementation and the least reported number of facilitators. Some studies reported concerns that teledermatology was not meeting the needs of patients. Additionally, there were reimbursement and liability challenges that some faced, predominantly in the USA and Canada, but also some European countries like Austria. In the USA for example, there are barriers to obtaining reimbursement across state-lines due to state-specific reimbursement policies. The Netherlands, however, has integrated telemedicine into its national healthcare system, and offers full reimbursement for telemedicine services [65]. Of the 3 studies conducted in the Netherlands in this review, none reports reimbursement barriers. These findings corroborate existing studies that have argued for improving economic, political, and social factors to facilitate teledermatology implementation [9]. With the recent expansion of telemedicine reimbursement in the USA [12, 13], and the call for widespread adoption of telemedicine in response to COVID-19, barriers related to reimbursement will hopefully begin to resolve in North America and elsewhere.
During article review, we excluded studies that reported aspects of teledermatology implementation (e.g., feasibility, patient satisfaction) but did not further explore the role those factors played in facilitating or hindering implementation. For instance, we reasoned that patients who reported being satisfied with teledermatology services may still prefer in-person services, in which case, patient satisfaction is not a facilitator to teledermatology adoption. Thus, without an explicit exploration of practitioner and patient viewpoints on how individual factors impact teledermatology adoption, these factors cannot be considered facilitators or barriers to implementation.
This systematic review highlighted an existing gap in the teledermatology implementation literature. Namely, many studies have been conducted without consideration of implementation models or frameworks. This poses significant challenges in drawing meaningful conclusions both within and across studies. First, the lack of framework use draws into question the comprehensiveness of reported implementation factors within studies [21]. For instance, it is difficult to ascertain whether barriers or facilitators reported in a study are exhaustive or if they merely reflect investigators’ specific areas of interest (e.g., teledermatology usability and workplace climate). As a result, some implementation factors may exist but were not investigated in the primary studies included in this review. Second, the lack of framework use hinders comparison across studies. Without a standardized taxonomy of definitions of barriers and facilitators, it is difficult to organize findings across studies to identify commonly occurring barriers. This, in turn, creates challenges for identifying solutions or to allocate funding to overcoming commonly encountered barriers. This review is a first attempt to summarize and organize the existing literature using well-defined implementation constructs. Lastly, if future research is designed based on the existing reports of facilitators and barriers, it will perpetuate a restricted understanding of implementation factors. The use of a framework to inform data collection would increase the comprehensiveness, clinical applicability and validity of data collection tools, which will reduce the bias in study design [24].
Comprehensive theories to support the adoption of evidence-based practice or human behavior change existed before studies included in the current review were published [29]. For instance, the CFIR framework used in this study was published in 2009. While we cannot be certain of the reasons why existing teledermatology implementation research does not use an empirical framework, we hope this systematic review serves to highlight the importance of framework-driven research. We recommend future work to be carried out with consideration to theoretical and empirical understanding of implementation. To facilitate this uptake, the CFIR also provides a series of tools for investigators to employ when conducting implementation studies, including a semi-structured interview guide, which has questions probing all implementation constructs and domains in the CFIR [66]. Once barriers have been identified, construct-matched, evidence-based implementation strategies may be used to guide barrier resolution [25–27].
The experience of many healthcare settings transitioning to telehealth during the COVID-19 pandemic offers a unique opportunity for a comprehensive evaluation of teledermatology implementation. Using a comprehensive framework, this systematic review organized and aggregated the existing teledermatology implementation literature. Findings suggest future research would benefit from the use of an implementation framework to ensure an unbiased, comprehensive representation of barriers and facilitators in real-world teledermatology practices.
Supplementary Information
(DOCX 14 kb)
(XLSX 18 kb)
(DOCX 17 kb)
(XLSX 77 kb)
(DOC 64 kb)
Acknowledgments
We would like to thank Ms. Jennifer Westrick from Rush University library and librarians at the University of Western Ontario for their assistance in database searches.
Data Availability
Data used in these studies were from published journal articles.
Compliance with Ethical Standards
Conflict of Interest
All authors (Edwin Dovigi, Elaine Kwok, Joseph English III) declare that they have no conflict of interest.
Code Availability
Search strategies are available in the “Appendix 1” seciton.
Footnotes
This article is part of the Topical Collection on Teledermatology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bramanti A, Bonanno L, Celona A, Bertuccio S, Calisto A, Lanzafame P, et al. GIS and spatial analysis for costs and services optimization in neurological telemedicine. 2010 Annu Int Conf IEEE Eng Med Biol Soc EMBC’10. 2010;2204–7. [DOI] [PubMed]
- 2.Brown EM. The Ontario telemedicine network: a case report. Telemed e-Health. 2013;19(5):373–376. doi: 10.1089/tmj.2012.0299. [DOI] [PubMed] [Google Scholar]
- 3.Costello CM, Cumsky HJL, Maly CJ, Harvey JA, Buras MR, Pallagi PJ, et al. Improving access to care through the establishment of a local, teledermatology network. Telemed e-Health. 2019;00(00):1–6. doi: 10.1089/tmj.2019.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zakaria A, Maurer T, Su G, Amerson E. Impact of teledermatology on the accessibility and efficiency of dermatology care in an urban safety-net hospital: a pre-post analysis. J Am Acad Dermatol. 2019;81(6):1446–1452. doi: 10.1016/j.jaad.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Rothstein BE, Gonzalez J, Cunningham K, Saraiya A, Dornelles AC, Nguyen BM. Direct and indirect patient costs of dermatology clinic visits and their impact on access to care and provider preference. Cutis. 2017;100(6):405–410. [PubMed] [Google Scholar]
- 6.Datta SK, Warshaw EM, Edison KE, Kapur K, Thottapurathu L, Moritz TE, Reda DJ, Whited JD. Cost and utility analysis of a store-and-forward teledermatology referral system: a randomized clinical trial. JAMA Dermatology. 2015;151(12):1323–1329. doi: 10.1001/jamadermatol.2015.2362. [DOI] [PubMed] [Google Scholar]
- 7.Trettel A, Eissing L, Augustin M. Telemedicine in dermatology: findings and experiences worldwide – a systematic literature review. J Eur Acad Dermatology Venereol. 2018;32(2):215–224. doi: 10.1111/jdv.14341. [DOI] [PubMed] [Google Scholar]
- 8.Gordon AS, Adamson WC, DeVries AR. Virtual visits for acute, nonurgent care: a claims analysis of episode-level utilization. J Med Internet Res. 2017;19(2):1–11. doi: 10.2196/jmir.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JJ, English JC., III Teledermatology: a review and update. Am J Clin Dermatol. 2018;19(2):253–260. doi: 10.1007/s40257-017-0317-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhai Y, Wang Y, Zhang M, Gittell JH, Jiang S, Chen B, et al. From isolation to coordination: how can telemedicine help combat the COVID-19 outbreak? medRxiv [Internet]. 2020 Jan;2020.02.20.20025957. Available from:http://medrxiv.org/content/early/2020/02/23/2020.02.20.20025957.abstract
- 11.American Medical Associataion. AMA quick guide to telemedicine in practice [Internet]. 2020. Available from: https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice
- 12.U.S. Centers for Medicare & Medicaid Servicces. President Trump expands telehealth benefits for medicare beneficiaries during COVID-19 outbreak. 2020.
- 13.U.S. Department of Health & Human Services. Secretary Azar announces historic expansion of telehealth access to combat COVID-19 [Internet]. [cited 2020 Mar 19]. Available from:https://www.hhs.gov/about/news/2020/03/17/secretary-azar-announces-historic-expansion-of-telehealth-access-to-combat-covid-19.html
- 14.Mounessa JS, Chapman S, Braunberger T, Qin R, Lipoff JB, Dellavalle RP, Dunnick CA. A systematic review of satisfaction with teledermatology. J Telemed Telecare. 2018;24(4):263–270. doi: 10.1177/1357633X17696587. [DOI] [PubMed] [Google Scholar]
- 15.Bashshur RL, Shannon GW, Tejasvi T, Kvedar JC, Gates M. The empirical foundations of teledermatology: a review of the research evidence. Telemed e-Health. 2015;21(12):953–979. doi: 10.1089/tmj.2015.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deshpande A, Khoja S, Lorca J, McKibbon A, Rizo C, Husereau D, et al. Asynchronous telehealth: a scoping review of analytic studies. Open Med. 2009;3(2):39–61. [PMC free article] [PubMed] [Google Scholar]
- 17.Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff. 2018;37(12):1923–1930. doi: 10.1377/hlthaff.2018.05077. [DOI] [PubMed] [Google Scholar]
- 18.Song E, Amerson E, Twigg AR. Teledermatology in medical and continuing education. Curr Dermatol Rep. 2020;9(2):136–140. [Google Scholar]
- 19.Ehrlich A, Kostecki J, Olkaba H. Trends in dermatology practices and the implications for the workforce. J Am Acad Dermatol [Internet] 2017;77(4):746–752. doi: 10.1016/j.jaad.2017.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Warshaw EM, Hillman YJ, Greer NL, Hagel EM, MacDonald R, Rutks IR, et al. Teledermatology for diagnosis and management of skin conditions: a systematic review. J Am Acad Dermatol. 2011;64(4):759–772.e21. doi: 10.1016/j.jaad.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4(1):1–15. doi: 10.1186/1748-5908-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone AB, Yuan CT, Rosen MA, Grant MC, Benishek LE, Hanahan E, Lubomski LH, Ko C, Wick EC. Barriers to and facilitators of implementing enhanced recovery pathways using an implementation framework: a systematic review. JAMA Surg. 2018;153(3):270–278. doi: 10.1001/jamasurg.2017.5565. [DOI] [PubMed] [Google Scholar]
- 23.Jones CM, Stewart C, Roszell SS. Beyond best practice implementing a unit-based CLABSI project. J Nurs Care Qual. 2015;30(1):24–30. doi: 10.1097/NCQ.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 24.Cilenti D, Assistant C, Health C, Hill C, Brownson RC, Sciences PH, et al. Information-seeking behaviors and other factors contributing to successful implementation of evidence-based practices in local health departments. J Public Heal Manag Pr. 2012;18(6):571–576. doi: 10.1097/PHH.0b013e31825ce8e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CFIR. Research team-Center for Clinical Management Research. CFIR-ERIC Matching Tool.
- 26.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. 2015;10(1):1–14. doi: 10.1186/s13012-015-0209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waltz TJ, Powell BJ, Matthieu MM, Damschroder LJ, Chinman MJ, Smith JL, et al. Use of concept mapping to characterize relationships among implementation strategies and assess their feasibility and importance: results from the Expert Recommendations for Implementing Change (ERIC) study. Implement Sci. 2015;10(1):1–8. doi: 10.1186/s13012-015-0295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirk MA, Kelley C, Yankey N, Birken SA, Abadie B, Damschroder L. A systematic review of the use of the consolidated framework for implementation research. Implement Sci. 2016;11(1). [DOI] [PMC free article] [PubMed]
- 29.Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10(1):1–13. doi: 10.1186/s13012-015-0242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eccles M, Grimshaw J, Walker A, Johnston M, Pitts N. Changing the behavior of healthcare professionals: the use of theory in promoting the uptake of research findings. J Clin Epidemiol. 2005;58(2):107–112. doi: 10.1016/j.jclinepi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510–520. doi: 10.1258/jrsm.2011.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Organization WH . Telemedicine, opportunities and developments in member states: report on the second global survey on eHealth. 2010. [Google Scholar]
- 33.Armstrong AW, Wu J, Kovarik CL, Goldyne ME, Oh DH, McKoy KC, et al. State of teledermatology programs in the United States. J Am Acad Dermatol [Internet] 2012;67(5):939–944. doi: 10.1016/j.jaad.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Agarwald A, Guyatt G, Busse J. Methods commentary: risk of bias in cross-sectional surveys of attitudes and preferences. 2019. pp. 3–7. [Google Scholar]
- 35.Liberati A, Altman DGDG, Tetzlaff J, of internal … CM-A, 2009 U. Mulrow C, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong AW, Sanders C, Farbstein AD, Wu GZ, Lin SW, Liu FT, Nesbitt TS. Evaluation and comparison of store-and-forward teledermatology applications. Telemed e-Healt. 2010;16(4):424–438. doi: 10.1089/tmj.2009.0133. [DOI] [PubMed] [Google Scholar]
- 37.Van Der Heijden JP, De Keizer NF, Witkamp L, Spuls PI. Evaluation of a tertiary teledermatology service between peripheral and academic dermatologists in the Netherlands. Telemed e-Health. 2014;20(4):332–337. doi: 10.1089/tmj.2013.0197. [DOI] [PubMed] [Google Scholar]
- 38.van der Heijden JP, de Keizer NF, Voorbraak FP, Witkamp L, Bos JD, Spuls PI. A pilot study on tertiary teledermatology: feasibility and acceptance of telecommunication among dermatologists. J Telemed Telecare. 2010;16(8):447–453. doi: 10.1258/jtt.2010.091205. [DOI] [PubMed] [Google Scholar]
- 39.Kips J, Lambert J, Ongenae K, De Sutter A, Verhaeghe E. Teledermatology in Belgium: a pilot study. Acta Clin Belg. 2019;75(2):116–122. doi: 10.1080/17843286.2018.1561812. [DOI] [PubMed] [Google Scholar]
- 40.Delaigue S, Morand JJ, Olson D, Wootton R, Bonnardot L. Teledermatology in low-resource settings: the MSF experience with a multilingual tele-expertise platform. Front Public Heal. 2014;2(NOV):1–9. doi: 10.3389/fpubh.2014.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lasierra N, Alesanco A, Gilaberte Y, Magallón R, García J. Lessons learned after a three-year store and forward teledermatology experience using internet: strengths and limitations. Int J Med Inform. 2012;81(5):332–343. doi: 10.1016/j.ijmedinf.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Orruño E, Gagnon MP, Asua J, Abdeljelil AB. Evaluation of teledermatology adoption by health-care professionals using a modified technology acceptance model. J Telemed Telecare. 2011;17(6):303–307. doi: 10.1258/jtt.2011.101101. [DOI] [PubMed] [Google Scholar]
- 43.Janda M, Horsham C, Koh U, Gillespie N, Vagenas D, Loescher LJ, et al. Evaluating healthcare practitioners’ views on store-and-forward teledermoscopy services for the diagnosis of skin cancer. Digit Heal. 2019;5:1–11. doi: 10.1177/2055207619828225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ford AR, Gibbons CM, Torres J, Kornmehl HA, Singh S, Young PM, Chambers CJ, Maverakis E, Dunnick CA, Armstrong AW. Access to dermatological care with an innovative online model for psoriasis management: results from a randomized controlled trial. Telemed e-Health. 2019;25(7):619–627. doi: 10.1089/tmj.2018.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manahan MN, Soyer HP, Nissen LM, Peter Soyer H, Nissen LM. Teledermatology in pharmacies: a pilot study. J Telemed Telecare. 2011;17(7):392–396. doi: 10.1258/jtt.2011.110315. [DOI] [PubMed] [Google Scholar]
- 46.Kaliyadan F, Amin TT, Kuruvilla J, Ali WHAB. Mobile teledermatology - patient satisfaction, diagnostic and management concordance, and factors affecting patient refusal to participate in Saudi Arabia. J Telemed Telecare. 2013;19(6):315–319. doi: 10.1177/1357633X13501778. [DOI] [PubMed] [Google Scholar]
- 47.Ludwick DA, Lortie C, Doucette J, Rao J, Samoil-Schelstraete C. Evaluation of a telehealth clinic as a means to facilitate dermatologic consultation: pilot project to assess the efficiency and experience of teledermatology used in a primary care network. J Cutan Med Surg. 2010;14(1):7–12. doi: 10.2310/7750.2010.09012. [DOI] [PubMed] [Google Scholar]
- 48.Von Wangenheim A, Nunes DH. Creating a web infrastructure for the support of clinical protocols and clinical management: an example in teledermatology. Telemed e-Health. 2019;25(9):781–790. doi: 10.1089/tmj.2018.0197. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama I, Matsumura T, Kamataki A, et al. Development of a teledermatopathology consultation system using virtual slides. Diagn Pathol. 2012;7(1):1–9. 10.1186/1746-1596-7-177. [DOI] [PMC free article] [PubMed]
- 50.Spinks J, Janda M, Soyer HP, Whitty JA. Consumer preferences for teledermoscopy screening to detect melanoma early. J Telemed Telecare. 2016;22(1):39–46. 10.1177/1357633X15586701. [DOI] [PubMed]
- 51.Armstrong AW, Kwong MW, Chase EP, Ledo L, Nesbitt TS, Shewry SL. Teledermatology operational considerations, challenges, and benefits: the referring providers’ perspective. Telemed e-Health. 2012;18(8):580–584. doi: 10.1089/tmj.2011.0241. [DOI] [PubMed] [Google Scholar]
- 52.Barbieri JS, Nelson CA, Bream KD, Kovarik CL. Primary care providers’ perceptions of mobile store-and-forward teledermatology. Dermatology online journa. 2015;21(8). [PubMed]
- 53.O’Toole A, Joo J, DesGroseilliers JP, Liddy C, Glassman S, Afkham A, et al. The association between question type and the outcomes of a Dermatology eConsult service. Int J Dermatol. 2017;56(8):836–841. doi: 10.1111/ijd.13628. [DOI] [PubMed] [Google Scholar]
- 54.Eber EL, Janda M, Arzberger E, Hofmann-Wellenhof R. Survey on the status of teledermatology in Austria. JDDG - J Ger Soc Dermatology. 2019;17(1):25–31. doi: 10.1111/ddg.13729. [DOI] [PubMed] [Google Scholar]
- 55.Ariens LFMM, Schussler-Raymakers FMLL, Frima C, Flinterman A, Hamminga E, Arents BWMM, et al. Barriers and facilitators to eHealth use in daily practice: perspectives of patients and professionals in dermatology. J Med Internet Res. 2017;19(9):2. doi: 10.2196/jmir.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X, Oliveria SA, Yagerman S, Chen L, Defazio J, Braun R, et al. Feasibility and efficacy of patient-initiated mobile teledermoscopy for short-term monitoring of clinically atypical nevi. JAMA Dermatology. 2015;151(5):489–496. doi: 10.1001/jamadermatol.2014.3837. [DOI] [PubMed] [Google Scholar]
- 57.Fiks AG, Fleisher L, Berrigan L, Sykes E, Mayne SL, Gruver R, Halkyard K, Jew OS, FitzGerald P, Winston F, McMahon P. Usability, acceptability, and impact of a pediatric teledermatology mobile health application. Telemed e-Health. 2018;24(3):236–245. doi: 10.1089/tmj.2017.0075. [DOI] [PubMed] [Google Scholar]
- 58.Kong F, Horsham C, Rayner J, Simunovic M, O’hara M, Soyer HP, et al. Consumer preferences for skin cancer screening using mobile teledermoscopy: a qualitative study. Dermatology. 2020;236(2):1–8. doi: 10.1159/000505620. [DOI] [PubMed] [Google Scholar]
- 59.Pathipati AS, Ko JM. Implementation and evaluation of Stanford Health Care direct-care teledermatology program. SAGE Open Med. 2016;4:205031211665908. doi: 10.1177/2050312116659089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horsham C, Loescher LJ, Whiteman DC, Soyer HP, Janda M. Consumer acceptance of patient-performed mobile teledermoscopy for the early detection of melanoma. Br J Dermatol. 2016;175(6):1301–1310. doi: 10.1111/bjd.14630. [DOI] [PubMed] [Google Scholar]
- 61.Al Quran HA, Khader YS, Ellauzi ZM, Shdaifat A. Effect of real-time teledermatology on diagnosis, treatment and clinical improvement. J Telemed Telecare. 2015;21(2):93–99. doi: 10.1177/1357633X14566572. [DOI] [PubMed] [Google Scholar]
- 62.Armstrong AW, Kwong MW, Ledo L, Nesbitt TS, Shewry SL. Practice models and challenges in teledermatology: a study of collective experiences from teledermatologists. PLoS One. 2011;6(12):1–7. doi: 10.1371/journal.pone.0028687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walters LEM, Scott RE, Mars M. A teledermatology scale-up framework and roadmap for sustainable scaling: evidence-based development. J Med Internet Res. 2018;20(6):1. doi: 10.2196/jmir.9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romero G, de Argila D, Ferrandiz L, et al. Practice models in teledermatology in Spain: longitudinal study, 2009-2014. Actas Dermosifiliogr. 2018;109(7):624–30. 10.1016/j.adengl.2018.06.013. [DOI] [PubMed]
- 65.Campagna M, Naka F, Lu J. Teledermatology: an updated overview of clinical applications and reimbursement policies. Int J Women’s Dermatology. 2017;3(3):176–179. doi: 10.1016/j.ijwd.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Consolidated framework for implementation research: tools [Internet]. [cited 2020 Sep 6]. Available from:https://cfirguide.org/tools/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)
(XLSX 18 kb)
(DOCX 17 kb)
(XLSX 77 kb)
(DOC 64 kb)
Data Availability Statement
Data used in these studies were from published journal articles.