Abstract

The search for alternative, biodegradable, and sustainable raw materials to replace finite petrochemicals is an area of great interest. Triglycerides obtained from oilseed crops are such potential raw materials. In this study, sesame oil was trans-esterified to sesame fatty methyl esters (SEFAMEs) that were used as precursors in the synthesis of sesame fatty methyl esters sulfonate (SEFAMESO) surfactant. SEFAME and SEFAMESO surfactants were characterized by high-performance liquid chromatography–mass spectrometry (HPLC-MS), 1H NMR, and Fourier transform infrared (FTIR) spectra. HPLC-MS, 1H NMR, and FTIR spectra indicated successful trans-esterification and conversion of SEFAMEs to SEFAMESO. Solution properties of the SEFAMESO surfactant including hydrophilic–lipophilic balance (HLB) value, Krafft point, foam-ability, critical micelle concentration (CMC), counterion degree of binding and thermodynamic parameters such as ΔG°mic, ΔH°mic, ΔS°mic, ΔH°mic,* and TC were evaluated. The CMC values of SEFAMESO at 298.15 K were relatively lower than that of the sodium dodecyl sulfate (SDS) standard, and these values decreased to a minimum at 303.15 K and then increased with an increase in temperature. ΔG°mic values were negative indicating a spontaneous micellization process. ΔH°mic and ΔS°mic values revealed that micellization was entropy-driven at low temperatures and both entropy- and enthalpy-driven at high temperatures. ΔH°mic,* values were negative suggesting formation of stable micelles. The evaluated properties revealed the potential application of the synthesized surfactant as a cleansing agent.
1. Introduction
The search for renewable and biodegradable raw materials for industrial productions has been an area of intensive research interest owing to increased demand to conserve finite petrochemical resources and the need to protect the environment from persistent petrochemical products.1−6 Vegetable oil crops such as sesame (Sesamum L. indicum) represent renewable and biodegradable raw materials that can supplement or replace finite petrochemical sources. Sesame is extensively used as food in nutraceutical and pharmaceutical applications because of its high oil, protein, and antioxidant contents. It is mainly cultivated for grains and oil in East Africa and used ethnobotanically against health problems such as cancer, cold, and colic in Germany, China, India, and Turkey.7−9 This plant is highly resistant to drought and can produce good yields at adequate soil moisture content and takes about 3–4 months to mature. Sesame seed has an oil content ranging from 35 to 63% being among the highest of any oil crop.7,10 Nevertheless, the International Plant Genetic Resources Institute (IPGRI) has listed it among the neglected and underutilized crops with high potential.11 Thus, the utilization of sesame oil as a feedstock in industrial production of fatty methyl esters for surfactant synthesis can stimulate rural economic development.
Surfactants can either be derived from petrochemicals or oleochemicals such as vegetable oils.1,4,12−17 Surfactants from the oleochemical origin are considered nontoxic and biodegradable, hence eco-friendly, besides being derived from renewable resources.1,3,6,14−18 The use of oleochemical feedstock to produce surfactants is a technology that has been around for many years; however, its potential has not been exploited because they could not compete with low-cost petrochemical products.15 One of the structural drawbacks limiting the use of vegetable oils (or triglycerides) as raw materials is their thermal stability due to the presence of H on the β-C of the glycerol backbone.19 Consequently, chemical modification has to be carried out to enable the industrial production of valuable commercial products. The routes to chemical modification of triglycerides include hydrolysis, esterification, trans-esterification, and hydrogenation, among others.6,20 Some of these processes introduce branched (or bulky) moieties into a fatty acid structure, which improves fundamental properties required for a variety of applications.14,17,19,20 Trans-esterification has been noted as one of the important processes of transforming vegetable oils due to lower energy consumption during the production of fatty esters as compared to the splitting of fats and oils to produce fatty acids.14,21 Fatty esters are also better chemical intermediates than fatty acids in some applications such as the production of alkanolamide esters.14,22 They are often applied as biodiesel, which has been found to exhibit physical properties that are similar to those of petroleum-derived diesel, though its emission properties are superior.2,22 They are also used as solvents, lubricants, and carrier fluids and can be transformed into oleochemical derivatives such as fatty alcohols, which are used as intermediates in the production of special esters.14,23
The present study aimed at utilizing sesame oil in the synthesis of a biodegradable surfactant. Anionic fatty acid methyl ester sulfonate surfactants synthesized using biological oils are considered to exhibit excellent surface activity and self-assembly behavior.3,24 These unusual properties of surfactants and their phase behavior are essential for various applications.13,25−28 For instance, the hydrophilic–hydrophobic balance (HLB) value is a numerical correlation, which provides solubilizing and emulsifying properties of a surfactant between oil–water and water–oil phases.27 Surfactants with low HLB values in the range of 0–8 are more soluble in oil and hence, good for forming water in oil emulsions, while those with high values in the range of 8–18 solubilize in water and are good for the formulation of oil in water emulsifiers such as wetting agents and detergents.25,27 Therefore, HLB values are of significance in determining quality, control, and suitability of a surfactant as moisturizing lotions, creams, or shampoos in cosmetics and as dispersants, wetting and solubilizing agents in drug delivery.25,26
The critical micelle concentration (CMC) is also one of the important properties of a surfactant. CMC is a specific concentration at which surfactant monomer molecules self-assemble into nanostructures called micelles.24,28 CMC represents surfactant concentration at which nearly maximum adsorption occurs; hence, it is of great interest regarding the practical use of surfactants.25,27 Physicochemical, transport, and thermodynamic properties of surfactants such as activity, enthalpy, surface tension, solubilization, equivalent conductivity, density, viscosity, osmotic pressure, and turbidity, among others, are important in understanding useful surfactant interactions and behavior in solution.12,13 These properties vary markedly below and above the CMC, and plotting them as a function of surfactant concentration or its logarithm, show a sharp break in the curves that indicate formation of micelles.12,13,27,29 In this study, the potentiality of sesame oil was evaluated as an alternative feedstock in the synthesis of eco-friendly surfactants. The oil was trans-esterified to sesame fatty methyl esters (SEFAMEs), which was then used as a precursor in the synthesized sesame fatty methyl esters sulfonate (SEFAMESO) surfactant. SEFAME and SEFAMESO surfactants were characterized by high-performance liquid chromatography–mass spectrometry (HPLC-MS), Fourier transform infrared (FTIR), and 1H NMR spectra. Surfactant properties of the SEFAMESO surfactant such as HLB values, foam-ability, Kraft temperature, CMC values, counterion degree of binding, and thermodynamic parameters (ΔG°mic, ΔH°mic and ΔS°mic, ΔH°mic,*, TC) were evaluated at 298.15, 303.15, 313.15, and 323.15 K.
2. Materials and Methods
2.1. Materials
Analytical grade chemicals were used in all of the experiments. They include n-hexane (99%), methanol (99.8%), sodium hydroxide (98%), sodium hydrogen carbonate (99%), carbon tetrachloride (99.9%), chloro-sulfonic acid (98%), n-butanol (99.5%), diethyl ether (99.5%), and SDS (98.5%) obtained from Sigma-Aldrich. Sesame oil was extracted from sesame seeds obtained from Busia County, Western Kenya. Double-distilled deionized water with specific conductivity values <2.0 μS/cm at 298.15–323 K was used to prepare solutions for conductivity measurements.
2.2. Extraction of Sesame Oil
Briefly, 300 g of sesame seeds were weighed and pulverized using pestle and mortar then placed in a 2.5 L glass bottle. Typically, 1.5 L of hexane was added to the powder and mixed properly and then corked and kept for 3 days. The mixture was filtered and the filtrate concentrated using a rotary evaporator set at 60 °C. The extracted sesame oil weighed 138 g, which was a 46% yield. This was stored in a freezer at −2 °C for subsequent experiments.
Physicochemical properties of oil were determined according to the standard methods of American Oil Chemists’ Society.30
2.3. Trans-esterification of Sesame Oil
Single-step base trans-esterification catalysis was carried out according to Knothe and Steidley.21 A 1:6 sesame oil (168 g, 0.6 mol)/methanol (115.2 g, 3.6 mol) molar ratio was transferred into a 500 mL three-necked flask with a magnetic rod and reflux setup. The mixture was added 1% (w/w) NaOH catalyst and heated under reflux in a water bath at 70 °C for 3 h, while vigorously stirring at 250 rmp. The reaction products were allowed to cool and then transferred into a separating funnel and left to stand for 12 h to allow gravitational separation. The lower layer of glycerol and the upper layer of SEFAMEs were drained into a separate flask for further purification. SEFAMEs were washed to remove remaining glycerin, methanol, catalyst, soap, and any other impurities. The initial washings were done using warm distilled water containing 10% H3PO4 acid, followed by repeated washing using warm distilled water to a relatively neutral pH. The purified SEFAMEs were heated in a hot plate to 100 °C for 1 h while stirring to expel water/moisture and then transferred into a clean bottle and kept in a freezer at −2 °C for further use. The obtained SEFAME measured 159.6 g and this was 95% yield.
2.4. Synthesis of the SEFAMESO Surfactant
Typically, 15.2 g (0.053 moles) of SEFAMEs was transferred into a three-necked round bottom flask and added 17 mL of tetra-chloromethane and then placed in an ice bath with a temperature range of 0–4 °C. Briefly, 0.06 moles of ClSO3H was added dropwise into the mixture with continuous stirring for 30 min. It was then warmed to a clear solution in a steam bath after which the reaction temperature was raised and maintained at 60 °C for 3 h under reflux and vigorous stirring. The reaction mixture was quenched and poured into ice-cooled aqueous NaHCO3 to convert the product into sodium salt. The crude product was transferred into a separating funnel, extracted twice using 40 mL of n-butanol. The solvent was removed from the product using a rotary evaporator. SEFAMESO surfactant was redissolved in distilled water and purified further by extracting with diethyl ether, then concentrated using rotary evaporator, and dried under vacuum. Typically, 18.5 g of SEFAMESO was obtained as a yellow solid and this was an 89.5% yield. The synthesized surfactant was kept in the freezer for further use.
2.5. Structural Characterization of SEFAME and SEFAMESO Surfactants
2.5.1. Ultra HPLC (UHPLC)-MS Spectroscopy
UHPLC-MS spectroscopy analysis was carried out on an Agilent 1290 Infinity UHPLC coupled with an electrospray ionization (ESI) tandem mass spectrometer (Bruker microOTOF-Q II HR MS w) in a low–low mass positive ion mode loop. Samples preparation for UHPLC analysis was carried out following the reported protocol by Agilent 1290 UHPLC manufacturer for fatty methyl esters.31 The retention times and peak integrations obtained from HPLC chromatograms were used to estimate the content of each fatty acid from the total composition of fatty acids present in sesame oil.
2.5.2. FTIR Spectroscopy
FTIR analysis was carried out using a Bruker Equinox 55 FTIR spectrometer with the OPUS/IR software. The background was scanned without the sample. The sample was placed on the sample area, pressed against the diamond crystal, and scanned between 4000 and 400 cm–1 at 1 cm–1 resolution and 15 scans.
2.5.3. 1H NMR Spectroscopy
1H NMR spectra were recorded on a Bruker Avance Hg400b spectrometer operating at 400 MHz equipped with a BBO ATM 5 mm Z-gradient probe. All free induction decays were acquired at 298.15 K under steady-state conditions using standard Bruker parameter sets. Chemical shifts were referenced internally to deuterated chloroform (CDCl3) and also used as the solvent.
2.6. Determination Solution Properties of the SEFAMESO Surfactant
The HLB value was estimated, and solution properties including Krafft point and foam-ability were evaluated. The relationship between specific conductivity and surfactant concentration was used to determine CMC values, counterion degree of binding, and thermodynamic properties of micellization such as Gibbs free energy, enthalpy, entropy, and enthalpy–entropy compensation parameters.
2.6.1. Estimation of the HLB Value
HLB value of the SEFAMESO surfactant was estimated following the Davies method.32 This was done by summing up the group numbers of hydrophilic and hydrophobic groups HLB values according to eq 1.
| 1 |
Since the chemical structures of the SEFAMESO surfactant constitute a mixture of fatty methyl esters sodium sulfonate groups, the overall contribution of each fatty methyl ester derivative was considered according to eq 2.33
| 2 |
where HLBT is the overall HBL value of the surfactant mixture and HLBa, HLBb, to HLBn represent the HLB values of each individual surfactant in the mixture with Sa, Sb, to Sn denoting their respective proportion (%) in the mixture.
2.6.2. Krafft Point
Typically, 0.2% (w/v) of aqueous SEFAMESO surfactant solution was gradually heated to a temperature at which the dispersion turned clear. This procedure was repeated several times until a clear dispersion temperature was obtained.
2.6.3. Foam-ability
Typically, 50 mL of 0.1% (w/v) aqueous SEFAMESO surfactant solution was vigorously shaken in a 500 mL glass cylinder at 25 °C. The initial and the final heights of the foam after 5 min were noted. This procedure was repeated several times until clear foam heights were obtained. Foam stability was determined by subtracting the final from the initial foam height that was stable for 5 min.
2.6.4. Conductivity Measurement
The conductance measurements were carried out using a digital conductivity meter (Hanna instrument model HI-8033) and a dipping-type conductivity cell with platinized electrodes. The cell constant was calibrated using aqueous KCl solutions and the measurement of conductivities of surfactant solutions was carried out by continuous dilution of a concentrated solution. The conductivity measurements as a function of the SEFAMESO surfactant concentration were performed at 298.15, 303.15, 313.15, and 323 K. The conductivity value was recorded when its fluctuation was <1% within 2 min, and the temperature was controlled at a precision of 0.02 K. The obtained absolute conductivity values were multiplied by cell constant to obtain specific conductivity.
2.6.5. Determination of Critical Micelle Concentration (CMC) and Counterion Degree of Binding of the SEFAMESO Surfactant
The average specific conductivity values were plotted against surfactant concentration using the Origin 6.0 program (OriginLab Corporation) to obtain the intersecting lines (CMC point). The linear relationship between specific conductivity and concentration of a surfactant is given by eq 3.
| 3 |
where k0 is the specific conductivity at infinite dilution, k is the specific conductivity of the surfactant, s is the slope, and C is the concentration of surfactant.27,28
The ratio of the slope of the postmicellar region to that of the premicellar region of specific conductivity versus surfactant concentration curves was used to determine counterion degree of binding or dissociation constant (β), according to eq 4.27,28,34
| 4 |
2.6.6. Determination of Thermodynamic Properties of the SEFAMESO Surfactant
The obtained CMC data as a function of temperature were used to determine thermodynamic properties of micellization including Gibbs free energy (ΔG°mic), enthalpy (ΔH°mic), entropy (ΔS°mic), and enthalpy–entropy compensation parameters such as intrinsic enthalpy (ΔH°mic,*) and compensation temperature (Tc).
The standard free energy values were calculated based on the pseudophase separation model, eq 5(6,27−29,35,36)
| 5 |
where XCMC surfactant CMC in terms of mole fraction, β is the counterion degree of binding, R is the gas constant, and T is the temperature in K.
The standard enthalpy change of micellization was determined using eq 6.6,28,29
| 6 |
The XCMC is dependent on temperature, and plots of ln XCMC against temperature display a U-shaped curve or parabolic arc according to eq 7.
| 7 |
where A, B, and C are constants obtained from performing least squares regression fit on the ln XCMC versus T polynomial curve, which was then used to obtain ΔH°mic according to eq 8.
| 8 |
The ΔG°mic and ΔH°mic values at each temperature were used to evaluate the standard entropy change (ΔS°mic) according to eq 9.6,17,28,29,35,36
| 9 |
The enthalpy–entropy compensation parameters were determined from the linear relationship between ΔH°mic and ΔS°mic, eq 10.28,29,36
| 10 |
where ΔH°mic,* is the intrinsic enthalpy and Tc is the compensation temperature.
3. Results and Discussion
3.1. Physicochemical Properties of Extracted Sesame Oil
Physicochemical properties of extracted sesame oil and reported literature values are given in Table 1. The oil yield and the measured physicochemical properties compared well with reported values for sesame oil.7,8,37−39,42−44
Table 1. Physicochemical Properties of Sesame Oil and Reported Literature Values.
| property | present study | reported values |
|---|---|---|
| oil yield (%) | 46 | 34–63 |
| pH | 7.5 | |
| density (kg/m3) at 25 °C | 880 | 880–940 |
| specific gravity at 25 °C | 0.88 | 0.88–0.941 |
| kinematic viscosity (mm2/s) at 25 °C | 50 | 48–56 |
| free fatty acid value (mg/g of KOH) | 0.56 | 0.2–3 |
| iodine value (g I2/100 g of oil) | 105 | 103–120 |
| saponification value (mg of KOH/g of oil) | 167 | 160–197 |
| unsaponifiable matter content (%) | 1.67 | 1.5–2.3 |
| peroxide value, (mequiv O2/kg of oil) | 1.84 | 1.84–16 |
The fatty acid composition of sesame oil obtained by HPLC-MS is shown in Table 2. Sesame oil mainly constituted of unsaturated acids including linoleic and oleic acids representing about 85% of total fatty acids. Other unsaturated fatty acids include linolenic, eicosenoic, and palmitoleic acids making about 1.5%. Saturated fatty acids present are palmitic and stearic acids making about 13%. The unsaturated fatty acids being the main constituents in sesame oil have also been extensively reported by many co-workers7−10,37,38
Table 2. Fatty Acid Composition in Sesame Oila.
| fatty acid | NAC/DB | % composition |
|---|---|---|
| myristic fatty acid | 14:0 | 0.10 |
| palmitoleic fatty acid | 16:1 | 0.16 |
| palmitic fatty acid | 16:0 | 8.92 |
| linoleic fatty acid | 18:2 | 40.36 |
| linolenic fatty acid | 18:3 | 0.40 |
| oleic fatty acids | 18:1 | 44.62 |
| stearic fatty acid | 18:0 | 4.61 |
| eicosenoic fatty acid | 20:1 | 0.15 |
| eicosanoic fatty acid | 20:0 | 0.53 |
| behenic fatty acid | 22:0 | 0.15 |
NAC/DB = number of acyl carbons to the number of double bonds.
3.2. Reaction Mechanism for Trans-esterification and Synthesis of the SEFAMESO Surfactant
The reaction mechanism for trans-esterification of sesame oil is shown in Scheme 1. Sesame oil triglyceride was esterified in a base-catalyzed reaction in the presence of methanol. The base (NaOH) de-protonates methanol to produce a methoxide ion, which then attacks the carbonyl carbon of the triglyceride ester group leading to the formation of fatty acid methyl esters, di-glyceride, mono-glyceride, and glycerol anions. These anions extract protons from H2O molecules in the presence of Na+ producing glycerol and regenerating NaOH catalyst. The reaction product (SEFAMEs) is, however, composed of a mixture of methyl esters of corresponding fatty acid chains (Table 2).
Scheme 1. Proposed Reaction Mechanism for Trans-esterification of Sesame Oil to SEFAMEs.

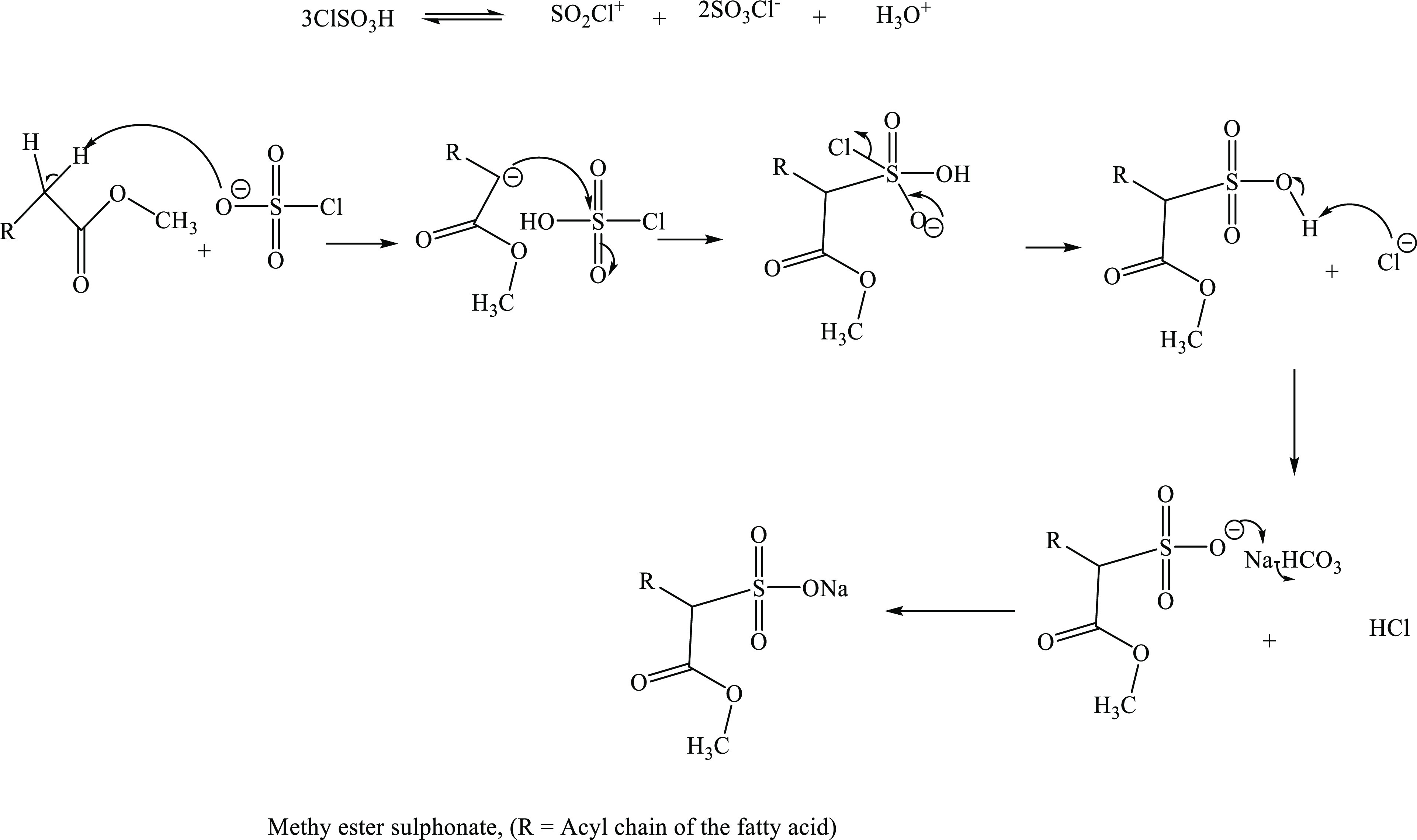
The reaction mechanism for the sulfonation of SEFAMEs is shown in Scheme 2. Chlorosulphonic anion abstracts a proton on the α-carbon of fatty acid methyl ester producing a nucleophilic carbon, which attacks the partially positively charged S of ClSO3H. The chloride ion is eliminated in the process, resulting in the formation of fatty acid methyl ester sulfonic acid, which is then neutralized using NaHCO3 to produce fatty acid methyl ester sulfonate. Like the precursor (SEFAMEs), this product is composed of a mixture of surfactants of corresponding acyl chains of the fatty acid.
Scheme 2. Proposed Reaction Mechanism for the Synthesis of the SEFAMESO Surfactant.
3.3. Structural Characteristics of SEFAME and SEFAMESO Surfactants
3.3.1. HPLC-MS Spectroscopy of SEFAME
The HPLC-MS spectrum of SEFAME is shown in Figure 1. The peaks in HPLC-chromatogram showed an elution time of less than 2 min, though the peaks were no well resolved, which could be attributed to inconsistencies of the mobile phase and the column used. Significant molecular ions in the MS spectrum were observed at m/z 263, 277.2, 295.5, 309.2, and 357.3. The molecular ion at m/z 295.5 [M]+ corresponds to linoleic fatty methyl esters with low abundance at m/z 263 [M + H – 32]+ due to the loss of the CH3OH group. Other molecular ions observed include m/z 277.2 [M – H]+ corresponding to linolenic fatty methyl ester, m/z 309.2 [M + H – 32]+ signed to eicosenoic fatty methyl ester due to loss of CH3OH, and m/z 357.3 [M + H] denoting behenic fatty methyl esters. These ionic fragments indicated a mixture of methyl esters of different fatty acid chains. This observation is similar to other reported spectra of vegetable oil fatty methyl esters.40,41
Figure 1.
HPLC-MS spectrum of SAFAMESO.
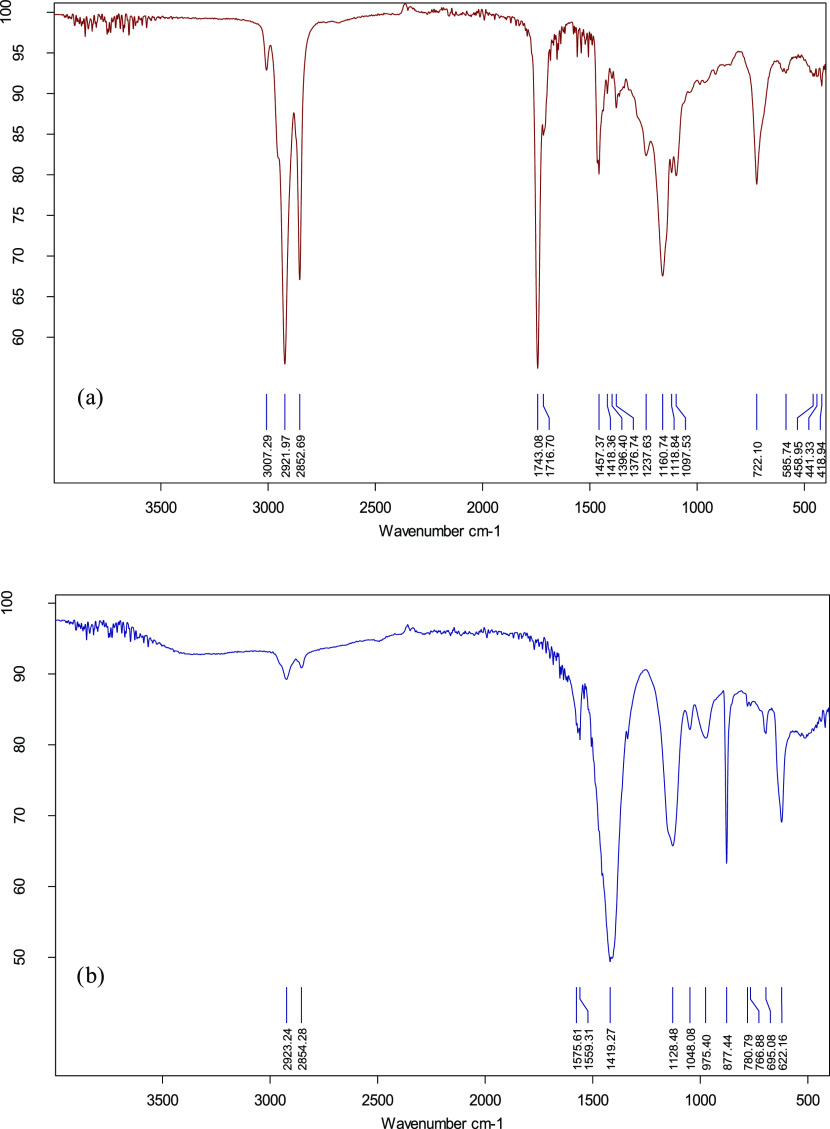
3.3.2. FTIR Analysis
FTIR spectrum of SEFAMEs (biodiesel) and that of the SEFAMESO surfactant are shown in Figure 2. The spectrum of SEFAMEs (Figure 2a) showed a weak absorption peak at 3007 cm–1 corresponding to olefinic =C–H stretch of unsaturated bonds. The intense peaks observed at 2922 and 2853 cm–1 correspond to aliphatic −C–H stretch of saturated bonds. The strong spectral bands at 1743 and 1717 cm–1 were assigned to ester C=O stretch, revealing the conversion of triglycerides to methyl esters. The peak observed at 1457 cm–1 was assigned to aliphatic −CH2 and −CH3 bending vibrations for a saturated chain. The bands around 1418–1376 cm–1 correspond to the methyl ester (−O–CH3) group, which confirmed successful trans-esterification of oil to sesame fatty methyl esters. This was in agreement with other reported IR spectra of biodiesel40,45−48 that the bands of this region indicate the existence of methyl esters as well as differentiate biodiesels from neat vegetable oils. The peaks observed at 1238–1098 cm–1 were assigned to C–O and C–O–C stretching vibrations of ester functionalities. The peak at 722 cm–1 was assigned to methylene [−(CH2)n−] out-of-plane stretch indicative of a long-chain aliphatic structure.
Figure 2.
FTIR spectrum of (a) SEFAME and (b) SEFAMESO surfactants.
The FTIR spectrum of the SEFAMESO surfactant (Figure 2b) showed weak spectral bands at 2923 and 2854 cm–1 assigned to C–H stretching of saturated carbon–carbon bonds. The weak absorption bands at 1576 and 1559 cm–1 correspond to an asymmetric stretch of COO– (carboxylate) ester bonds. The intense band observed at 1419 cm–1 was assigned to S=O stretching vibration, indicating the presence of the sulfonate group. The spectral bands at 1129–1048 cm–1 also corresponded to S=O and S–O stretching vibrations, suggesting the successful conversion of fatty methyl esters to fatty methyl ester sulfonates. These results agreed with other findings that this region accounts for the existence of the sulfonate group.3,6,18,49
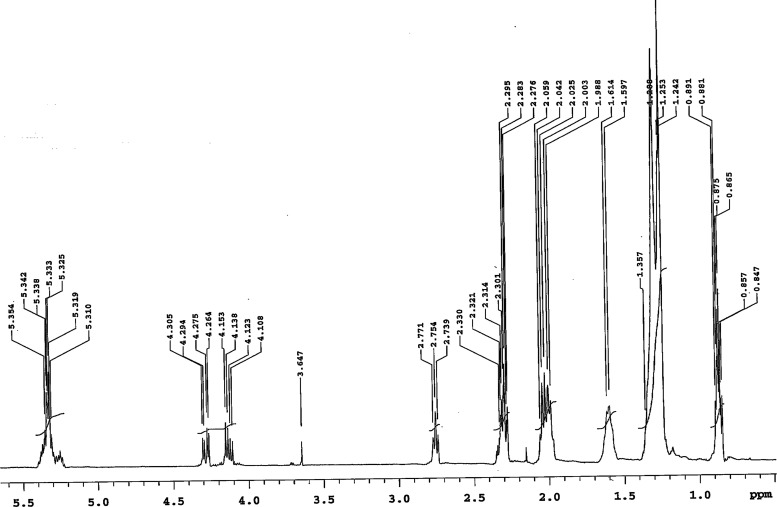
3.3.3. 1H NMR Analysis
1H NMR spectrum of SEFAMEs is shown in Figure 3. The signals observed at δ 0.847–0.891 ppm were assigned to methyl (CH3−) protons of the fatty acid chains. The signals at δ 1.242–1.28 ppm corespond to aliphatic methylene [−(CH2)n−] protons and those at δ 1.346–2.330 ppm were assigned to methylene (−CH2−) protons of saturated acyl chains. The signals at δ 2.739–2.771 ppm and those ranging from δ 4.108 to 5.354 were assigned to olefinic (−CH = CH−) protons of unsaturated fatty acid chains. The signal observed at δ 3.647 ppm correspond to terminal methyl (−COO–CH3) protons of the ester group; this peak confirmed successful trans-esterification of sesame oil. The results were in agreement with related works for trans-esterified vegetable oil.18,45,49−53
Figure 3.
1H NMR spectrum of SEFAMEs in CDCl3.
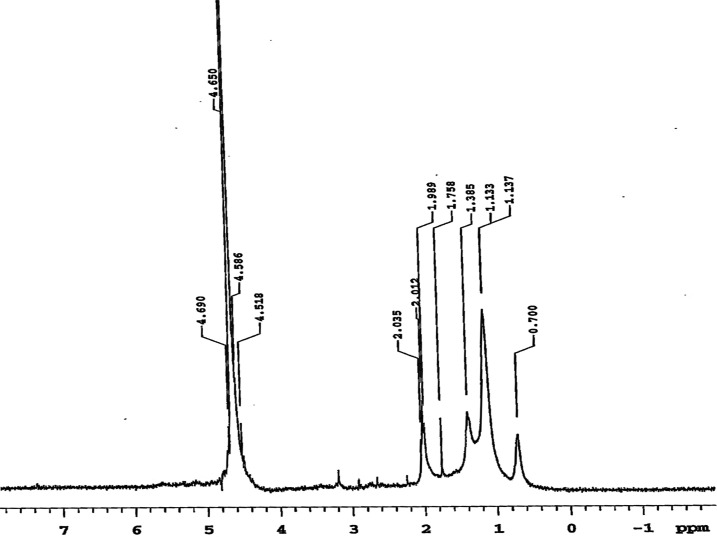
1H NMR spectrum of SEFAMESO is shown in Figure 4. The signals observed at δ 0.700 ppm correspond to terminal methyl (CH3−) protons and those at δ 1.137–1.785 ppm were assigned to aliphatic methylene [−(CH2)n−] protons of saturated acyl chains. The signals in the range of δ 1.989–2.035 ppm were assigned to allylic (=CH–CH2–CH=) protons and those ranging from δ 4.518 to 4.690 ppm correspond to olefinic (−CH=CH−) protons of unsaturated acyl chains.15,18,49,51 The spectrum indicated that the synthesized surfactant constitutes methyl ester sulfonates of saturated and unsaturated acyl chains.
Figure 4.
1H NMR spectrum of SEFAMESO Surfactant in D2O.
3.4. Solution Properties of the SEFAMESO Surfactant
3.4.1. HLB Value of the SEFAMESO Surfactant
The HLB value of the SEFAMESO surfactant was estimated according to Guo et al.54 and is presented in Table 3. This value was obtained by summing up the contributions from each individual fatty methyl ester sulfonate based on the percentage composition of their respective fatty acids in sesame oil. The calculated HLB value of SEFAMESO was 13.00, and this was much lower than the HLB value of 40 for SDS,50 a widely known anionic surfactant in the formulation of many wetting agents, detergents, and drugs.29,55,56 The lower HLB value of SEFAMESO relative to that of SDS could be attributed to longer acyl chains predominantly C:18 compared to C:12 in SDS. The difference could also be related to varied anionic head group contributions with the sodium sulfonate group in SEFAMESO contributing 11 and sodium sulfate in SDS contributing 38.7.57 The HLB value of SEFAMESO suggested that the synthesized surfactant is potentially suitable for use as a wetting or solubilizing agent in the formulation of shampoos.27,56 Studies have also shown that mixed surfactant systems derived from edible oleochemical fatty methyl esters have excellent properties in the formulation of stable food emulsifiers as well as enhanced ability to form complex condensed films at liquid–liquid interfaces,56 including formulation of cosmetics,58 herbicides,59 and drugs.60,61 This, therefore, suggests that the SEFAMESO surfactant ought to be explored beyond the potential use as a cleansing agent to drug formulation, among other applications.
Table 3. Estimated HLB Value of the SEFAMESO Surfactanta,b.
| |
groups
in SEFAMESO |
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| lipophilic
groups |
hydrophilic
groups |
|
|||||||
| fatty acid | NAC/DB | CH3– (−0.475) | –CH=CH (−0.475) | –CH2 (−0.475) | –COOCH3 (2.4) | –SO3Na (11) | fatty methyl ester HLB value | quantity (%) | HLB value |
| myristic | 14:0 | 1 | 0 | 12 | 1 | 1 | 14.23 | 0.10 | 0.01 |
| palmitoleic | 16:1 | 1 | 1 | 12 | 1 | 1 | 13.75 | 0.18 | 0.02 |
| palmitic | 16:0 | 1 | 0 | 14 | 1 | 1 | 13.28 | 8.92 | 1.18 |
| linoleic | 18:2 | 1 | 2 | 12 | 1 | 1 | 13.28 | 40.36 | 5.36 |
| linolenic | 18:3 | 1 | 3 | 10 | 1 | 1 | 13.75 | 0.50 | 0.07 |
| oleic | 18:1 | 1 | 1 | 14 | 1 | 1 | 12.80 | 44.62 | 5.71 |
| stearic | 18:0 | 1 | 0 | 16 | 1 | 1 | 12.33 | 4.61 | 0.57 |
| eicosenoic | 20:1 | 1 | 1 | 16 | 1 | 1 | 11.85 | 0.25 | 0.03 |
| eicosanoic | 20:0 | 1 | 0 | 18 | 1 | 1 | 11.38 | 0.21 | 0.03 |
| behenic | 22:0 | 1 | 0 | 20 | 1 | 1 | 10.43 | 0.18 | 0.02 |
| SEFAMESO | 13.00 | ||||||||
The group numbers in brackets were adopted from Guo et al.54
NAC/DB = number of acyl carbons to the number of double bonds.
3.4.2. Krafft Temperature of SEFAMESO
Krafft point is important as regards the solubility of surfactants because micelles are formed above this temperature.17 The Krafft temperature of 0.2% (w/v) SEFAMESO surfactant was 19.75 °C indicating its potential application as a detergent that could be more effective in warm water washing. This value also indicated the absence of di-salts in the synthesized surfactant, which was attributed to the use of NaHCO3 in neutralizing fatty methyl ester sulfonic acid (Scheme 2) The di-salts are side-reaction products, which result from hydrolysis of the ester groups during sulfonation of fatty methyl esters using NaOH as neutralizing base.62 They often cause an increase in Krafft point (or decreased solubility) of oleochemical surfactants.1,62 The Krafft temperature obtained was within the range of reported values of 17–30 °C for a mixture of C16–18 methyl esters sulfonates.3,51,62
3.4.3. Foam-ability of SEFAMESO
Foam-ability and foam stability are the two parameters often used to report the forming power of a detergent.3,5,6 The initial foam height of 0.1% (w/v) aqueous solution of the SEFAMESO surfactant was 145 cm3 at 25 °C of which after 5 min decreased to 66 cm3. The foam stability was 79 cm3 and this was equivalent to 54.48% of the initial foam height. In a similar study, Tai et al.63 evaluating foam height of 0.25% (w/v) of methyl ester sulfonates and 0.25% (w/v) of linear alkylbenzene sulfonate, observed slightly higher foam stability values ranging from 120 to 170 cm3. The higher values obtained by these authors relative to the present study may be attributed to the amount of surfactant used in the experiment. The films formed as foam aid in elevating soil particles, stripping off soil particles from the fabric surface when rinsing and acting against the redeposition of soil.5 The evaluated parameter, thus, revealed cleansing or detergent property of the synthesized surfactant.
3.4.4. CMC and Counterion Degree of Binding of the SEFAMESO Surfactant
The plot of specific conductivity against anionic SEFAMESO surfactant concentration at 298.15 K is shown in Figure 5. The specific conductivity changed linearly with surfactant concentration at both postmicellar and premicellar regions. The point at which an abrupt break in the curve is observed represents the critical micelle concentration (CMC) point (or value).24 The change in conductivity of aqueous ionic surfactant solutions at CMC is attributed to the mobility of ions29,36 and the difference in the degree of ionization below and above the CMC.27,64 Below the CMC, surfactant monomers are mobile and act as strong electrolytes because both alkyl chains (anions) and counterions (cations) contribute independently to ionization. This results in the observed rapid increase in specific conductivity with increased surfactant concentration up to the CMC. Above the CMC, the micelles are partially ionized and of relatively lower mobility than the monomeric surfactant molecules.29 The specific conductivity, therefore, fairly increases with increased surfactant concentration, resulting in a lower slope at postmicellar than the premicellar region.13,24,29
Figure 5.
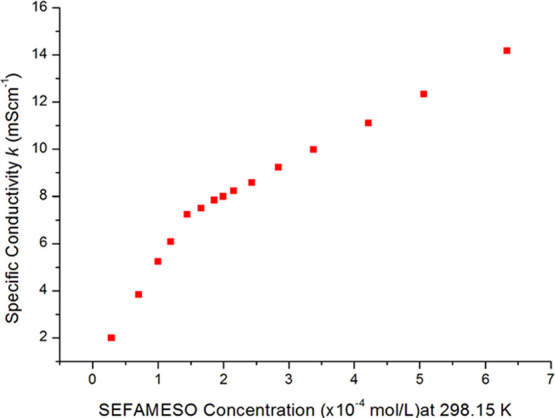
Specific conductivity against SEFAMESO concentration at 298.15 K.
Figure 6 shows a change in specific conductivity against the SEFAMESO surfactant concentration at different temperatures. The CMC values and the counterion degree of binding at different temperatures are given in Table 4. The specific conductivity increased with an increase in temperature (Figure 6), an observation ascribed to the increased thermal energy of molecular entities.13,25 As observed in Table 4, there was an initial decrease in CMC values between 298.15 and 303.15 K, after which an increase was observed at higher temperatures of 313.15 and 323.15 K. The effect of temperature on CMC values has been attributed to two factors, namely, hydrophilic and hydrophobic hydrations, which decrease with an increase in temperature.13,29,36,64,65 Both types of hydrations are possible in a monomeric surfactant, whereas hydrophilic hydration is likely in a micellized surfactant system. The hydrophilic dehydration favors micellization at low temperatures, while hydrophobic dehydration disfavors micellization with an increase in temperature; hence, the magnitude of these opposing effects determine either an increase or a decrease of CMC values over a particular temperature range.24,29,65 The observed decrease in CMC values at lower temperatures in the present study may be attributed to dominant hydrophilic dehydration, whereas the increase at higher temperatures could be due to dominant hydrophobic dehydration.24,65 A similar observation of U-shaped CMC-temperature profile of ionic surfactants has been made by other workers.24,29,36,64,65 The increase in CMC values at higher temperatures has also been linked to increased thermal motion of surfactant and solvent molecules.65 The kinetic energy that increases with an increase in temperature destroys ordered micelle structures causing a decrease in aggregation number of micelles and hence, higher CMC values.
Figure 6.
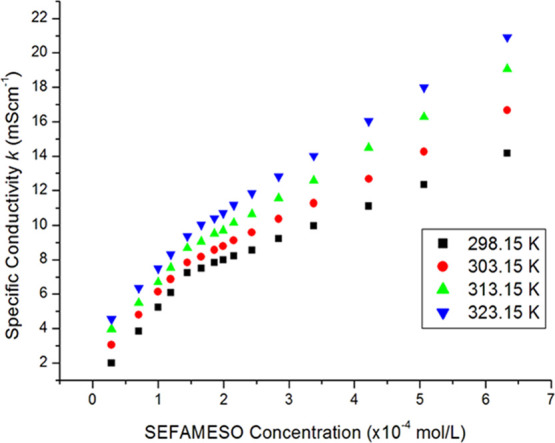
Effect of temperature on the specific conductivity against SEFAMESO concentration.
Table 4. CMC Values and Counterion Degree of Binding of the SEFAMESO Surfactant and the SDS Standard.
| temp (K) | CMC
values |
β |
||
|---|---|---|---|---|
| values and counterion degree | SEFAMESO(×10–4 mol/L) | SDS(×10–3 mol/L) | SEFAMESO | SDS |
| 298.15 | 1.435 | 8.318 | 0.382 | 0.407 |
| 303.15 | 1.418 | 8.200 | 0.205 | 0.420 |
| 313.15 | 1.471 | 8.530 | 0.278 | 0.423 |
| 323.15 | 1.518 | 8.757 | 0.342 | 0.417 |
The CMC values of the SEFAMESO surfactant were lower than those of the SDS standard at the studied temperature range (Table 4). SDS is a common ingredient in detergents and is highly effective in oily stain removal.29,36 The lower CMC values of SEFAMESO relative to those of SDS implies that it can be used in lower quantities as a cleansing agent and still remain effective in addition to its advantage of eco-friendliness due to biodegradation in the environment. In related studies, Saxena et al.6 using conductivity and surface tension measurements of anionic (soap–nut) surfactant derived from Sapindus laurifolius obtained higher values ranging from 9.3 to 11.3 mM/L at 298–348 K. Pal et al.17 obtained comparable values of 0.14–0.38 mM/L on evaluating nonionic Gemini surfactants derived from sunflower oil by surface tension measurement.
The values of counterion degree of binding of the SEFAMESO surfactant showed a marked decrease with an increase in temperature (Table 4). This suggested that with an increase in temperature, the self-assembly of surfactant molecules was less cooperative due to the exothermic nature of the micellization process35 that led to the formation of loosely held aggregates at higher temperatures. The β values of SEFAMESO were also lower than those of SDS standard, an observation attributed to longer hydrophobic alkyl chains in SEFAMESO relative to that of SDS which resulted in chain folding inside the micelle core.66
3.4.5. Thermodynamic Properties of the SEFAMESO Surfactant
Thermodynamic parameters of micellization of SEFAMESO and that of the SDS standard as a function of temperature are presented in Table 5. ΔG°mic values at studied temperature range were negative in both surfactant systems, an indication of thermodynamically spontaneous the micellization process.6,29,35,36 SEFAMESO recorded more negative G°mic values than the SDS standard. This was ascribed to the longer hydrocarbon chain length (hydrophobic portion) of SEFAMESO relative to that of SDS, which caused an increase in the free energy of micellization.27,66G°mic values of SEFAMESO were less negative with an increase in temperature to a minimum at 303.15 K after which more negative values were recorded above this temperature, supporting U-shaped micellization behavior of the CMC (Table 4). This trend was not observed in the SDS system though more negative values were recorded with an increase in temperature, an observation attributed to increased spontaneity of the micellization process.36 The ΔG°mic values of SEFAMESO obtained in the present study were comparable to reported values of −30.07–45.72 kJ/mol for C12, C14, C16, and C16/18 methyl ester sulfonates.67,68 The ΔG°mic values were, however, dependent on ΔH°mic and ΔS°mic values.
Table 5. Thermodynamic Parameters of Micellization of the Anionic SEFAMESO Surfactant and the SDS Standard.
| ΔG°mic(kJ/mol) |
ΔH°mic (kJ/mol) |
ΔS°mic (J/(mol K)) |
||||
|---|---|---|---|---|---|---|
| temp (K) | SEFAMESO | SDS | SEFAMESO | SDS | SEFAMESO | SDS |
| 298.15 | –44.06 | –0.72 | 4.50 | 2.61 | 162.87 | 111.80 |
| 303.15 | –39.12 | –1.57 | 0.34 | –2.27 | 130.16 | 96.66 |
| 313.15 | –42.72 | –2.53 | –8.02 | –13.1 | 110.80 | 62.07 |
| 323.15 | –46.19 | –3.32 | –18.38 | –25.2 | 86.07 | 25.14 |
The ΔH°mic values were positive at lower temperatures and negative at high temperatures for both surfactants. This indicated that micellization was endothermic at low temperatures and exothermic at high temperatures. The ΔH° mic value is the sum of enthalpies contributed by hydrophobic and hydrophilic interactions, counterion degree of binding, and hydration of polar head groups of a surfactant.29,35 The negative ΔH°mic values observed at high temperatures were related to favored hydration of hydrophilic heads groups than the disruption of the water structure around hydrophobic chains.6,27−29,35,66 ΔS°mic values were positive for both surfactant systems and decreased with increased temperature. The decrease in ΔS°mic values was attributed to an ordered structure of water molecules around the hydrophobic portion and the mobility constraints of hydrophobic groups.27,35,66 The observed positive values of both ΔH°mic and ΔS°mic at lower temperatures suggested dominant hydrophobic interactions between surfactant chains.35 Thus, the micellization process was entropy-controlled at low temperatures and both entropy- and enthalpy-controlled at high temperatures.
The enthalpy–entropy compensation parameters for SEFAMESO and SDS are presented in Table 6 and the plots of ΔH°mic against ΔS°mic are displayed in Figure 7. ΔH°mic,* and Tc values provide information on solute–solute and solute–solvent interactions, respectively.29,36 Both SEFAMESO and SDS surfactant systems yielded high R2 values of 0.929 and 0.999, respectively, suggesting a good fit of the data to the model (Figure 7a,b). ΔH°mic,* values were negative for both surfactant systems, an indication of stable micellar formation even at ΔS°mic = 0.24,28,29,36 The more negative value of ΔH°mic,* observed in the SEFAMESO surfactant indicated greater stability of its micelles relative to that of SDS. Tc of SEFAMESO was also lower than that of SDS.
Table 6. Enthalpy–Entropy Compensation Parameters.
| surfactant | ΔH°mic,* (kJ/(K mol)) | Tc (K) |
|---|---|---|
| SEFAMESO | –42.187 | 300.45 |
| SDS | –33.154 | 320.14 |
Figure 7.
Plots of ΔHmico versus ΔSmico for (a) the SAFAMESO surfactant and (b) the SDS standard.
4. Conclusions
Sesame oil was utilized in the synthesis of the SEFAMESO surfactant, and a reaction mechanism has been proposed. SEFAMEs constituted a mixture of methyl esters as indicated by an HPLC-MS spectrum. FTIR spectrum of SEFAMEs showed peaks at 1418–1376 cm–1 corresponding to methyl ester (−COOCH3) that indicated successful trans-esterification of oil. SEFAMESO showed spectral bands at 1419, 1129–1048 cm–1 corresponding to sulfonic (−S=O and S–O) groups, suggesting successful synthesis of the surfactant. 1H NMR spectrum further confirmed successful trans-esterification of oil and the presence of saturated and unsaturated chains in SEFAMESO and its precursor. The estimated HLB value was lower than that of SDS, while Krafft point and foam stability were comparable to reported values for cleaning and wetting agents containing methyl esters sulfonates. CMC and β values of SEFAMESO were lower than those of SDS revealing the possibility of greater performance. Negative values of ΔG°mic indicated thermodynamically spontaneous micellization process and this was entropy-driven at low temperatures and both entropy- and enthalpy-driven at high temperatures. The SEFAMESO system recorded more negative ΔH°mic,* value compared to that of SDS, suggesting greater stability of its micelles. The synthesized product could potentially be applied as a cleansing agent and its performance could be better than that of SDS. The results of evaluated solution properties were however based on freshly made surfactant and since it is an oleochemically derived product, evaluation should be carried out on its stability with time. The study also suggests the evaluation of other application properties such as wetting and emulsifying properties for pharmaceutical and cosmetic applications.
Acknowledgments
The authors are grateful to Dr. David Bwambok of the Department of Chemistry and Chemical Biology, Harvard University, USA, for performing spectroscopic measurements. The authors are indebted to the National Commission of Science, Technology and Innovation (NACOSTI, Kenya) for facilitating the study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03698.
Specific conductivity of varied concentration of SEFAMESO at different temperatures; representative extrapolation of CMC on the specific conductivity against the SEFAMESO concentration curve; specific conductivity of varied concentration of SDS at different temperatures; a plot of specific conductivity against the SDS concentration at a temperature range of 298.15–323.15 K. CMC values, counterion degree of binding (β), and thermodynamic properties of micellization of the SDS standard (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Surfactants from Renewable Resources; Kjellin M.; Johansson I., Eds.; John Wiley & Sons: London, 2010. [Google Scholar]
- Islam M. S.; Ahmed A. S.; Islam A.; Aziz S. A.; Xian L. C.; Mridha M. Study on emission and performance of diesel engine using castor biodiesel. J. Chem. 2014, 2104, 451526 10.1155/2014/451526. [DOI] [Google Scholar]
- Asselah A.; Tazerouti A. Photosulfochlorination synthesis and physicochemical properties of methyl ester sulfonates derived from lauric and myristic acids. J. Surfactants Deterg. 2014, 17, 1151–1160. 10.1007/s11743-014-1635-9. [DOI] [Google Scholar]
- Babu K.; Maurya N. K.; Mandal A.; Saxena V. K. Synthesis and characterization of sodium methyl ester sulfonate for chemically-enhanced oil recovery. Braz. J. Chem. Eng. 2015, 32, 795–803. 10.1590/0104-6632.20150323s00003642. [DOI] [Google Scholar]
- Maurad Z. A.; Idris Z.; Ghazali R. Performance of palm-based C16/18 methyl ester sulphonate (MES) in liquid detergent formulation. J. Oleo Sci. 2017, 66, 677–687. 10.5650/jos.ess16190. [DOI] [PubMed] [Google Scholar]
- Saxena N.; Pal N.; Ojha K.; Dey S.; Mandal A. Synthesis, characterization, physical and thermodynamic properties of a novel anionic surfactant derived from Sapindus laurifolius. RSC Adv. 2018, 8, 24485. 10.1039/C8RA03888K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Were B. A.; Onkware A. O.; Gudu S.; Welander M.; Carlsson A. S. Seed oil content and fatty acid composition in East African sesame (Sesamum indicum L.) accessions evaluated over 3 years. Field Crops Res. 2006, 97, 254–260. 10.1016/j.fcr.2005.10.009. [DOI] [Google Scholar]
- Ong’injo E. O.; Ayiecho P. O.. Genotypic variability in sesame mutant lines in Kenya Afri. Crop Sci. J. 2009, 17, 101–−107. 10.4314/acsj.v17i2.54203. [DOI]
- Gharby S.; Harhar H.; Bouzoubaa Z.; Asdadi A.; El Yadini A.; Charrouf Z. Chemical characterization and oxidative stability of seeds and oil of sesame grown in Morocco. J. Saudi Soc. Agric. Sci. 2017, 16, 105–111. 10.1016/j.jssas.2015.03.004. [DOI] [Google Scholar]
- Baydar H.; Turgut I.; Turgut K. Variation of certain characters and line selection for yield, oil, oleic and linoleic acids in the Turkish sesame (Sesamum indicum L.) populations. Turk. J. Agric. For. 1999, 23, 431–441. [Google Scholar]
- Hawtin G.Underutilized Plant Species Research and Development Activities–Review of Issues and Options, GFU/ICUC; A report submitted to the chairs of the GFU Steering Committee and the ICUC Scientific Advisory Board, 2007.
- Novel Surfactants: Preparation Applications and Biodegradability, 2nd ed.; Holmberg K., Ed.; CRC Press: New York, 2003. [Google Scholar]
- Holmberg K.; Lindman B.; Jonsson B.; Kronberg B.. Surfactants and Polymers in Aqueous Solution, 2nd ed.; John Wiley & Sons: West Sussex, 2003. [Google Scholar]
- Holser R.Synthesis of Surfactants from Vegetable Oil Feedstocks. In Industrial Uses of Vegetable Oils; Erhan S. Z., Ed.; AOCS Press: Champaign, 2005; pp 170–178. [Google Scholar]
- Kumar S.; Saxena N.; Mandal A. Synthesis and evaluation of physicochemical properties of anionic polymeric surfactant derived from Jatropha oil for application in enhanced oil recovery. J. Ind. Eng. Chem. 2016, 43, 106–116. 10.1016/j.jiec.2016.07.055. [DOI] [Google Scholar]
- Kumar S.; Kumar A.; Mandal A. Characterizations of surfactant synthesized from Jatropha oil and its application in enhanced oil recovery. AIChE J. 2015, 63, 2731–2741. 10.1002/aic.15651. [DOI] [Google Scholar]
- Pal N.; Samanta K.; Mandal A. A novel family of non-ionic gemini surfactants derived from sunflower oil: Synthesis, characterization and physicochemical evaluation. J. Mol. Liq. 2019, 275, 638–653. 10.1016/j.molliq.2018.11.111. [DOI] [Google Scholar]
- Jin Y.; Tian S.; Guo J.; Ren X.; Li X.; Gao S. Synthesis, characterization and exploratory application of anionic surfactant fatty acid methyl ester sulfonate from waste cooking oil. J. Surfactants Deterg. 2016, 19, 467–475. 10.1007/s11743-016-1813-z. [DOI] [Google Scholar]
- Salih N.; Salimon J.; Yousif E. Synthesis of oleic acid based esters as potential basestock for biolubricant production. Turk. J. Eng. Environ. Sci. 2011, 35, 115–123. [Google Scholar]
- Yao L.Synthesis of fatty acid derivatives as potential biolubricants and their physical properties and boundary lubrication performances. Ph.D. Thesis, Iowa State University, 2009. [Google Scholar]
- Knothe G.; Steidley K. R. Kinematic viscosity of biodiesel fuel components and related compounds; Influence of compound structure and comparison to petro-diesel fuel components. Fuel 2005, 84, 1059–1065. 10.1016/j.fuel.2005.01.016. [DOI] [Google Scholar]
- Kirk-Othmer. Chemical Technology of Cosmetics.;Seidel A., Eds.; John Wiley: New Jersey, 2013. [Google Scholar]
- Meher L. C.; Sagar D. V.; Naik S. N. Technical aspects of biodiesel production by trans-esterification - A review. Renewable Sustainable Energy Rev. 2006, 10, 248–268. 10.1016/j.rser.2004.09.002. [DOI] [Google Scholar]
- Mahbub S.; Rana S.; Rub M. A.; Hoque M. A.; Kabir S. E.; Asiri A. M. Influence of alcohol/temperature on the interaction of sodium dodecyl sulfate with cetyltrimethylammonium bromide: Experimental and theoretical study. J. Chem. Eng. Data 2019, 64, 4376–4389. 10.1021/acs.jced.9b00456. [DOI] [Google Scholar]
- Schramm L. L.; Stasiuk E. N.; Marangoni D. G. Surfactants and their applications. Annu. Rep. Prog. Chem., Sect. C: Phys. Chem. 2003, 99, 3–48. 10.1039/B208499F. [DOI] [Google Scholar]
- Handbook for Cleaning/Decontamination of Surfaces, 1st ed.; Johansson I.; Somasundaran P., Eds.; Elsevier: Amsterdam, 2007; Vol. 1. [Google Scholar]
- Rosen M. J.; Kunjappu J. T.. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons: New York, 2012. [Google Scholar]
- Ahsan S. M. A.; Amin M. R.; Mahbub S.; Molla M. R.; Aktar S.; Rub M. A.; Hoque M. A.; Arshad M. N.; Khan M. A. Interaction of ciprofloxacin hydrochloridewith sodiumdodecyl sulfate in aqueous/electrolytes solution at different temperatures and compositions. Chin. J. Chem. Eng. 2020, 28, 216–223. 10.1016/j.cjche.2019.03.019. [DOI] [Google Scholar]
- Mahbub S.; Akter S.; Luthfunnessa; Akter P.; Hoque M. A.; Rub M. A.; Kumar D.; Alghamdi Y. G.; Asiri A. M.; Džudžević-Čančar H. Effects of temperature and polyols on the ciprofloxacin hydrochloride-mediated micellization of sodium dodecyl sulphate. RSC Adv. 2020, 10, 14531–14541. 10.1039/D0RA00213E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOCS . Official Methods and Recommended Practices of the American Oil Chemists’ Society: Physical and Chemical Characteristics of Oils, Fats and Waxes, 5th ed.; American Oil Chemists’ Society: Champaign, 1998. [Google Scholar]
- Naegele E.Determination of FAMEs in AVTUR with the Agilent 1290 Infinity ELSD. Agilent Application Note, publication number 5991-2782EN, 2013.
- Davies J. T.A Quantitative Kinetic Theory of Emulsion Type. I. Physical Chemistry of the Emulsifying Agent, In Gas/Liquid and Liquid/Liquid Interface, Proceedings of the 2nd International Congress of Surface Activity, 1957; pp 426–438.
- Penagos-Calvete D.; Duque V.; Marimon C.; Parra D. M.; Restrepo-Arango S. K.; Scherf-Clavel O.; Holzgrabe U.; Montoya G.; Salamanca C. H. Glycerolipid composition and advanced physicochemical considerations of sacha inchi oil toward cosmetic products formulation. Cosmetics 2019, 6, 70 10.3390/cosmetics6040070. [DOI] [Google Scholar]
- Sristy S. I. H.; Mahbub S.; Alam M. M.; Wabaidur S. M.; Rana S.; Hoque M. A.; et al. Interaction of tetradecyltrimethylammonium bromide with sodium dodecyl sulfate in aqueous/urea medium at several temperatures and compositions. J. Mol. Liq. 2019, 284, 12–22. 10.1016/j.molliq.2019.03.142. [DOI] [Google Scholar]
- Akhtar F.; Hoque M. A.; Khan M. A. Interaction of cefadroxyl monohydrate with hexadecyltrimethyl ammonium bromide and sodium dodecyl sulphate. J. Chem. Thermodyn. 2008, 40, 1082–1086. 10.1016/j.jct.2008.03.001. [DOI] [Google Scholar]
- Mahbub S.; Mia M. L.; Roy T.; Akter P.; Uddin A. K. M. R.; Rub M. A.; Hoque M. A.; Asiri A. M. Influence of ammonium salts on the interaction of fluoroquinolone antibiotic drug with sodium dodecyl sulfate at different temperatures and compositions. J. Mol. Liq. 2020, 297, 111583 10.1016/j.molliq.2019.111583. [DOI] [Google Scholar]
- Rahman M. S.; Hossain M. A.; Ahmed G. M.; Uddin M. M. Studies on the characterization, lipids and glyceride compositions of Sesame (Sesamum indicum Linn.) Seed Oil. Bangladesh J. Sci. Indust. Res. 2007, 42, 67–74. 10.3329/bjsir.v42i1.357. [DOI] [Google Scholar]
- El Khier M. K. S.; Ishag K. E. A.; Yagoub A. E. A. Chemical composition and oil characteristics of sesame seed cultivars grown in Sudan. J. Agric. Biol. Sci. 2008, 4, 761–766. [Google Scholar]
- Mohammed M. I.; Hamza Z. U. Physicochemical properties of oil extracts from Sesamum Indicum L. Seeds grown in Jigawa State Nigeria. J. Appl. Sci., Environ. Manage. 2008, 12, 99–101. 10.4314/jasem.v12i2.55542. [DOI] [Google Scholar]
- Mok H. J.; Lee J. W.; Bandu R.; Kang H. S.; Kim K. H.; Kim K. P. A rapid and sensitive profiling of free fatty acids using liquid chromatography electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS) after chemical derivatization. RSC Adv. 2016, 6, 32130–32139. 10.1039/C6RA01344A. [DOI] [Google Scholar]
- MacDougall K. M.; McNichol J.; McGinn P. J.; O’Leary S. J.; Melanson J. E. Triacylglycerol profiling of microalgae strains for biofuel feedstock by liquid chromatography–high-resolution mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 2609–2616. 10.1007/s00216-011-5376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzikou J. M.; Matos L.; Bouanga-Kalou G.; Ndangui C. B.; Pambou-Tobi N. P. G.; Kimbonguila A.; Silou T.; Linder M.; Desobry S. Chemical composition on the seeds and oil of sesame (Sesamum indicum L.) grown in Congo-Brazzaville. Adv. J. Food Sci. Technol. 2009, 1, 6–11. [Google Scholar]
- Alyemeni M. N.; Basahy A. Y.; Sher H. Physico-chemical analysis and mineral composition of some sesame seeds (Sesamum indicum L.) grown in the Gizan area of Saudi Arabia. J. Med. Plants Res. 2011, 5, 270–274. 10.5897/JMPR.9000976. [DOI] [Google Scholar]
- Gulla S.; Waghray K. Effect of storage on physicochemical characteristics and fatty acid composition of selected oil blends. J. Life Sci. 2011, 3, 35–46. 10.1080/09751270.2011.11885167. [DOI] [Google Scholar]
- Ahmad M.; Ullah K.; Khan M. A.; Ali S.; Zafar M.; Sultana S. Quantitative and qualitative analysis of sesame oil biodiesel. Energy Sources, Part A 2011, 33, 1239–1249. 10.1080/15567036.2010.531510. [DOI] [Google Scholar]
- Zhang W. B. Review on analysis of biodiesel with infrared spectroscopy. Renewable Sustainable Energy Rev. 2012, 16, 6048–6058. 10.1016/j.rser.2012.07.003. [DOI] [Google Scholar]
- Donnell O. S.; Demshemino I.; Yahaya M.; Nwadike I.; Okoro L. A review on the spectroscopic analyses of biodiesel. Eur. Int. J. Sci. Technol. 2013, 2, 137–146. [Google Scholar]
- Oyerinde A. Y.; Bello E. I. Use of Fourier transformation infrared (FTIR) spectroscopy for analysis of functional groups in peanut oil biodiesel and its blends. Br. J. Appl. Sci. Technol. 2016, 13, 1–14. 10.9734/BJAST/2016/22178. [DOI] [Google Scholar]
- Pal N.; Saxena N.; Laxmi K. V. L.; Mandal A. Interfacial behaviour, wettability alteration and emulsification characteristics of a novel surfactant: Implications for enhanced oil recovery. Chem. Eng. Sci. 2018, 187, 200–212. 10.1016/j.ces.2018.04.062. [DOI] [Google Scholar]
- Silverstein R.; Webster F.; Kiemle D.. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: New York, 2005. [Google Scholar]
- Cohen L.; Soto F.; Melgarejo A.; Roberts D. W. Performance of Φ-sulfo fatty methyl ester sulfonate versus linear alkylbenzene sulfonate, secondary alkane sulfonate and α-sulfo fatty methyl ester sulphonate. J. Surfactant Deterg. 2008, 11, 181–186. 10.1007/s11743-008-1069-3. [DOI] [Google Scholar]
- Awang M.; Seng G. M. Sulfonation of phenols extracted from the pyrolysis oil of oil palm shells for enhanced oil recovery. ChemSusChem 2008, 1, 210–214. 10.1002/cssc.200700083. [DOI] [PubMed] [Google Scholar]
- Tariq M.; Ali S.; Ahmad F.; Ahmad M.; Zafar M.; Khalid N.; Khan M. A. Identification, FT-IR, NMR (1H and 13C) and GC/MS studies of fatty acid methyl esters in biodiesel from rocket seed oil. Fuel Process Technol. 2011, 92, 336–341. 10.1016/j.fuproc.2010.09.025. [DOI] [Google Scholar]
- Guo X.; Rong Z.; Ying X. Calculation of hydrophile–lipophile balance for polyethoxylated surfactants by group contribution method. J. Colloid Interface Sci. 2006, 298, 441–450. 10.1016/j.jcis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Piret J.; Lamontagne J.; Bestman-Smith J.; Roy S.; Gourde P.; Désormeaux A.; Omar R. F.; Juhász J.; Bergeron M. G. In vitro and in vivo evaluations of sodium lauryl sulfate and dextran sulfate as microbicides against herpes simplex and human immunodeficiency viruses. J. Clin. Microbiol. 2000, 38, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontogeorgis G. M.; Kiil S.. Introduction to Applied Colloid and Surface Chemistry; John Wiley & Sons: New York, 2016. [Google Scholar]
- Food Emulsifiers and Their Applications; Hasenhuettl G. L.; Hartel R. W., Eds.; Springer: New York, 2008. [Google Scholar]
- Arroyo Negrete M. A. A.; Wrobel K.; Aguilar F. J. A.; Barrientos E. Y.; Escobosa A. R. C.; Wrobel K. Determination of fatty acid methyl esters in cosmetic castor oils by flow injection–electrospray ionization–high-resolution mass spectrometry. Int. J. Cosmet. Sci. 2018, 40, 295–302. 10.1111/ics.12465. [DOI] [PubMed] [Google Scholar]
- Synowiec A.; Halecki W.; Wielgusz K.; Byczyńska M.; Czaplicki S. Effect of fatty acid methyl esters on the herbicidal effect of essential oils on corn and weeds. Weed Technol. 2017, 31, 301–309. 10.1017/wet.2016.17. [DOI] [Google Scholar]
- Butt U.; ElShaer A.; Snyder L. A.; Chaidemenou A.; Alany R. G. Fatty acid microemulsion for the treatment of neonatal conjunctivitis: quantification, characterisation and evaluation of antimicrobial activity. Drug Delivery Transl. Res. 2016, 6, 722–734. 10.1007/s13346-016-0338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viljoen J. M.; Cowley A.; du Preez J.; Gerber M.; du Plessis J. Penetration enhancing effects of selected natural oils utilized in topical dosage forms. Drug Dev. Ind. Pharm. 2015, 41, 2045–2054. 10.3109/03639045.2015.1047847. [DOI] [PubMed] [Google Scholar]
- Aparicio J.; MacArthur B. W.; Sheats W. B.; Brooks B. J.. MES -myths, mysteries and perspectives on properties and use J. Deterg. Cosmet. 2012, 35 (4), .
- Tai X.; Song J.; Du Z.; Liu X.; Wang T.; Wang G. The performance test of fatty acid methyl ester sulfonates and application in the dishwashing liquid detergent. J. Dispersion Sci. Technol. 2018, 39, 1422–1426. 10.1080/01932691.2017.1409633. [DOI] [Google Scholar]
- Mehta S. K.; Bhasin K. K.; Chauhan R.; Dham S. Effect of temperature on critical micelle concentration and thermodynamic behaviour of dodecyldimethylethylammonium bromide and dodecyltrimethylammonium chloride in aqueous media. Colloids Surf., A 2005, 255, 153–157. 10.1016/j.colsurfa.2004.12.038. [DOI] [Google Scholar]
- Chauhan S.; Sharma K. Effect of temperature and additives on the critical micelle concentration and thermodynamics of micelle formation of sodium dodecyl benzene sulfonate and dodecyltrimethylammonium bromide in aqueous solution: A conductometric study. J. Chem. Thermodyn. 2014, 71, 205–211. 10.1016/j.jct.2013.12.019. [DOI] [Google Scholar]
- De S.; Mondal S.. Micellar Enhanced Ultrafiltration: Fundamentals and Applications; CRC Press: New York, 2012. [Google Scholar]
- Lim W. H.; Ramle R. A. The behaviour of methyl esters sulphonate at the water–oil interface: Straight-chained methyl ester from lauryl to stearyl as an oil phase. J. Dispersion Sci. Technol. 2009, 30, 131–136. 10.1080/01932690802313451. [DOI] [Google Scholar]
- Prabha D. R.; Santhaanalakshmi J.; Prasath R. A. Analysis of micellar behaviour of a synthesized sodium itaconate monoesters with various hydrophobic chain lengths in aqueous media. Res. J. Chem. Sci. 2013, 3, 43–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.