Abstract
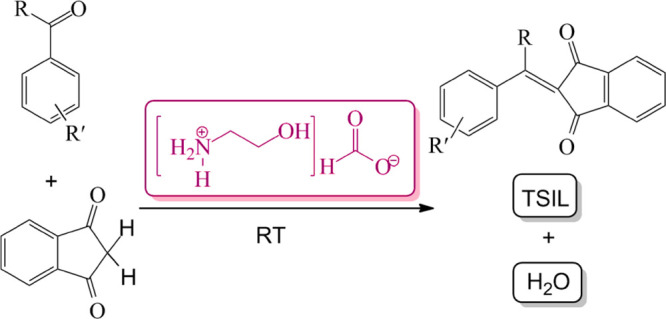
A novel method for condensation reaction of indan-1,3-dione with various aldehydes which are efficiently catalyzed by a task-specific ionic liquid, 2-hydroxyethylammonium formate, to provide the corresponding 2-arylidenindane-1,3-diones has been developed. This green, low-cost, high-yield, and fast reaction takes place at room temperature without the use of any solvent and catalyst. A plausible reaction mechanism that involves ionic liquid-assisted activation is also discussed. This work is the first report of ionic liquids as a reaction medium and catalyst for the synthesis of 2-arylidenindane-1,3-diones.
Introduction
2-Arylidenindane-1,3-diones are an important class of chemicals with many potential applications ranging from material to medical science. Some derivatives show antibacterial activities and nonlinear optical properties, and some have been used as electroluminescent devices, eye lens clarification agents,1 urease and protease inhibitors, and anticoagulant agents and alleviate symptoms of diabetes.2−4 They can be also considered as organic Lewis acids.5,6 Because of their low-lying lower unoccupied molecular orbitals, they are reactive Michael acceptors and have been used as heterodienes in cycloaddition reactions.7,8
Synthesis of 2-arylideneindane-1,3-diones through condensation reaction of indan-1,3-dione with aromatic aldehydes using piperidinein in organic solvents,8 pyridine,2 and aqueous sodium hydroxide9 has been reported. In addition, various amino acids and amines have been employed for synthesis of 2-arylidenindane-1,3-diones starting from aromatic aldehydes and indan-1,3-dione.10 Also, the microwave technique (250 watt, 150 °C) in water has been utilized to obtain 2-arylidenindane-1,3-diones.11 In addition, synthesis of 2-arilydeneindan-1,3-diones in the presence of calcium oxide,12 magnesium oxide or silica gel,13 and zirconium4 has been reported. Nevertheless, the reported methods have some flaws, namely, usage of costly reagents and toxic solvents, time- and resource-wasting procedures, poor yields, and harsh reaction conditions. Thus, a facile and green method for synthesis of 2-arylidenindan-1,3-diones is still challenging and necessary. Ionic liquids (ILs) have attracted a great deal of interest for organic transformations14 because of high ionic conductivity, solubility of different materials, and thermal, mechanical, chemical, and electrochemical stabilities.15 Unique properties of ILs such as low toxicity, nonflammability, nonvolatility, and ease of handling make them green media for organic synthesis.16 Inspired by the previous report of our group,17 we sought to rational design of a reaction for synthesis of 2-arylidenindan-1,3-diones by the IL. On this subject, in this study, a low-cost and task-specific IL, namely, 2-hydroxyethylammonium formate (2-HEAF),18 has been reported to form 2-arylidenindan-1,3-dione derivatives. To our knowledge, there is no report describing the synthesis of 2-arylidenindan-1,3-diones using ILs. 2-HEAF with unique properties, such as high conductivity, high solvation power, low melting point, and easy fabrication from inexpensive resources, is a very promising high-performance task-specific room temperature IL. Also, this method is the fastest reported so far for condensation reaction between aromatic aldehydes and indan-1,3-dione.
Results and Discussion
We started our investigation by considering indan-1,3-dione and benzaldehyde as model substrates. The progress of the reactions was checked with thin layer chromatography (TLC). Using 0.25 mmol of benzaldehyde and 0.25 mmol of indan-1,3-dione even after 72 h of stirring neat without adding 2-HEAF at room temperature, in ethanol at room temperature and also under reflux conditions, low yield was obtained (Table 1, entries 1–3). Accordingly, we carried out the reaction in the presence of 2-HEAF as an IL. Upon the addition of 1 mL of 2-HEAF, condensation immediately occurred and the reaction led to the desired product straightaway after less than 1 min in 80% yield without using any solvent or catalyst at room temperature (Table 1, entry 4). Briefly, the addition of water to the reaction mixture resulted in an immediate precipitation of the final products, and further washing with distilled water led to the desired pure 2-benzylidenindane-1,3-diones. The optimum amount of 2-HEAF was examined by the comparison of four different volumes (1, 0.5, 0.1, and 0.01 mL) which equals ∼11.2, 5.6, 1.1, and 0.1 mmol, respectively (Mw = 107.11 g/mol; density = 1.204 g/cm3), in this reaction.17 In these cases, the desired arylidenindane-1,3-dione was formed in 80, 80, 85, and 98% isolated yields after 1 min (Table 1, entries 4–7). In the presence of 0.01 mL of 2-HEAF, the desired product in 98% yield (monitored by TLC) was isolated, and therefore, we decided to use this minimum amount for the reaction. Among the various conditions, the neat condition was found to be the most convenient, affording the desired 2-benzylidenindane-1,3-diones in excellent yields at room temperature and in short reaction times. Full synthetic details are reported in the Experimental Section. The lack of the formation of the product in the absence of 2-HEAF reveals the crucial catalytic role of this IL to produce 2-arylidenindane-1,3-diones. Additional experiments were carried out to examine the influence of other ILs through some experiments. Among various ILs tested, [tetra-n-butylphosphonium-bromide (TBB), diethanolammonium formate (Di-Fo), diethanolammonium acrylate (Di-Acryl), and diethanolammonium acetate (Di-Ac)] gave poor to moderate yields of the corresponding product (Table 2, entries 1–5). Therefore, it was concluded that 2-HEAF was the best within the five ILs used in this study. To extend the scope of this method, the reaction was further investigated with various aryl and heteroaryl aldehydes to afford the corresponding products, and the results are summarized in Table 3. To the best of our knowledge, synthesized derivatives 15 and 17 are new.
Table 1. Synthesis of 2-Arylidenindan-1,3-dione by the Reaction of Indan-1,3-dione with Benzaldehyde under Various Conditions in the Presence of 2-HEAFa.
| entry | 2-HEAF (mmol) | solvent | temp. (°C) | time | yield (%)b |
|---|---|---|---|---|---|
| 1 | 0.0 | r.t. | 72 h | 25 | |
| 2 | 0.0 | EtOH | r.t. | 24 h | 25 |
| 3 | 0.0 | EtOH | reflux | 24 h | 25 |
| 4 | 11.2 | r.t. | 1 min | 80 | |
| 5 | 5.6 | r.t. | 1 min | 80 | |
| 6 | 1.1 | r.t. | 1 min | 85 | |
| 7 | 0.1 | r.t. | 1 min | 98 |
Reaction conditions: benzaldehyde (0.25 mmol), indan-1,3-dione (0.25 mmol), and 2-HEAF.
Yields refer to the pure isolated products.
Table 2. Catalytic Effect of Some ILs on Synthesis of 2-Arylidenindan-1,3-dione by the Reaction of Indan-1,3-dione with Benzaldehydea.
| entry | TSIL | IL (mL) | time | yield (%)b |
|---|---|---|---|---|
| 1 | TBB | 1 | 48 h | 25 |
| 2 | Di-Fo | 1 | 5 min | 25 |
| 3 | Di-Ac | 1 | 5 min | 75 |
| 4 | Di-Acryl | 1 | 10 min | 80 |
| 5 | 2-HEAF | 1 | 1 min | 98 |
Reaction conditions: benzaldehyde (0.25 mmol), indan-1,3 dione (0.25 mmol), and 2-HEAF at room temperature.
Yields refer to the pure isolated products.
Table 3. Synthesis of 2-Arylidenindane-1,3-diones Promoted by 2-HEAF.
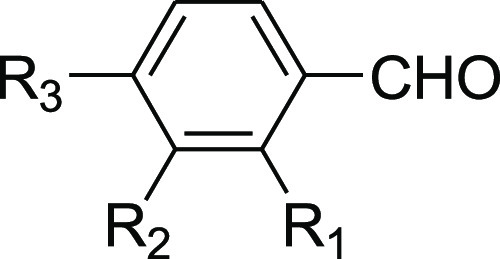
| entry | aldehyde | yield % | mp (°C) |
|---|---|---|---|
| 1 | R1, R2, R3 = H | 98 | 150 |
| 2 | R1, R2 = H; R3 = CH3 | 98 | 150–152 |
| 3 | R1, R2 = H; R3 = OCH3 | 98 | 159 |
| 4 | R1, R2 = H; R3 = NO2 | 98 | 230–232 |
| 5 | R1 = OCH3; R2, R3 = H | 85 | 168 |
| 6 | R1, R2 = OCH3; R3 = H | 98 | 159 |
| 7 | R1, R3 = OCH3; R2 = H | 98 | 204 |
| 8 | R1, R3 = H; R2 = NO2 | 98 | 251–252 |
| 9 | R1, R2 = H; R3 = Cl | 98 | 178 |
| 10 | R1, R3 = Cl; R2 = H | 88 | 194 |
| 11 | 4-(dimethylamino)-benzaldehyde | 98 | 205 |
| 12 | 5-methyl-2-furaldehyde | 98 | 169–170 |
| 13 | 2-thiophencarboxaldehyde | 98 | 176–178 |
| 14 | 1-naphthaldehyde | 98 | 175 |
| 15 | biphenyl-4-carbaldehyde | 90 | 238–240 |
| 16 | 4-(dimethylamino)-cinnammaldehyde | 98 | 252–253 |
| 17 | 4-(12-bromododecyloxy) benzaldehyde | 60 | 104–106 |
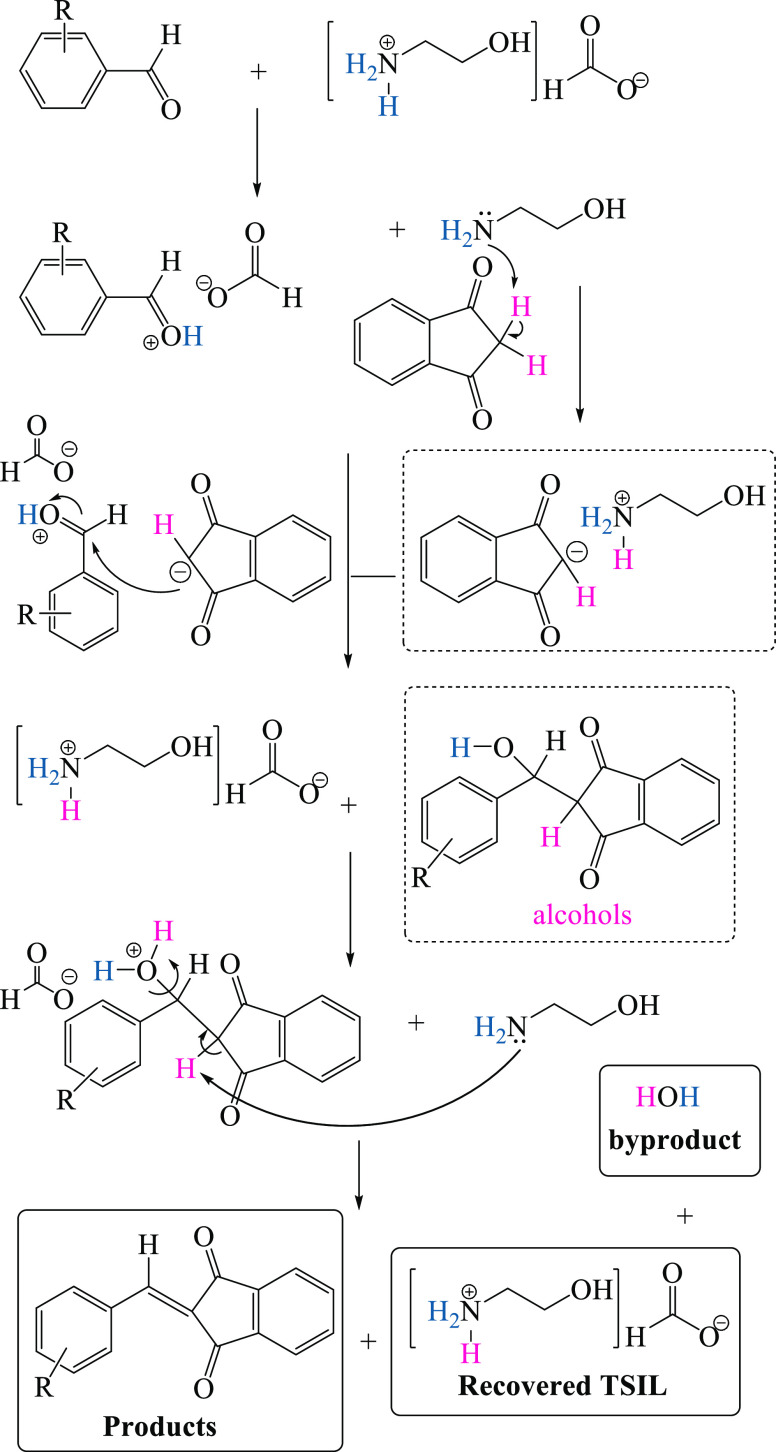
Based on our previous work,17 a step-by-step mechanism has been proposed for the IL-catalyzed condensation reaction of aromatic aldehydes with indan-1,3-dione, as outlined in Scheme 1.
Scheme 1. Proposed Mechanism for the Formation of 2-Arylidenindan-1,3-diones in the Presence of 2-HEAF (IL).
In the next step, the protonated aldehydes may undergo a nucleophilic attack by these nucleophilic intermediates, which affords alcohols. It important to point out that if acidic protons are available (with the protic solvent or catalyst), alcohols tend to remove water to generate 2-benzylidene-indan-1,3-diones. It is worth noting that no alcohols (monitored by TLC) were detected in this reaction and only 2-arylidenindane-1,3-diones in quantitative yield were obtained. 2-HEAF was recovered, and water was detected as the only byproduct of the reactions. We believe that the described reaction proceeds through a one-pot consecutive pathway affected by dual catalytic roles of 2-HEAF as a task-specific IL. As can be seen, the protonated carbonyl moiety of the aldehydes can be easily generated by means of the acidic fragment of 2-HEAF, implying that ethanol amine might serve as a base and therefore deprotonate C–H acids (indan-1,3-dione), giving active enolate.
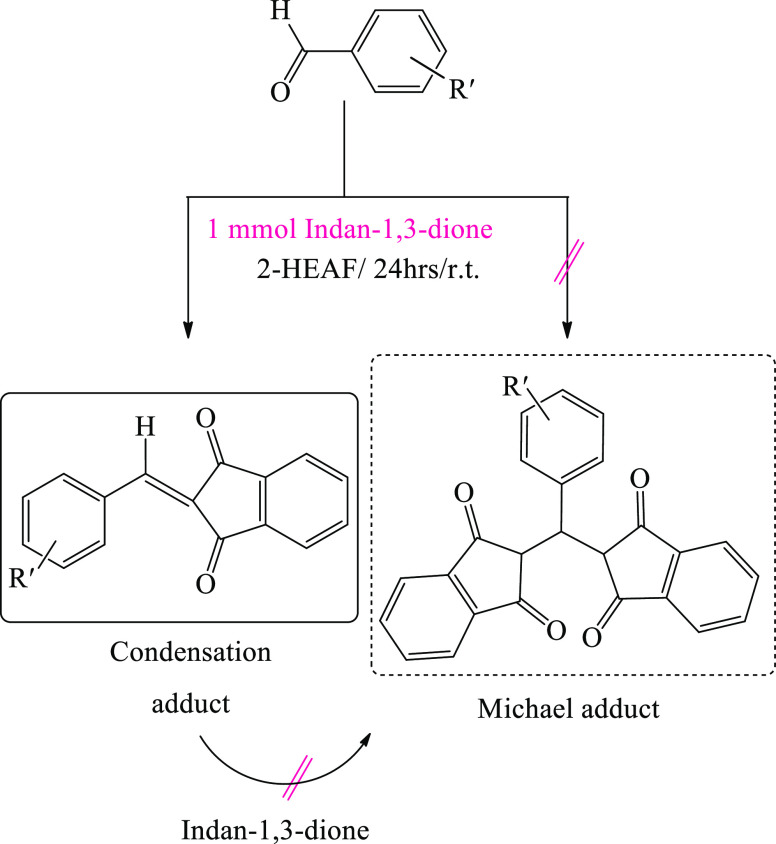
Extending the study further, we decided to perform the reaction in the presence of an excess amount of indan-1,3-dione to investigate if it was able to provide Michael adducts. For this purpose, as a pilot experiment, a mixture of indan-1,3-dione and benzaldehye (ratio 2:1) in 0.01 mL IL was stirred, the reaction was terminated after a certain time monitored by TLC, and the purified product was yielded after workup. Remarkably, even in the presence of an excess amount of indan-1,3-dione, only 2-arylidenindan-1,3-dione was observed and Michael adducts were not identified (monitored by TLC). Besides this, to prove the efficacy of the IL, the synthesis of 2-arylidenindan-1,3-dione in the presence of 2-HEAF was compared with some of the previously reported methods (Table 4).
Table 4. Comparison between Our Results for the Synthesis of Arylidenindane-1,3-diones and Other Reported Methods.
Conclusions
In general, 2-arylidenindan-1,3-dione derivatives are used as important intermediates in organic synthesis, and thus, a simple, green, and efficient protocol for their synthesis is essential. The present procedure that involves the use of a simply made and inexpensive ammonium-based IL provides a novel protocol for the generation of a library of diversely substituted 2-benzylidene-indan-1,3-diones under neat conditions at room temperature in high yields. Attractive features such as an eco-friendly, environmentally friendly, and simple procedure combined with the ease of workup make this method economic, benign, and a waste-free chemical process. We also, encountered a remarkable acceleration in the rate of a reaction with the use of this IL. This novel method opens up a new access to this group of compounds (Scheme 2).
Scheme 2. Formation of 2-Arylidenindan-1,3-dione in the Presence of an Excess Amount of Indan-1,3-dione.
Experimental Section
Ethanolamine, formic acid, indan-1,3-dione, aldehydes, and 12-dibromododecane were all reagent-grade materials and used without further purification. Throughout all experiments, distilled water was used, and all the experiments were carried out at room temperature. 1H NMR spectra were recorded on a 200 MHz spectrometer in deuteriochloroform solution and are reported in parts per million with respect to the chloroform peak at 7.26 ppm. 13C NMR spectra were recorded on a 200 MHz spectrometer in deuteriochloroform solution and are reported in parts per million with respect to the chloroform peak at 77.0. The units of the coupling constants (J) are given in Hz. Melting points are uncorrected. Fourier transform infrared spectra were recorded using a drop casting technique on KBr plates and are reported in wavenumbers (cm–1).
General Procedure for the Synthesis of 2-Arylidenindane-1,3-diones
(Representative procedure for synthesis of 2-benzylidene-1H-indene-1,3-(2H)-dione). In a reaction vial (in a round bottom glass test tube), benzaldehyde (0.106 g, 0.25 mmol), indan-1,3-dione (0.036 g, 0.25 mmol), and 2-HEAF (0.1 mmol) were mixed together and stirred for 1 min at room temperature until completion (TLC) of the reaction. Then, the addition of water (5 mL) led to the immediate formation of the pure product of 2-benzylidene-1H-indene-1,3-(2H)-dione (0.230 g, 98%). The precipitated condensation product was filtered off and washed with water to afford the desired adducts. After completion of the reaction, water was removed via rotary evaporation, and the remaining IL was rinsed with ethyl acetate. In the following cycles (2, 3, ...) a small portion of the fresh IL was added each time to compensate the loss of IL and reach its optimized amount (0.1 mmol). This procedure was followed for all the reactions listed in Table 3.
General Procedure for the Synthesis of 4-(12-Bromododecyloxy)-benzaldehyde
To a solution of 4-hydroxybenzaldehyde (2.0 g, 16.40 mmol) and K2CO3 (3.97 g, 28.7 mmol) in 20 mL of acetone, 1,12-dibromododecane (16.14 g, 49.2 mmol) in 5 mL of acetone was added dropwise at room temperature, and the resulting mixture was refluxed for 17 h. The solvent was removed via rotary evaporation, and the residue was dissolved in CH2Cl2 (20 mL) and washed several times with water. After drying over MgSO4, the solvent was evaporated, and the residue purified by column chromatography on silica gel, using a solvent gradient from hexane/CH2Cl2 (2:8, v/v), afforded 4-(12-bromododecyloxy)-benzaldehyde as a crystalline white solid (5.58 g, 92%). IR νmax (KBr): 3112, 2910, 2780 (HCO), 1687 (CO), 1610–1450, 1210 cm–1. 1H NMR (600 MHz; CDCl3): δH (ppm) 1.28–1.45 (m, 16H), 1.79–1.85 (m, 4H), 3.39 (t, J = 9.7 Hz, 2H, CH2Br), 4.01 (t, J = 9.6 Hz, 2H, CH2O), 6.98 (d, J = 12.5 Hz, 2H, Ph), 7.81 (d, J = 12.5 Hz, 2H, Ph), 9.87 (s, 1H, HC=O). 13C NMR (150 MHz; CDCl3): δC (ppm) 26.1, 28.3, 28.9, 29.2, 29.5, 29.6, 29.7, 33.0, 34.1, 68.5, 114.8, 129.9, 132.0, 164.3, 190.6; m/z: (EI) 369.9 (M+, 22%), 289(9), 123(100), 83.9(45), 69(30). HRMS (EI): m/z calcd for C19H29BrO2, 368.1351; found, 368.1358.
2(3-(12-Bromododecyloxy)benzylidene)-1H-indene-1,3(2H)-dione (Table 3, Entry 17)
Pale-green powder, mp 104–106 °C. IR νmax (KBr) 2918, 2850, 1680, 1579, 1552, 1271, 1207, 1178, 742 cm–1. 1H NMR (200 MHz; CDCl3): δH (ppm) 1.30–2.02 (m, 20H, CH2), 3.42 (t, J = 6.5 Hz, 2H, CH2Br), 4.08 (t, J = 6.0 Hz, 2H, CH2O), 7.01 (d, J = 8.5 Hz, 2H, Ph), 7.81–8.16 (m, 5H, Ar–H, Hvinyl), 7.99 (d, J = 8.4 Hz, 2H, Ph). 13C NMR (50 MHz; CDCl3): δC (ppm) 25.96, 28.18, 28.77, 29.06, 29.33–29.51 (t), 32.84, 34.04, 68.44, 111.97, 114.85, 123.05, 126.38, 134.82, 135.03, 137.28, 140, 146.99, 162.83, 163.77, 190.88. Mass 29, 41, 55, 165, 233, 249, 250, 496, 499.
2(Biphenyl-3-methylene)-1H-indene-1,3(2H)-dione (Table 3, Entry 15)
Yellow powder, mp 238–240 °C. 1H NMR (200 MHz; CDCl3): δH (ppm) 7.37–8.58 (m, 14H, Ph). 13C NMR (50 MHz; CDCl3): δC (ppm) 123.32, 127.26, 127.34, 128.42, 129, 132.12, 134.89, 135.17, 135.37, 139.77, 140.05, 142.52, 145.77, 146.43, 189.16, 190.38. Mass 77, 104, 233, 252, 309, 310.
Acknowledgments
The financial support of Razi University and Maa-ja vesitekniikan tuki ry (MVTT) to accomplish the project is appreciated.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03645.
1H and 13C NMR and mass spectra of the new synthesized products (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Salama M. A.; Yousif N. M.; Ahmed F. H.; Hammam A. G. Some reactions with 2-arylidene-1,3-indandiones and related derivatives with antibacterial activities. Pol. J. Chem. 1988, 62, 243–250. [Google Scholar]; b Afsah E. M.; Etman H. A.; Hamama W. S.; Sayed-Ahmed A. F. A study on the reaction of 1,3-indandione with Schiff bases: synthesis of new 1,3-indandiones with expected psychopharmacological and anticoagulant activity. Boll. Chim. Farm. 1998, 137, 244–248. [Google Scholar]; c Osman S. A. M.; Yousif N. M.; Ahmed F. H.; Hammam A. G. Some reactions with 2-arylidene-1,3-indandiones for preparation of derivatives possessing antibacterial activity. Egypt. J. Chem. 1988, 31, 727–734. [Google Scholar]; d Afsah E. M.; Hammouda M.; Zoorob H.; Khalifa M. M.; Zimaity M. Introduction of some Pharmaceutically Active Heterocycles into the Benzylic moiety of 2-Benzyl-1,3-indandione. Pharmazie 1990, 45, 255–257. [Google Scholar]; e Yoakim C.; Hache B.; Ogilvie W.; O’Meara J. A.; White P. W.; Goudreau N.. Inhibitors of papilloma virus. WO2002050082A2, 2002; Chem. Abstr. 2002, 137, 63181.; f Szymusiak H.; Zieliński R.; Domagalska B. W.; Wilk K. A. Electronic structure and nonlinear optical properties of model push–pull polyenes with modified indanone groups: a theoretical investigation. Comput. Chem. 2000, 24, 369–380. 10.1016/s0097-8485(99)00082-0. [DOI] [PubMed] [Google Scholar]; g Aziz A. B. M. S. A.; Mohamed E. S. Eur. Pat. Appl. 1992, 14 pp. CODEN: EPXXDW EP 489991 A1 19920617.
- Khan G.; Aftab M. F.; Bano B.; Khan K. M.; Murtaza M.; Siddiqui S.; Rehman M. H.; Waraich R. S. A New Indanedione Derivative Alleviates Symptoms of Diabetes by Modulating RAGE-NF-kappaB Pathway in db/db Mice. Biochem. Biophys. Res. Commun. 2018, 501, 863–870. 10.1016/j.bbrc.2018.05.043. [DOI] [PubMed] [Google Scholar]
- a Bano B.; Kanwal; Khan K. M.; Begum F.; Lodhi M. A.; Salar U.; Khalil R.; Ul-Haq Z.; Perveen S. Benzylidine indane-1,3-diones: As Novel Urease Inhibitors; Synthesis, in Vitro, and in Silico Studies. Bioorg. Chem. 2018, 81, 658–671. 10.1016/j.bioorg.2018.09.030. [DOI] [PubMed] [Google Scholar]; b Mitka K.; Kowalski P.; Pawelec D.; Majka Z. Synthesis of Novel Indane-1,3-dione Derivatives and Their Biological Evaluation as Anticoagulant Agents. Croat. Chem. Acta 2009, 82, 613–618. [Google Scholar]
- Oliveira A. F. C. D. S.; de Souza A. P. M.; de Oliveira A. S.; da Silva M. L.; de Oliveira F. M.; Santos E. G.; da Silva Í. E. P.; Ferreira R. S.; Villela F. S.; Martins F. T.; Leal D. H. S.; Vaz B. G.; Teixeira R. R.; de Paula S. O. Zirconium catalyzed synthesis of 2-arylidene Indan-1,3-diones and evaluation of their inhibitory activity against NS2B-NS3 WNV protease. Eur. J. Med. Chem. 2018, 149, 98–109. 10.1016/j.ejmech.2018.02.037. [DOI] [PubMed] [Google Scholar]
- a Cammi R.; Ghio C.; Tomasi J. Neutral organic lewis acids of π type. Int. J. Quantum Chem. 1986, 29, 527–539. 10.1002/qua.560290325. [DOI] [Google Scholar]; b Liedl E.; Wolschann P. The reaction of activated nitrones to C-C-double bonds of organicLewis acids. OrganicLewis acids 37. Monatsh. Chem. 1982, 113, 1067–1071. 10.1007/bf00799249. [DOI] [Google Scholar]; c Goerner H.; Leitich J.; Polansky O. E.; Riemer W.; Ritter-Thomas U.; Schlamann B. Die Photoenolisierung, Autoxidation und Dimerisation von 2-Isobutyliden-1,3-indandion. Monatsh. Chem. 1980, 111, 309–329. 10.1007/BF00938736. [DOI] [Google Scholar]; d Margaretha P. Die reaktion von Meldrumsäure mit isobutylidenmeldrumsäure : Ein reaktionskinetischer beitrag zur Michael-reaktion. Tetrahedron 1972, 28, 83–87. 10.1016/0040-4020(72)80057-7. [DOI] [Google Scholar]; f Margaretha P.; Polansky O. E. Anbadons originating from organic bases and electrically neutral organic Lewis acids. Monatsh. Chem. 1969, 100, 576–583. 10.1007/BF00904105. [DOI] [Google Scholar]
- Duan J.; Cheng J.; Li B.; Qi F.; Li P. Enantioselective synthesis of spiro[1,3-indanedione-tetrahydrothiophene]s by organocatalytic Sulfa-Michael domino reaction. Eur. J. Org. Chem. 2015, 2015, 6130–6134. 10.1002/ejoc.201500837. [DOI] [Google Scholar]
- Lantaño B.; Aguirre J. M.; Drago E. V.; Bollini M.; Faba D. J.; de la Faba J. D. Synthesis of Benzylidenecycloalkan-1-ones and 1,5-diketones under Claisen-Schmidt reaction: Influence of the temperature and electronic nature of arylaldehydes. Synth. Commun. 2017, 47, 2202–2214. 10.1080/00397911.2017.1367819. [DOI] [Google Scholar]
- a Trost B. M.Comprehensive Organic Synthesis; Pergamon Press: Oxford, 1991; pp 341–394. [Google Scholar]; b Inayama S.; Mamoto K.; Shibata T.; Hirose T. Structure and antitumor activity relation of 2-arylidene-4-cyclopentene-1,3-diones and 2-arylideneindan-1,3-diones. J. Med. Chem. 1976, 19, 433–436. 10.1021/jm00225a022. [DOI] [PubMed] [Google Scholar]
- Tugrak M.; Inci Gul H.; Sakagami H.; Gulcin I.; Supuran C. T. New azafluorenones with cytotoxic and carbonic anhydrase inhibitory properties: 2-Aryl-4-(4-hydroxyphenyl)-5H-indeno[1,2-b]pyridin-5-ones. Bioorg. Chem. 2018, 81, 433–439. 10.1016/j.bioorg.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Ramachary D. B.; Anebouselvy K.; Chowdari N. S.; Barbas C. F. Direct organocatalytic asymmetric heterodomino reactions: The Knoevenagel/Diels-Alder/Epime sequence for the highly diastereoselective synthesis of symmetrical and nonsymmetrical synthons of benzoannelated centropolyquinanes. J. Org. Chem. 2004, 69, 5838–5849. 10.1021/jo049581r. [DOI] [PubMed] [Google Scholar]
- Tu S.-J.; Jiang B.; Zhang J.-Y.; Jia R.-H.; Zhang Y.; Yao C.-S. Efficient and direct synthesis of poly-substituted indeni[1,2-b]quinolines assisted by p-toluene sulfonic acid using high-temperature water and microwave heating via one-pot, three-component reaction. Org. Biomol. Chem. 2006, 4, 3980–3985. 10.1039/b611462h. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Ren Z.; Cao W.; Tong W.; Gao M. Solvent-Free Synthesis of Ethyl α-Cyanocinnamate in the Presence of CaO. Synth. Commun. 2004, 34, 2047–2051. 10.1081/scc-120037918. [DOI] [Google Scholar]
- Wu D.; Ren Z.; Cao W.; Tong W. Solvent-Free Synthesis of 2-Arylideneindan-1,3-diones in the Presence of Magnesium Oxide or Silica Gel Under Grinding. Synth. Commun. 2005, 35, 3157–3162. 10.1080/00397910500282968. [DOI] [Google Scholar]
- a Cao P.; Yuan Y.; Huang C.; Sun W.; Zhao L. Promoting the sulfuric acid catalyzed isobutane alkylation by quaternary ammonium ionic liquids. AIChE J. 2020, 66, e16979 10.1002/aic.16979. [DOI] [Google Scholar]; b Zhang X.; Li H.; Li X.; Liu Y.; Li X.; Guan J.; Long J. Glucose Aqueous Isomerization Catalyzed by Basic Ionic Liquids. ACS Sustainable Chem. Eng. 2019, 7, 13247–13256. 10.1021/acssuschemeng.9b02495. [DOI] [Google Scholar]; c Wang A.; Zhu X.; Yin H.; Fu Y.; Hou X. Chlorination of Toluene to o-Chlorotoluene Catalyzed by Ionic Liquids. Catalysts 2018, 8, 532–547. 10.3390/catal8110532. [DOI] [Google Scholar]; d Tong J.; Li Y.-W.; Xu D.-Z. Solvent-Controlled Friedel-Crafts Reaction for the Synthesis of 3-Indolyl-3-hydroxy Oxindoles and 3,3-Diindolyl Oxindoles Catalyzed by Dabco-Base Ionic Liquids. Chemistry Select 2017, 2, 3799–3803. 10.1002/slct.201700603. [DOI] [Google Scholar]; e Wang S.; Liu L.; Jiang Y.; Hu M.; Li S.; Zhai Q. Enzymatic Polymerization of Phenols Catalyzed by Chloroperoxidase in the Presence of Ionic Liquids/Quaternary Ammonium Salts. Chem. J. Chin. Univ. 2016, 37, 1733–1739. 10.7503/cjcu20160236. [DOI] [Google Scholar]; f Li L.; Liu Y.; Yu S.-T.; Liu S.-W.; Xie C.-X.; Liu F.-S. Hydration of Alpha-Pinene Homogenous Catalyzed by Acidic Polyether-Modified Ammonium Salt Ionic Liquid in “Microreactor”. Res. Chem. Intermed. 2015, 41, 2407–2414. 10.1007/s11164-013-1355-1. [DOI] [Google Scholar]; g Zhang J.; Zhang Y.; Zhou Z. Hydroxyl Ammonium Ionic Liquid-Catalyzed Simple and Efficient Synthesis of 5-arylidene-2,4thiazolidinediones under Solvent-Free Conditions. Green Chem. Lett. Rev. 2014, 7, 90–94. 10.1080/17518253.2014.895866. [DOI] [Google Scholar]; h Zhang B.-H.; He J.-Y.; Liu S.-J.; Shi L.-X. Asymmetric Aldol Reactions in Caprolactam-Quaternary Ammonium Salt Coordination Ionic Liquid Catalyzed by L-Pro-L-Trp. Helv. Chim. Acta 2013, 96, 1266–1268. 10.1002/hlca.201200457. [DOI] [Google Scholar]; i Ghosh S. K.; Qiao Y.; Ni B.; Headley A. D. Asymmetric Michael reactions catalyzed by a highly efficient and recyclable quaternary ammonium ionic liquid-supported organocatalyst in aqueous media. Org. Biomol. Chem. 2013, 11, 1801–1804. 10.1039/c3ob27398a. [DOI] [PubMed] [Google Scholar]
- a Singh S. K.; Savoy A. W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq 2020, 297, 112038. 10.1016/j.molliq.2019.112038. [DOI] [Google Scholar]; b Wang B.; Qin L.; Mu T.; Xue Z.; Gao G. Are ionic liquids chemically stable?. Chem. Rev. 2017, 117, 7113–7131. 10.1021/acs.chemrev.6b00594. [DOI] [PubMed] [Google Scholar]; c Dong K.; Liu X.; Dong H.; Zhang X.; Zhang S. Multiscale studies on ionic liquids. Chem. Rev. 2017, 117, 6636–6695. 10.1021/acs.chemrev.6b00776. [DOI] [PubMed] [Google Scholar]; d Zhang S.; Zhang Q.; Zhang Y.; Chen Z.; Watanabe M.; Deng Y. Beyond solvents and electrolytes: Ionic liquids-based advanced functional materials. Prog. Mater. Sci. 2016, 77, 80–124. 10.1016/j.pmatsci.2015.10.001. [DOI] [Google Scholar]; e Zhang J.; Liu H.; Jiang L. Membrane-Based Strategy for Efficient Ionic Liquids/Water Separation Assisted by Superwettability. Adv. Funct. Mater. 2017, 27, 1606544. 10.1002/adfm.201606544. [DOI] [Google Scholar]
- Ahmed A. In Sustainable Organic Synthesis in Ionic Liquids; Inamuddin A. A., Ed.; Applications of Nanotechnology for Green Synthesis. Nanotechnology in the Life Sciences; Springer: Cham, 2020. [Google Scholar]
- Alizadeh A.; Khodaei M. M.; Eshghi A. Ambiphilic dual activation role of a task-specific ionic liquid: 2-hydroxyethylammonium formate as a recyclable promoter and medium for the green synthesis of β–Nitrostyrenes. J. Org. Chem. 2010, 75, 8295–8298. 10.1021/jo101696z. [DOI] [PubMed] [Google Scholar]
- Bicak N. A new ionic liquid: 2-hydroxy ethylammonium formate. J. Mol. Liq. 2005, 116, 15–18. 10.1016/j.molliq.2004.03.006. [DOI] [Google Scholar]
- Okukawa T.; Suzuki K.; Sekiya M. Formic acid reduction. XX. Reduction of 2-Benzylidene-1,3-indandiones. Chem. Pharm. Bull. 1974, 22, 448–451. 10.1248/cpb.22.448. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.