Abstract
The United Nations Office on Drugs and Crime designated twenty psychoactive botanical species as “plants of concern” because of their increased recreational abuse. Four of these are used to prepare ayahuasca brews. The complexity of the plant matrices, as well as the beverage itself, make the identification and quantification of the Schedule I component, N,N-dimethyltryptamine (DMT), a time-consuming and resource-intensive endeavor when performed using conventional approaches previously reported. Reported here is the development of a rapid validated method for the quantification of DMT in ayahuasca by direct analysis in real time-high-resolution mass spectrometry (DART-HRMS). This ambient ionization approach also enables identification of ayahuasca through detection of the secondary metabolites associated with its plant constituents. Analysis of six ayahuasca brews created using different combinations of DMT/harmala alkaloid-containing plants resulted in beverages with DMT levels of 45.7–230.5 mg/L. The detected amounts were consistent with previously reported values determined by conventional approaches.
Introduction
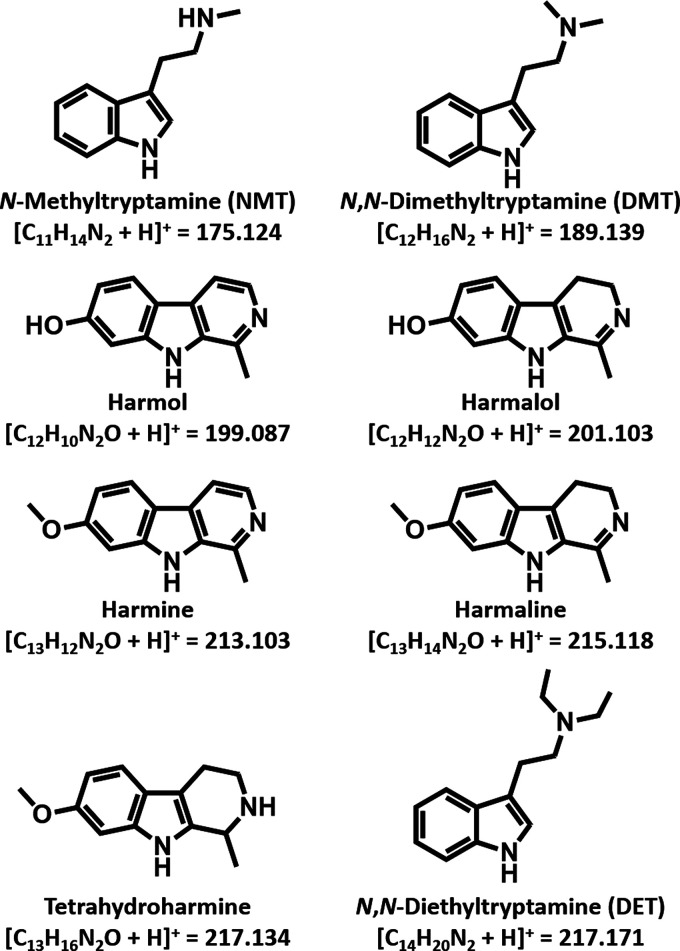
In the 2019 World Drug Report, the United Nations Office on Drugs and Crime (UNODC) noted with concern the global increase in seizures of plant-based new psychoactive substances (NPSs), which ballooned by 78% between 2013 and 2017.1 Of the products that were highlighted, ayahuasca stood out because in contrast to the others, it is not a plant species, but rather a hallucinogenic beverage of South American origin that is made using multiple plants.2 While its consumption has most often been associated with religious practices and traditional medicine, there has been a sharp rise in recent years in its recreational use by those wishing to access altered states of consciousness. Traditionally, the brew is made using a combination of two types of plants: one that contains the Schedule I psychoactive alkaloid N,N-dimethyltryptamine (DMT) and another that contains harmala alkaloids with a β-carboline skeleton (Figure 1).3 DMT was noted by the UNODC as the most seized hallucinogen in 2017,1 with the largest quantity of seizures reported by the US.1 The necessity of preparing ayahuasca with a combination of a DMT-containing plant as well as a harmala alkaloid-containing plant derives from the fact that the hallucinogenic effects of DMT are not normally experienced when it is ingested by the oral route. This is because it is rapidly deactivated by liver monoamine oxidase (MAO) enzymes, which deaminate the molecule, converting it to a nonpsychoactive derivative.4 However, this deamination can be circumvented if the MAOs are inactivated, thereby preventing DMT degradation. Harmala alkaloids are well-known MAO-inhibitors (MAOIs),5,6 and therefore, ingestion of a plant combination that contains harmala alkaloids and DMT will in principle result in a hallucinogenic effect. Common plant sources of MAOIs include Banisteriopsis caapi and Peganum harmala,5,6 which contain the β-carbolines harmalol, harmine, and harmaline. DMT-containing plants include Mimosa hostilis (aka M. tenuiflora), Psychotria viridis, and Diplopterys cabrerana.3 Accordingly, ayahuasca recipes featuring any one of six pairs of DMT/harmala alkaloid-containing plant combinations can be found.7 Several of these species including B. caapi, P. harmala, M. hostilis/tenuiflora, and P. viridis were identified by the UNODC in its 2013 report as “plants of concern” that, in addition to D. cabrerana, are often used in the preparation of ayahuasca brews.8 To prepare ayahuasca, the DMT-containing and MAOI-containing plants are brewed together for several hours.2,5,9−11 The combination most commonly used is that of B. caapi vines and P. viridis leaves, with other admixtures being utilized based on plant ingredient accessibility.3
Figure 1.
Structures, [M + H]+ chemical formulas, and corresponding calculated masses of psychoactive alkaloids and β-carboline MAOIs reported to be found in ayahuasca brews, as well as the synthetic internal standard diethyltryptamine (DET) used in this study.
While the compound DMT is scheduled, neither the DMT- nor the β-carboline-containing plants are specifically regulated as controlled substances. The dramatic rise in law enforcement encounters with ayahuasca and the plants from which it is made, particularly in a forensic context, have increased the demand for methods to assist forensic crime labs in the efficient analysis and identification of the plant ingredients, as well as the ayahuasca beverage itself. Although reports of quantification of psychoactive molecules for forensic purposes have become more prevalent with the increasing recreational use of the plants identified by the UNODC,12−14 few reports involve the quantification of these molecules in plant-based complex mixtures. Various gas chromatography- (GC)10,11,15−18 and liquid chromatography-mass spectrometry (LC-MS)7,9,10,19−21 methods have been developed for the analysis of ayahuasca and quantification of DMT in the plant material and brews. The successful implementation of these methods generally requires significant time-consuming sample preparation steps, human resource investments, and lengthy instrument run-times, all of which are disadvantageous, given the sample analysis backlog challenges that plague many drug analysis laboratories.
It has previously been reported that the unique attributes of direct analysis in real time-high-resolution mass spectrometry (DART-HRMS)22 can enable confirmation of the presence of DMT and β-carboline biomarkers in ayahuasca brews using a rapid screening approach.23 These compounds can be detected by direct analysis of the materials, with no required sample preparation, thereby dramatically reducing sample analysis time. However, to be optimally useful in a forensic context, it is necessary that the technique enables quantification of its Schedule I component, DMT. We report here the development of a rapid validated DART-HRMS method for the quantification of DMT using a structurally related compound, N,N-diethyltryptamine (DET) as the internal standard, and demonstrate its application to DMT quantification in ayahuasca brews made from six different combinations of DMT/harmala alkaloid-containing plants.
Results
Confirmation of DMT and Harmala Alkaloids in Botanical Products and Ayahuasca Brews
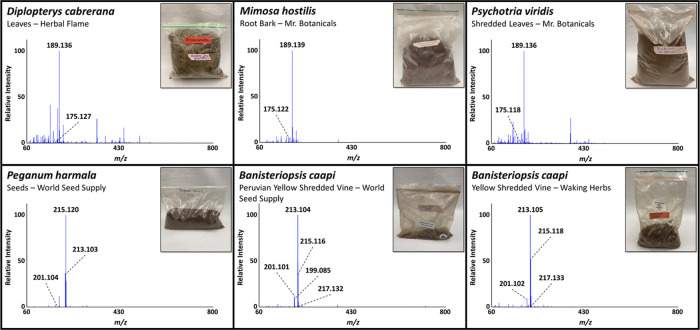
Ayahuasca brews are prepared by combining a DMT source with an MAOI source. Common DMT sources include M. hostilis, P. viridis, and D. cabrerana plant materials. B. caapi and P. harmala are two plants that contain MAOIs that are required for DMT to be orally active.6,23 Harmalol, harmine, and harmaline are three naturally occurring MAOIs, all of which are present in B. caapi and P. harmala.4 The study was initiated using DART-HRMS to confirm the presence of DMT and β-carboline alkaloids in the purchased plant materials, and compound identities were confirmed based on their fragmentation patterns observed in collision-induced dissociation (CID) experiments performed by DART-HRMS.23M. hostilis, P. viridis, and D. cabrerana products were screened for the presence of DMT, while B. caapi and P. haramala products were screened for harmine and harmaline. The results are shown in Figure 2. The DART-HRMS spectra for the D. cabrerana leaves, M. hostilis root bark, and shredded P. viridis leaves all contained DMT ([M + H]+ at m/z 189.139). In addition to DMT, a peak at m/z 175.124 was detected in these three products, which is consistent with the protonated mass of N-methyltryptamine (NMT), a structurally related compound. Harmalol, harmine, and harmaline ([M + H+] at m/z 201.103, 213.103, and 215.118, respectively) were detected in the two B. caapi products and P. harmala seeds. High-resolution masses consistent with the presence of the additional harmala alkaloids harmol and trace levels of tetrahydroharmine ([M + H]+ at m/z 199.087 and 217.134, respectively) were also observed. Tetrahydroharmine was detected in both B. caapi products, while harmol was detected in only the B. caapi vines purchased from World Seed Supply. The structures of the various alkaloids are shown in Figure 1.
Figure 2.
DART-HRMS spectra of plant material ingredients used to prepare ayahuasca brews. Images of the corresponding commercial products are shown in the insets.
Once the identities of the plant materials were confirmed and the ayahuasca brews were prepared as described in the Materials and Methods section, the brews were screened for DMT and MAOIs, to verify that their presence was still detectable by DART-HRMS in a complex mixture containing multiple plants. Brews 1–6 all contained two plants: one DMT source and one MAOI source. High-resolution masses consistent with the presence of NMT, DMT, and the three harmalol alkaloids previously mentioned were all detected by DART-HRMS in each of the six brews, as illustrated in Figure 3, with the mass measurement data presented in Table S1. The botanical species composition of the six ayahuasca brews are displayed in Figure 3, and the images showing the corresponding ayahuasca brews are presented in the insets.
Figure 3.
DART-HRMS spectra for six ayahuasca brews prepared with various UNODC-identified plants of concern. Images of the corresponding brews are shown in the insets.
Validation of a DART-HRMS Protocol for Quantification of DMT
The US Food and Drug Administration (FDA) Guidelines for the Development and Validation of Bioanalytical Methods have been used to validate DART-HRMS protocols to quantify the psychoactive molecules in commercial botanical products and plant preparations.12−14 These guidelines include: (1) demonstration that the method is selective, sensitive, and reproducible; (2) development of standard curves using a minimum of six nonzero calibrators; (3) a blank calibrator and a zero calibrator (i.e., a blank calibrator with internal standard) that do not interfere with the analyte of interest and do not exceed 5% of the average responses in the curve calibrators and quality control (QC) standards; (4) the lowest point of the standard curve being the lower limit of quantification (LLOQ); and (5) utilization of QC standards at four levels (high, medium, low, and LLOQ) incorporated into the curve.24 Criteria that an analysis must meet to be declared validated are as follows: (1) nonzero calibrators should be within 15% of nominal (theoretical) concentrations, except for the LLOQ calibrator, which can be within 20% of its nominal value; (2) 75%, and a minimum of six nonzero calibrators are required to meet the previous guideline; (3) ≥67% of the QC standards should be within 15% of their nominal values; and (4) ≥50% of the QC standards at each level must be within 15% of their nominal values.24 Each of the standard curves used to validate this method had R2 values of >0.99. For each standard curve analysis, all calibrators from 10 to 150 mg/L fell within acceptable percentages of their theoretical values. Of the QC standards, ≥50% at each level passed, as did ≥67% of the QC standards overall. The three validated standard curves and QC standards are displayed in Figure 4. After three standard curves were completed on separate days and successfully validated according to the FDA guidelines, the method was used to determine the DMT content in six ayahuasca brews.
Figure 4.
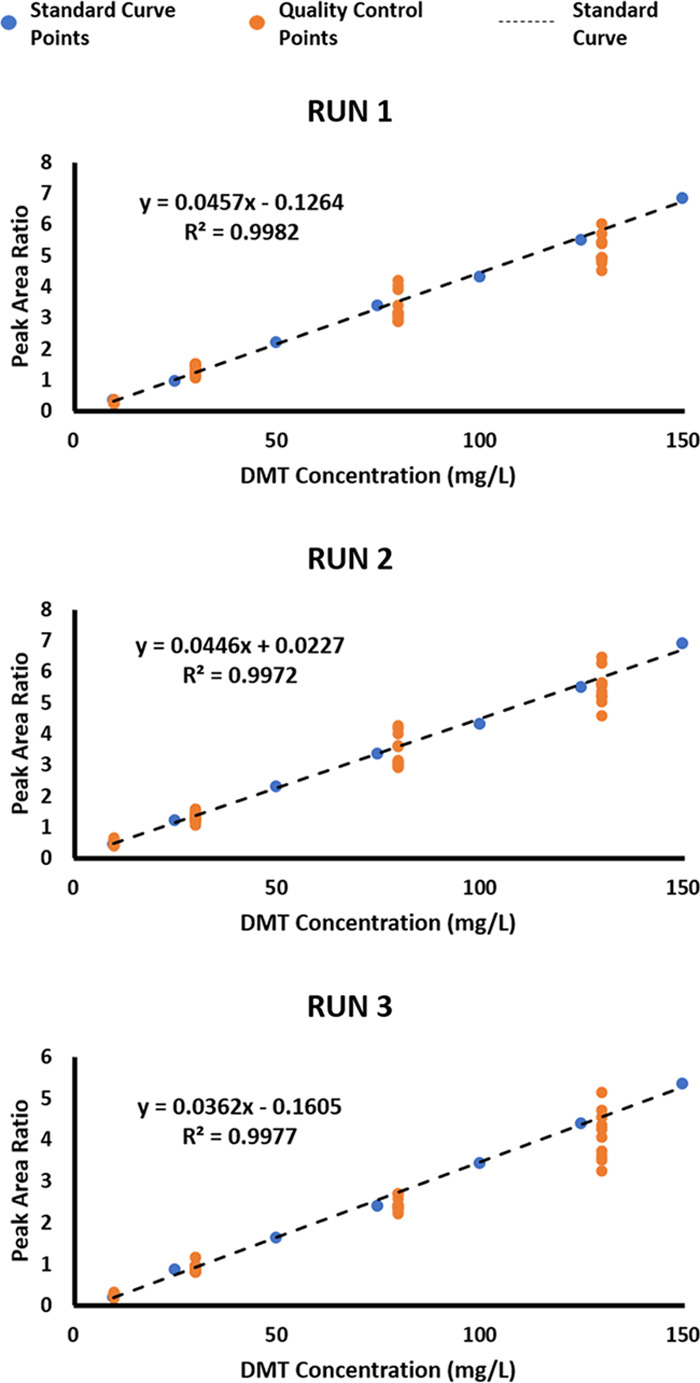
Validated standard curves for DMT quantification developed from DART-HRMS data, using DET as the internal standard.
In previously reported studies of the quantification of DMT, deuterated DMT is most often used as the internal standard. We found N,N-diethyltryptamine (DET) to serve as a suitable alternative to the more expensive deuterated DMT, and it exhibited a response in the DART gas stream similar to that of DMT. It should be noted, however, that the protonated precursors of both DET and tetrahydroharmine, a compound that was observed in trace amounts in the B. caapi starting material and not at all in any of the ayahuasca brews themselves, have the same nominal mass (i.e., 217 Da). However, the DET internal standard could still be distinguished from any tetrahydroharmine (were the latter to be present in measurable quantities), by detection of its high-resolution mass as distinguished from that of tetrahydroharmine (i.e., calc. 217.171 versus 217.134, respectively). With a high-resolution mass spectrometer such as the type used in this study, both compounds are readily differentiated. In any case, in this study, as well as studies previously conducted in our laboratory, tetrahydroharmine was not detected in the ayahuasca brews prepared.23
Quantification of DMT in Ayahuasca Plant Mixtures
Six ayahuasca brews were made from the following pairs of plant materials: (1) M. hostilis root bark and B. caapi vines; (2) M. hostilis root bark and P. harmala seeds; (3) P. viridis leaves and B. caapi vines; (4) P. viridis leaves and P. harmala seeds; (5) D. cabrerana leaves and B. caapi vines; and (6) D. cabrerana leaves and P. harmala seeds. Brews are usually prepared with water and a small amount of an acidic component such as lemon juice. In this work, brews were made using water and the addition of 1 mL of 10% H2SO4 to mimic the acid component (to ensure that the resulting brew was not fit for human consumption).3 Following straining, a 1 mL aliquot of each of the brews was centrifuged at 5000 g for 10 min. Brews 1, 2, and 6 required additional dilution due to high concentrations of DMT that fell above the linear range of the standard curve. Aliquots of 150 μL of all brew solutions were then spiked with 150 μL of 100 mg/L DET internal standard. When the brews were analyzed with the standard curve and QC standards, the peak area ratio of the DMT peak (m/z 189.139) to the internal standard DET peak (m/z 217.171) was used to determine the concentration of the brews in units of mg/L based on the standard curve linear range. Once all of the brew solutions fell within the linear range of the standard curve, these solutions, the curve, and freshly prepared QC standards were analyzed on three different days. As with the validation process, the DMT concentrations of the brews were only considered accurate if the standard curve met the requirements of the FDA guidelines, and the brew solutions fell within the curve range. The average DMT concentrations for the six brews are listed in Table 1. Once the DMT content in the ayahuasca brews was determined, it was used to calculate the approximate concentration of DMT in the material used to prepare each of the brews. These values are also displayed in Table 1, and they show that M. hostilis root bark exhibited the highest concentration of DMT at 3.8 mg/g, followed by D. cabrerana leaves at 2.4 mg/g. P. viridis leaves contained the lowest level of DMT at a concentration of 0.94 mg/g.
Table 1. Botanical Species Compositions and Measured DMT Concentrations of the Six Ayahuasca Brews.
| brew | DMT-containing species | MAOI-containing species | average DMT concentration in brew (mg/L) | standard deviation | average DMT concentration in DMT-containing plant material (mg/g) |
|---|---|---|---|---|---|
| brew 1 | M. hostilis | B. caapi | 224.1 | 36.7 | 3.8 |
| brew 2 | M. hostilis | P. harmala | 230.5 | 32.5 | |
| brew 3 | P. viridis | B. caapi | 48.3 | 9.6 | 0.94 |
| brew 4 | P. viridis | P. harmala | 45.7 | 7.2 | |
| brew 5 | D. cabrerana | B. caapi | 82.4 | 17.8 | 2.4 |
| brew 6 | D. cabrerana | P. harmala | 155.1 | 43.9 |
Discussion
The quantification of DMT in a variety of matrices is of relevance in toxicology, pharmacology, medicinal chemistry, and forensic investigations and has been reported previously. DMT levels observed in various materials and the methods by which they were determined are presented in Table 2.
Table 2. Previously Reported DMT Content in Ayahuasca Brews and Assorted Plant Material.
| matrix type | botanical species/plant organ | method | DMT concentration |
|---|---|---|---|
| ayahuasca brew | B. caapi and P. viridis | HPLC-FD | 160–5840 mg/L19 |
| SPE GC-NPD | 310–730 mg/L15 | ||
| GC-MS | 240 mg/L11 | ||
| LC-ESI-MS/MS | 117.1–3190 mg/L20 | ||
| SPME GC-IT-MS | 170–1140 mg/L16 | ||
| HPLC-DAD | 324.3–2070.3 mg/L21 | ||
| LC-MS/MS | 62–340 mg/L7 | ||
| TLC; HPLC; GC-MS | 510–700 mg/L10 | ||
| GLC-MS | 40–180 mg/L17 | ||
| unprocessed plant material | M. tenuiflora stem bark | GC-MS | 1.35–9.35 mg/g18 |
| M. tenuiflora root bark | GC-MS | 1.26–7.20 mg/g18 | |
| P. viridis leaves | HPLC | 0.0–17.75 mg/g9 | |
| lyophilized B. caapi and P. viridis mixture | TLC; HPLC; GC-MS | 5.2–7.2 mg/g10 | |
| P. viridis leaves | TLC; HPLC; GC-MS | 1.0–1.6 mg/g10 | |
| D. cabrerana leaves | TLC; HPLC; GC-MS | 1.74 mg/g10 | |
| jurema wine | M. tenuiflora/hostilis | SPME GC-IT-MS | 100–1810 mg/L16 |
The results of this study are consistent with several aspects of previously reported investigations into ayahuasca and its associated plant material. The concentrations of DMT in the ayahuasca beverages described herein ranged from 45.7 to 230.5 mg/L. In ayahuasca brews prepared by the primary method, which utilizes B. caapi vines and P. viridis leaves, the DMT content previously reported varied from 40 to 5840 mg/L.7,10,11,15−17,19−21 Brews 1–6 all exhibited DMT content that fell within this range. It has been reported that D. cabrerana leaves contain higher concentrations of DMT than P. viridis leaves.10 The results presented here confirm that ayahuasca brews prepared with D. cabrerana leaves (brews 5 and 6) had a much higher DMT content than the brews prepared with P. viridis leaves (brews 3 and 4). Even though brews 5 and 6 were made with the same amount of raw D. cabrerana leaves, the DMT concentration in brew 5 was nearly half that of brew 6. It is possible that the difference between the DMT content in brews 5 and 6 reflects differences in the DMT concentrations in the raw plant material used to prepare the beverages. Even though the D. cabrerana leaves used in both preparations were from the same vendor, each brew was prepared from a different batch (in each case, the raw materials were weighed and ground separately). Thus, differences in the DMT content of the starting material, which are a consequence of its having been derived from plants grown in different geographical locations, harvested at different times and/or seasons, kept under different storage conditions,6 or derived from combined plant parts (e.g., leaves and vines versus only leaves) would manifest in terms of different DMT levels in the brews.18 Illustrative of this is the range of concentrations of DMT that have been reported in plant materials (e.g., 0.0–17.75,9,10 1.74,10 and 1.26–9.35 mg/g18 in P. viridis, D. cabrerana, and M. hostilis/tenuiflora unprocessed plant materials, respectively). When using the DMT concentrations observed in the ayahuasca brews to determine the approximate DMT content in the plant material from which it was made, the concentrations fell within the literature reported ranges for B. caapi, P. viridis, and M. hostilis. A corollary to this is that the intensity of the psychoactive effect is impacted by the DMT levels of the starting materials.
Although B. caapi is the plant species usually used in ayahuasca brews as the source of the β-carboline MAOIs,10P. harmala seeds also contain many of the same chemical markers.6 Therefore, ayahuasca brews were prepared using not only B. caapi, but also ground P. harmala seeds instead, to assess the versatility of the developed DART-HRMS method. Furthermore, while P. viridis and D. cabrerana are the most common DMT-containing plants used in ayahuasca,3 reports of the use of M. hostilis, which contains high DMT levels, have also appeared in recent years.3 There are numerous online recipes and how-to blogs that provide ayahuasca brew experimentation instructions using different plant materials, ratios, solvents, and admixtures.3 The method presented here was successfully used to analyze all six different ayahuasca brews. This high-resolution, ambient ionization technique eliminates the typical need for a chromatography step and permits direct analysis of the mixture for the detection of compounds, including DMT, harmine, and harmaline. In cases where quantification is necessary, the implementation of complex extraction sequences prior to DMT measurement by DART-HRMS can be avoided.
Conclusions
DART-HRMS analysis of six ayahuasca brews demonstrated the rapid detection of relevant biomarkers in the various preparations, including NMT and DMT, as well as the MAOIs harmol, harmine, and harmaline. Screening for these compounds confirmed previously reported results in which these molecules were detected and identified in ayahuasca brews by DART-HRMS under CID conditions.23 The DART-HRMS approach enabled facile quantification of DMT in six different ayahuasca formulations prepared using various pairs of plant species constituents. The method, validated according to the FDA guidelines for the analysis of ayahuasca brews, and utilizing a nondeuterated internal standard, offers forensic analysts with a more efficient, rapid, cost-effective, and streamlined approach to DMT quantification that circumvents the challenges encountered with more labor- and resource-intensive conventional approaches. Furthermore, it demonstrates that psychoactive molecules in complex plant-based mixtures can be analyzed and quantified using DART-HRMS, without the traditional chromatography step that often accompanies these types of analyses.
Materials and Methods
Botanical Material
All plant material was purchased online and photographed in their original packaging. D. cabrerana shredded leaves were purchased from Herbal Flame (Hollywood, FL). M. hostilis bark and P. viridis leaves were purchased from Mr. Botanicals (Newstead, QLD, Australia). B. caapi yellow vines were purchased from Waking Herbs (Haarlem, Netherlands). P. harmala seeds and B. caapi Peruvian yellow shredded vines were purchased from World Seed Supply (Mastic Beach, NY).
Chemical Standards
N,N-dimethyltryptamine (DMT) and N,N-diethyltryptamine (DET) were purchased from Cayman Chemical (Ann Arbor, MI) as the certified reference material (CRM) standards. HPLC-grade methanol was purchased from Pharmco Aaper (Brookfield, CT). High-purity helium was purchased from Airgas (Albany, NY). Sulfuric acid was purchased from Sigma-Aldrich (St. Louis, MO).
Ayahuasca Brew Preparation
Whole plant material and seeds were ground using a coffee grinder (Hamilton Beach, Southern Pines, NC). In all, 6 brews representing different combinations of pairs of plants were made as described previously,23 and these were designated as brews 1–6. Brews 1 and 2 were prepared with 15 g of M. hostilis root bark and 15 g of B. caapi vines from World Seed Supply or 15 g of P. harmala seeds, respectively, in 250 mL of water and 1 mL of 10% H2SO4. Brews 3 and 4 were prepared with 10 g of P. viridis leaves and 10 g of B. caapi vines from World Seed Supply or 10 g of P. harmala seeds, respectively, in 200 mL of water and 1 mL of 10% H2SO4. Brews 5 and 6 were prepared with 10 g of D. cabrerana leaves and either 10 g of B. caapi vines from Waking Herbs or 10 g of P. harmala seeds, respectively, in 200 mL of water and 1 mL of 10% H2SO4. All suspensions were refluxed for 4 h and strained through cheesecloth as is customarily done with home brews of ayahuasca. The resulting brews were photographed, as shown in Figure 3.
DART-HRMS Mass Spectral Data Acquisition and Data Analysis
DART-high-resolution mass spectra of standards, plant material, and ayahuasca brews were obtained using a DART-SVP ion source (IonSense, Saugus, MA) coupled to an AccuTOF high-resolution time-of-flight mass spectrometer (JEOL USA, Peabody, MA). All data were collected in positive-ion mode with the following DART ion source parameters: grid voltage, 250 V, and heater temperature, 350 °C. Settings for the mass spectrometer were as follows: ring lens voltage, 5 V; orifice 1 voltage, 20 V; orifice 2 voltage, 5 V; peak voltage, 600 V; and detector voltage, 2050 V. The mass spectra were collected over a range of m/z 60–1000 at a rate of 1 spectrum per s. The DART ion source helium flow rate was 2 L/min. The mass spectrometer had a resolving power of 6000 FWHM and a mass accuracy of 5 millimass units (mmu). Mass spectral data calibration, spectral averaging, background subtraction, and peak centroiding and integration were performed using TssPro 3 software (Shrader Software Solutions, Detroit, MI). Mass calibration for all calibrators, quality control (QC) standards, plant material, and ayahuasca brews was accomplished using polyethylene glycol (PEG 600). The processing of the mass spectra was performed using the Mass Mountaineer software suite (RBC Software, Portsmouth, NH).
Rapid screening of the plant materials was conducted to confirm previous results23 showing the presence of various biomarkers. Whole plant materials, such as vines, leaves, and root bark, were presented in their native form via tweezers to the open-air space between the DART ion source and mass spectrometer inlet for approximately 5 s. For the analysis of the shredded leaves, the closed end of a melting point capillary tube was immersed into the leaf material and the coated surface of the tube was suspended in the DART gas stream for approximately 5 s. To conduct a thorough spectral analysis of the seeds, a coffee grinder was used to render the seeds into a powdered form, which was sampled in the same manner as the shredded leaves. All plants were analyzed in replicates of three, meaning that in a single DART-HRMS acquisition, three replicates of a single plant type or brew were introduced into the DART gas stream. Images of all of the purchased plant materials that were analyzed are shown in the insets of Figure 2.
To quantify the psychoactive DMT content in various preparations of ayahuasca brews, standard curves were developed using 1 mg/mL (1000 mg/L) CRM standards of DMT and DET, with the latter serving as the internal standard. For DMT, seven calibrators with the following concentrations were prepared: 150, 125, 100, 75, 50, 25, and 10 mg/L. The lower limit of quantification (LLOQ), which was found to be 10 mg/L, was then used as the lowest point on the standard curve. A 1000 mg/L DET CRM standard was diluted with methanol to a concentration of 100 mg/L to prepare an internal standard solution. Therefore, when added in a 1:1 ratio with the calibrators and beverage solutions, its final concentration was 50 mg/L in all standards and solutions. Two DMT CRM standards were used as the QC1 and QC2 stock solutions to develop the QC standards to satisfy the US Food and Drug Administration (FDA) guidelines for method validation. Two sets of QC standards were prepared at high (130 mg/L), medium (80 mg/L), low (30 mg/L), and LLOQ (10 mg/L) concentrations on the curve, with the QC standards at the 30 mg/L level being 3 times the LLOQ. All QC standards were prepared fresh daily.
For efficient semiautomated quantification, a 24-Pin Liquid Sampler (IonSense, Saugus, MA) was used to facilitate the mass spectrometric measurements that were made for the development of the standard curves and for the quantification of DMT in the calibrators, QC standards, and ayahuasca brews. This apparatus, photographed and featured in Figure 5, consists of 24 small metal pins affixed to a linear rail system that enter into the DART gas stream at a user-defined speed for ionization of the analytes. Aliquots (10 μL) of the calibrators, QC standards, and ayahuasca solutions were dispensed into a 384-well plate. The pins in the 24-Pin Sampler were then briefly dipped into the wells of the plate and then, with the pins now coated with the sample, the 24-Pin Sampler was fastened to the linear rail system. All calibration standards were analyzed in triplicate, while QC standards were analyzed in replicates of five, all at a speed of 0.8 mms–1. DART-HRMS enables the integration of individual solutions based on the selected m/z values. The peak area ratio between the DMT and DET [M + H]+ peaks at m/z 189.139 and 217.171, respectively, was used to develop a standard curve to confirm the DMT concentration in QC standards and accurately determine the unknown DMT content in six ayahuasca brews.
Figure 5.
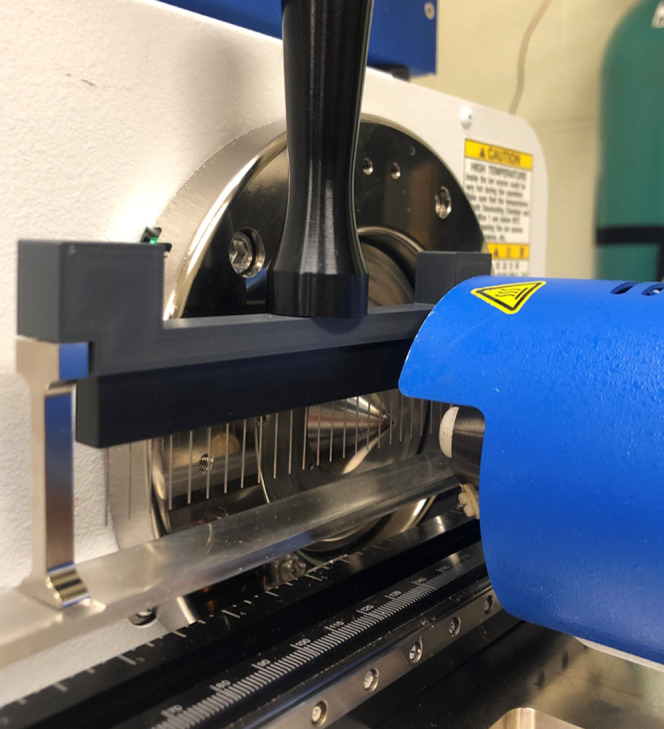
DART-HRMS linear rail system, with a 24-Pin Liquid Sampler, placed between the DART ion source and the mass spectrometer inlet for semiautomated analysis.
After the standard curve was validated, the method was applied to various formulations of ayahuasca brews prepared as described above, to quantify their DMT content. A 1 mL aliquot of each brew was centrifuged at 5000 g for 10 min to remove any residual plant material solids. After centrifugation, 150 μL of brews 3, 4, and 5 were diluted with 150 μL of the DET internal standard solution. Aliquots of 150 μL of brews 1, 2, and 6, which displayed high DMT concentrations that extended beyond the range in the standard curve, were further diluted with 150 μL of methanol. Then, a 150 μL aliquot of the diluted samples was spiked with 150 μL of the internal standard. As was done for the method validation experiments, the standard curve, QC standards, and ayahuasca brews were analyzed on three different days, with the concentrations of DMT averaged over the three-day period.
Acknowledgments
This work was supported by the National Institute of Justice (NIJ), Office of Justice Programs, US Department of Justice (DOJ) under Grant Nos. 2015-DN-BX-K057, 2017-R2-CX-0020, and 2018-R2-CX-0012, and the US National Science Foundation (NSF) under Grant No. 1429329. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the DOJ and the NSF.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03196.
Table containing the mass measurements and relative intensities of tryptamines and β-carbolines in ayahuasca brews (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- UNODC (United Nations Office on Drugs and Crime). Global Overview of Drug Demand and Supply. Wolrd Drug Report: Booklet 2. 2019. Available at https://wdr.unodc.org/wdr2019/prelaunch/WDR19_Booklet_2_DRUG_DEMAND.pdf. Accessed Feb 13, 2020.
- Riba J.; McIlhenny E. H.; Valle M.; Bouso J. C.; Barker S. A. Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test. Anal. 2012, 4, 610–616. 10.1002/dta.1344. [DOI] [PubMed] [Google Scholar]
- Thies T. A.Legally Stoned: 14 Mind Altering Substances You Can Obtain and Use Without Breaking the Law; Citadel Press Books, 2008. [Google Scholar]
- Oliveira C. D. R.; Okai G. G.; da Costa J. L.; de Almeida R. M.; Oliveira-Silva D.; Yonamine M. Determination of dimethyltryptamine and β-carbolines (ayahuasca alkaloids) in plasma samples by LC-MS/MS. Bioanalysis 2012, 4, 1731–1738. 10.4155/bio.12.124. [DOI] [PubMed] [Google Scholar]
- Samoylenko V.; Rahman M. M.; Tekwani B. L.; Tripathi L. M.; Wang Y. H.; Khan S. I.; Khan I. A.; Miller L. S.; Joshi V. C.; Muhammad I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. 10.1016/j.jep.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable R. S. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 2007, 102, 24–34. 10.1111/j.1360-0443.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Souza R. C. Z.; Zandonadi F. S.; Freitas D. P.; Tófoli L. F. F.; Sussulini A. Validation of an analytical method for the determination of the main ayahuasca active compounds and application to real ayahuasca samples from Brazil. J. Chromatogr. B. 2019, 1124, 197–203. 10.1016/j.jchromb.2019.06.014. [DOI] [PubMed] [Google Scholar]
- Hammond B.; Crean C.; Levissianos S.; Mermerci D.; Tun Nay S.; Otani T.; Park M.; Pazos D.; Piñeros K.; Umapornsakula A.; Wong Y. L.; Chawla S.. The Challenges of New Psychoactive Substances; UNODC Global SMART Programme, 2013. [Google Scholar]
- Callaway J. C.; Brito G. S.; Neves E. S. Phytochemical analyses of Banisteriopsis caapi and Psychotria viridis. J. Psychoactive Drugs 2005, 37, 145–150. 10.1080/02791072.2005.10399795. [DOI] [PubMed] [Google Scholar]
- McKenna D. J.; Towers G. H. N.; Abbott F. S. Monoamine oxidase inhibitors in South American hallucinogenic plants: Tryptamine and β-carboline constituents of ayahuasca. J. Ethnopharmacol. 1984, 10, 195–223. 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Gambelunghe C.; Aroni K.; Rossi R.; Moretti L.; Bacci M. Identification of N,N-dimethyltryptamine and β-carbolines in psychotropic ayahuasca beverage. Biomed. Chromatogr. 2008, 22, 1056–1059. 10.1002/bmc.1023. [DOI] [PubMed] [Google Scholar]
- Chambers M. I.; Osborne A. M.; Musah R. A. Rapid detection and validated quantification of psychoactive compounds in complex plant matrices by direct analysis in real time-high resolution mass spectrometry – Application to “Kava” psychoactive pepper products. Rapid Commun. Mass Spectrom. 2019, 33, 1915–1925. 10.1002/rcm.8532. [DOI] [PubMed] [Google Scholar]
- Longo C. M.; Musah R. A. An efficient ambient ionization mass spectrometric approach to detection and quantification of the mescaline content of commonly abused cacti from the Echinopsis genus. J. Forensic Sci. 2020, 65, 61–66. 10.1111/1556-4029.14134. [DOI] [PubMed] [Google Scholar]
- Fowble K. L.; Musah R. A. A validated method for the quantification of mitragynine in sixteen commercially available Kratom (Mitragyna speciosa) products. Forensic Sci. Int. 2019, 299, 195–202. 10.1016/j.forsciint.2019.04.009. [DOI] [PubMed] [Google Scholar]
- Pires A. P. S.; De Oliveira C. D. R.; Moura S.; Dörr F. A.; Silva W. A. E.; Yonamine M. Gas chromatographic analysis of dimethyltryptamine and β-carboline alkaloids in ayahuasca, an Amazonian psychoactive plant beverage. Phytochem. Anal. 2009, 20, 149–153. 10.1002/pca.1110. [DOI] [PubMed] [Google Scholar]
- Gaujac A. N.; Dempster N.; Navickiene S. D.; Brandt S. D.; de Andrade J. B. Determination of N,N-dimethyltryptamine in beverages consumed in religious practices by headspace solid-phase microextraction followed by gas chromatography ion trap mass spectrometry. Talanta 2013, 106, 394–398. 10.1016/j.talanta.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Rivier L.; Lindgren J. E. “Ayahuasca,” the South American hallucinogenic drink: An ethnobotanical and chemical investigation. Econ. Bot. 1972, 26, 101–129. 10.1007/BF02860772. [DOI] [Google Scholar]
- Gaujac A.; Aquino A.; Navickiene S.; de Andrade J. B. Determination of N,N-dimethyltryptamine in Mimosa tenuiflora inner barks by matrix solid-phase dispersion procedure and GC-MS. J. Chromatogr. B. 2012, 881–882, 107–110. 10.1016/j.jchromb.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Callaway J. C. Various alkaloid profiles in decoctions of Banisteriopsis caapi. J. Psychoactive Drugs 2005, 37, 151–155. 10.1080/02791072.2005.10399796. [DOI] [PubMed] [Google Scholar]
- McIlhenny E. H.; Pipkin K. E.; Standish L. J.; Wechkin H. A.; Strassman R.; Barker S. A. Direct analysis of psychoactive tryptamine and harmala alkaloids in the Amazonian botanical medicine ayahuasca by liquid chromatography-electrospray ionization-tandem mass spectrometry. J. Chromatogr. A. 2009, 1216, 8960–8968. 10.1016/j.chroma.2009.10.088. [DOI] [PubMed] [Google Scholar]
- Lanaro R.; de Aquino Calemi D. B.; Togni L. R.; Costa J. L.; Yonamine M.; de Oliveira Santos Cazenave S.; Linardi A. Ritualistic use of ayahuasca versus street use of similar substances seized by the police: A key factor involved in the potential for intoxications and overdose?. J. Psychoact. Drugs 2015, 47, 132–139. 10.1080/02791072.2015.1013202. [DOI] [PubMed] [Google Scholar]
- Cody R. B.; Laramée J. A.; Durst H. D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- Lesiak A. D.; Musah R. A. Application of ambient ionization high resolution mass spectrometry to determination of the botanical provenance of the constituents of psychoactive drug mixtures. Forensic Sci. Int. 2016, 266, 271–280. 10.1016/j.forsciint.2016.06.009. [DOI] [PubMed] [Google Scholar]
- FDA (Food and Drug Administration) Bioanalytical Method Validation Guidance for Industry. 2018. Available at https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. Accessed Nov 21, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.