Abstract
The mechanochemical preparation of silver sulfadiazine and dantrolene, two marketed active pharmaceutical ingredients, was investigated by in situ Raman spectroscopy. For the first time, the mechanochemical transformations involving highly fluorescent compounds could be studied in situ with a high-resolution Raman system combined with a unique suitable Raman probe. Moreover, the kinetic features of the mechanochemical process were examined by a mathematical model allowing to describe the chemical changes under mechanical stress. This approach is promising both to broaden the scope of Raman in situ investigations that would otherwise be impossible and for process optimization at any scale.
Introduction
Mechanochemistry, as a method of synthesis that uses solid reactants to prepare solid products without intermittent dissolution, has recently earned significant interest in the context of resource-efficient and low-waste manufacturing.1−3 Currently, it encompasses transformations of inorganic,4 organic,5−8 organometallic,9−11 metal–organic,12,13 and supramolecular materials,14 and it has been used in screening for novel pharmaceutical forms,15 targeted synthesis,16,17 and transformations of active pharmaceutical ingredients (APIs).18,19 An innovative area of investigation is medicinal mechanochemistry(18) providing access to potential APIs as well as marketed drugs in a sustainable way, with cleaner reaction profiles and simplified work-up procedures, as well as with an improved reagent, solvent, energy, and waste economy. Despite the widely recognized benefits of mechanochemistry, its application is limited due to the lack of mechanistic knowledge and in situ process understanding. Mechanochemical milling reactions are normally conducted in closed and rapidly moving reaction vessels, preventing a direct insight into the reaction course and, for decades, limited reaction monitoring to enable stepwise ex situ analysis.20−23
This situation was only recently remedied with the development of the first two in situ monitoring techniques probing the chemical composition of the reaction mixture, which are based on powder X-ray diffraction (PXRD)24 and Raman spectroscopy25 as well as their use in tandem.26,27 Both techniques are enabled by the use of poly(methyl methacrylate) (PMMA) plastic reaction vessels. The translucent PMMA vessel enables the Raman laser light to penetrate the vessel walls, scatter at the sample, and exit the vessel, enabling collection of the mixture’s Raman spectrum. Both in situ techniques are suited for uninterrupted reaction monitoring on a vibratory ball mill while it is in operation and provide diffraction patterns and Raman spectra with time resolution in seconds. They are complementary in the sense that PXRD is sensitive to bulk crystalline species, while Raman is sensitive to changes at the molecular level. In the case when the reaction mixture is highly crystalline, the reaction profiles extracted from both techniques coincide. Milled reaction mixtures, however, often become amorphous or partially amorphous, rendering PXRD monitoring limited to determination of the amount of the amorphous phase.28 In such cases, Raman spectroscopy may provide a more complete information on the chemical composition and molecular structure of the products and reactants because it does not require a sample to be crystalline.29 In addition, PXRD requires a synchrotron source,30 while in situ Raman monitoring is a laboratory technique that can employ fiber-optic sampling probes for remote monitoring.
Application of mechanochemistry to synthesis or screening of pharmaceutical compounds is now well known, but it largely remains within academic circles.31−39 Several examples were reported for the mechanochemical preparation of API,16,18,40,41 including metallodrugs42−44 and metallopharmaceuticals.45−47 However, before the mechanochemical approach could be exploited in large-scale industrial preparation of pharmaceutical products, better understanding of the underlying processes needs to be accomplished including detailed mapping of reaction mechanisms and kinetics, full characterization of products in terms of their particulate properties, identification of any contamination, and an understanding of how the reaction mechanism can be altered.5
Here, we study mechanochemical processes by means of in situ reaction monitoring using Raman spectroscopy and involving the formation of pharmaceutically relevant materials. We previously reported the mechanochemical preparation of metallodrugs (e.g., the gastrointestinal drug bismuth subsalicylate, Pepto-Bismol)44 and marketed hydantoin-based API16 (phenytoin,48 ethotoin,49,50 nitrofurantoin, and dantrolene51). Next, to show that not only pharmaceutical materials can readily be prepared by mechanochemistry, we show how one can better understand reaction mechanisms based on kinetic analysis of reaction profiles extracted from time-resolved in situ Raman spectra. In situ Raman spectroscopy is particularly suitable for studying pharmaceutical materials since these may experience partial or full amorphization, limiting usefulness of in situ PXRD monitoring.
To achieve these goals, we selected marketed drugs such as silver sulfadiazine (Silvadene) and dantrolene (Dantrium) as benchmarks (Figure 1).
Figure 1.
Active pharmaceutical ingredients selected for in situ monitoring studies.
Herein, we introduce for the first time the use of a large volumetric Raman probe to carry out in situ measurements in the mechanochemical preparation of highly fluorescent pharmaceutical materials (e.g., dantrolene). Based on the real-time Raman data, it was possible to discern, for the first time, mechanistic information (e.g., kinetic constants). The newly derived kinetic model allowed us to link experimental observations to local microscopic processes taking place during individual collisions.52,53
Experimental Section
Syntheses
Mechanochemical-activated reactions are represented using the formalism first introduced by Hanusa and Rightmire.11
Dantrolene Synthesis
Dantrolene CAS [7261-97-4] was prepared, adapting a previously published procedure.51 1-Aminohydantoin hydrochloride (75.8 mg, 0.5 mmol) and 5-(4-nitrophenyl)furfural (108.6 mg, 0.5 mmol) were ground in a 14 mL PMMA jar with two stainless steel balls (7 mm in diameter, weight of each ball m = 1.4 g) at 30 Hz for 30–120 min. For liquid-assisted grinding (LAG), acetonitrile (50 μL, η = 0.27 μL/mg) was used, with the η value15 defined as the volume of the solvent (expressed in μL)/the sample weight (expressed in mg).
Silver Sulfadiazine Synthesis
Silver sulfadiazine CAS [22199-08-2] was prepared by mechanochemical treatment of AgNO3 (169.9 mg, 1.0 mmol) and sulfadiazine (250.3 mg, 1 mmol) using 20 μL of either 25 or 10% aqueous ammonia solution. Solid reactants were weighed in one-half of the PMMA reaction vessel (internal volume of 14 mL) together with the milling media (two 7 mm diameter stainless-steel milling balls, weight of each ball m = 1.4 g) while aqueous ammonia was added in the other half using an automatic pipette. The two halves were carefully closed so that the solid reactants did not come into contact with the ammonia solution before milling was started. The closed reaction vessel was positioned on the vibratory ball mill, and in situ Raman monitoring was initiated together with the start of milling, which was performed at 30 Hz for 30–90 min.
Product Identification by Nuclear Magnetic Resonance (NMR)
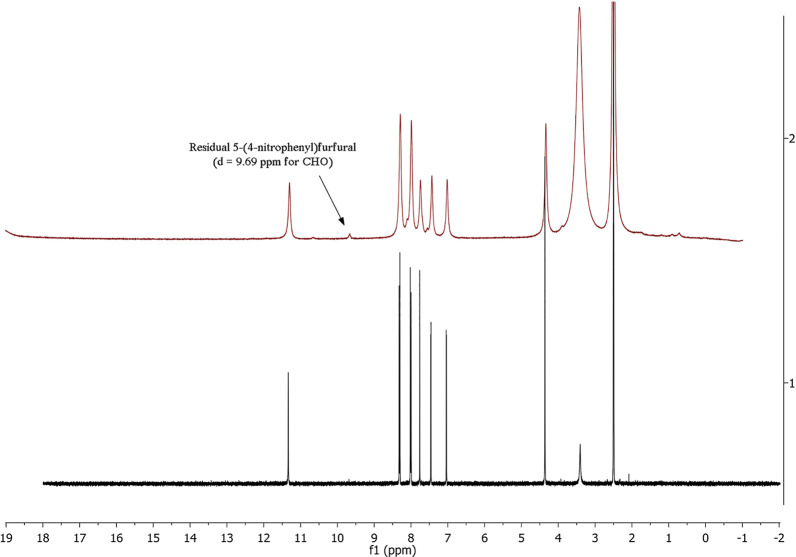
The identity of the final product dantrolene was confirmed with NMR and by comparing spectra with the NMR spectral data previously described in the literature.51 Chemical shifts (δ) of 1H NMR spectra are reported in ppm relative to residual solvent signals (DMSO in DMSO-d6: δ = 2.50 ppm). 1H NMR spectra were recorded at 400 MHz (Figure 6, black spectrum), 600 MHz (Figure 7, red spectrum), and 300 MHz (Figure 7).
Figure 6.
Assessment of dantrolene identity by comparison of 1H NMR spectra for LAG reaction (red spectrum) vs. the analytical sample prepared by planetary ball mill51 (black spectrum).
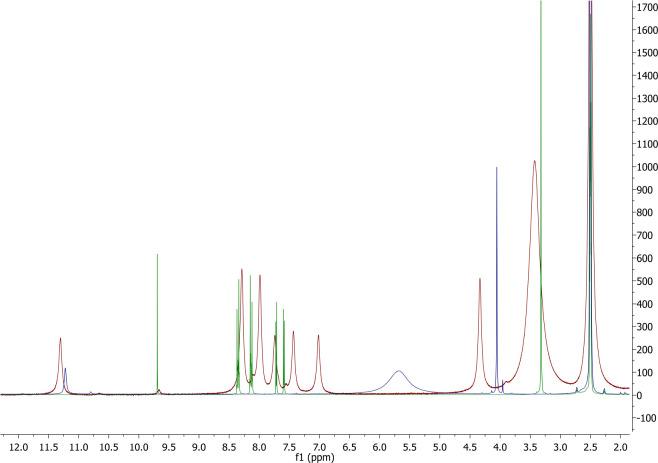
Figure 7.
1H NMR spectra of the final mixture (red), 5-(4-nitrophenyl)furfural (green), and 1-aminohydantoine hydrochloride (blue).
In Situ Raman Spectroscopy for Silver Sulfadiazine Synthesis
Raman spectroscopy measurements for the silver sulfadiazine synthesis employed a portable Raman system with a PD-LD (now Necsel) BlueBox laser source having an excitation wavelength of 785 nm and an OceanOptics Maya2000Pro spectrometer coupled with a B&W-Tek fiber optic BAC102 probe. The position of the probe was about 0.4 cm from the bottom of the vessel. Raman spectra were collected for 10 s with an acquisition time of 500 ms and summing 20 scans for each spectrum.
In Situ Raman Spectroscopy for Dantrolene Synthesis
Raman spectroscopy for real-time measurements of the dantrolene synthesis was carried out by using a 785 nm Kaiser Raman Rxn2 Hybrid instrument including a 785 nm laser at 400 mW power, a high-resolution spectrograph, and a cooled charged-coupled device (CCD) detector. The Kaiser Raman Rxn2 base unit was fitted with a PhAT large volumetric bulk sampling probe, providing a circular illumination area of 6 mm diameter and a sample penetration of 1–2 mm to cover a large sample area. Scattered light was collected by a bundle of 50 optical fibers dispersed through a high-performance spectrometer (f/1.8) and focused to a cooled charge-coupled device (CCD) detector.
In situ Raman spectra were collected across the range 1875–150 cm–1. A laser exposure time of 1 s with 10 accumulation was selected to collect one spectrum every 30 s during milling. The PhAT approach allowed a greater volume to be analyzed in a single measurement than measurements that use a backscattered probe geometry. This larger sampling volume allowed a more representative, repeatable, and robust measurement of the process because the measurement was not as sensitive to sample placement with regard to the laser focus.
Raman Data Analysis
Utilizing both the Kaiser Hololab calibration accessory and a Raman calibration standard, calibrations of the spectrograph, laser excitation wavelength, and instrument spectral response were performed to ensure high spectral quality. All Raman spectral data were processed by GramsAI (Thermo, Inc., Waltham, MA) for visual inspection. Chemometric calculations were performed by using the multivariate curve resolution-alternating least squares (MCR-ALS) in Matlab.
Multivariate Analysis of Raman Spectra
Chemometric analysis was performed by using the multivariate curve resolution-alternating least squares (MCR-ALS) method directly on the baseline-subtracted Raman spectra. MCR-ALS is based on a linear model assuming the generalized law of Lambert–Beer where the individual response of each component is addable. The aim of this method is the decomposition of the original data matrix, which contains all the spectra recorded during the dantrolene synthesis, into the product of two matrices, one that contains the concentration profiles and the other corresponding to the so-called reference Raman spectra.
Results and Discussion
Mechanochemical Preparation of the Metallodrug Silver Sulfadiazine
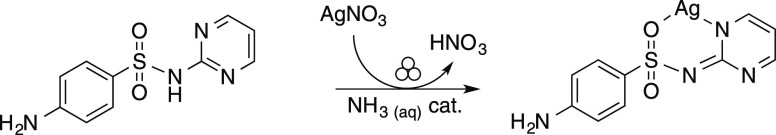
We studied the formation of silver sulfadiazine, a topical sulfa-antibiotic used as an antiseptic in creams and ointments in the treatment of extensive skin burn and surgery.54,55 Silver sulfadiazine is on the World’s Health Organization’s list of essential medicines56 because of its antibacterial properties against Gram-positive bacteria. Silver sulfadiazine has these properties because it is a metallodrug, which combines the antiseptic properties of bioactive silver ion57,58 with the sulfadiazine API.59 This association provides a new antibacterial agent having a broader spectrum of action, more recently finding new applications in coating for cardiac devices and indwelling catheters.57 Insoluble in water, it dissolves slowly only in biological fluids.54 In solution, silver sulfadiazine precipitates in good yield after mixing aqueous ammonia solutions of sulfadiazine and silver nitrate. In this study, we prepared silver sulfadiazine in the solid state in a mechanochemical reaction of silver nitrate and sulfadiazine using a catalytic amount of concentrated or diluted aqueous ammonia (Scheme 1). Proton abstraction from the sulfadiazine molecule is essential for product formation, so the reaction does not proceed without aqueous ammonia. The dry mixture of silver nitrate and sulfadiazine remained a solid mixture even after prolonged milling.
Scheme 1. Mechanochemical Preparation of Silver Sulfadiazine Using Catalytic Aqueous Ammonia.
Previous studies indicated that silver sulfadiazine is a 1:1 complex with sulfadiazine acting at the same time as the anion and the coordinating ligand, with silver coordinating both the sulfonamide group and the nitrogen atoms of the 2-aminopyrimidine ring.60−62
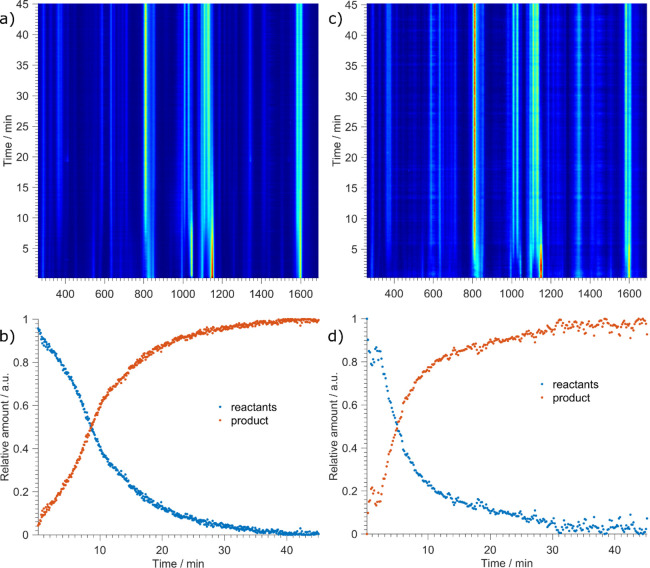
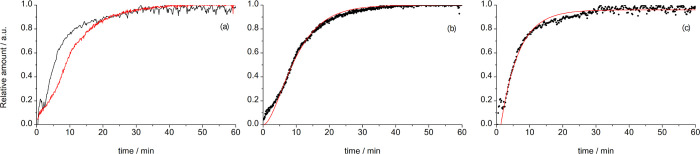
Upon addition of aqueous ammonia, the reaction on the 1 mmol scale proceeded rapidly and was complete in 10–20 min of milling (Figure 2). Reaction rates could be modified by using different concentrations of aqueous ammonia. Ammonia was essential for product formation, and without aqueous ammonia, solid sulfadiazine and AgNO3 would not react. However, optimization of the amount of ammonia was required since too much ammonia slows down the reaction as evidenced by comparing the reaction profiles for milling using equal volumes (20 μL) of either 25 or 10% aqueous ammonia solution (Figures 2 and 3a). While ammonia is likely necessary for deprotonation of sulfadiazine, it is well known that it efficiently binds to silver(I), which may thus stabilize it and slow down the formation of the product silver sulfadiazine.
Figure 2.
Time-resolved Raman monitoring and reaction profiles for mechanochemical synthesis of silver sulfadiazine from AgNO3 and sulfadiazine with (a, b) 25% aqueous ammonia and (c, d) 10% aqueous ammonia.
Figure 3.
Comparison of reaction profiles for the silver sulfadiazine formation using 10 and 25% aqueous ammonia and kinetic profile fitting. (a) Red represents 25% aqueous ammonia, and black represents 10% aqueous ammonia. (b) Fitting of the kinetic curve for reaction using 25% aqueous ammonia and (c) using 10% aqueous ammonia.
The two curves for the formation of silver sulfadiazine were collected under the same experimental conditions except for the initial concentration of the added aqueous ammonia and are well suited for a kinetic analysis and comparison. We find that the reaction using 10% NH3(aq) is faster and is best described with an exponential equation
which arises when one critical compression is required to achieve the reaction, where the final conversion (αmax) is allowed to be lower than unity and where an induction period (ti) compensates for the non-uniform mixing in the beginning of the reaction. The reaction constant k, which measures the amount of effectively processed material in a single compression (the volume fraction of the material compressed in a single ball impact), is determined to be 0.17 min–1.
The reaction using 25% NH3(aq) is best fitted using the expression
which is derived assuming that two critical compressions are required for the material to undergo a transformation. The rate constant k for this reaction equals 0.19 min–1. Importantly, the values of rate constants in these two experiments are similar, as they should be since the milling conditions (reaction vessel volume, amount of reactants, and type and number of milling balls) have not changed. This different behavior suggests a variation in the reaction mechanism, and we propose that the difference stems from stabilization of Ag+ with excess of ammonia due to the likely formation of a [Ag(NH3)2]+ species. Ammonia is essential for the reaction to facilitate proton abstraction from sulfadiazine, but excess ammonia seems to stabilize Ag+, thus slowing down the reaction.
Mechanochemical Preparation of Dantrolene
Dantrolene remains the only clinically available agent for the treatment of malignant hyperthermia (MH),63 a condition in which the body temperature is very high, by restoring normal calcium levels in the muscles. It contains the N-acylhydrazone moiety, a functional group extensively used in medicinal chemistry and marketed drugs.64
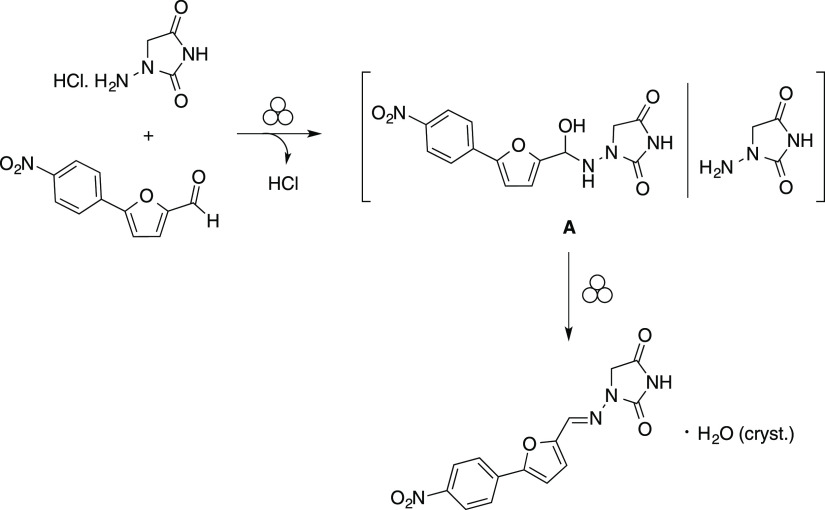
Dantrolene was previously prepared by mechanochemistry in a single-step condensation between 5-(4-nitrophenyl)furfural and 1-aminohydantoine hydrochloride (Scheme 2).51 The reaction displayed a highly improved environmental footprint and a reduced cost compared to classic solvent-based procedures. The strong activation provided by mechanochemistry avoided the use of an external base to generate the nucleophilic amine. Moreover, the hydrochloric acid generated in situ allowed the reaction to occur without the need for an additional Brønsted (e.g., hydrochloric, p-toluenesulfonic or acetic acid) or Lewis (e.g., scandium triflate) acid to promote the condensation reaction.51
Scheme 2. Mechanochemical Synthesis of Dantrolene with Two Potential Intermediate Species.
We encountered problems during our initial experiments due to fluorescence of the reaction mixture. We attributed this problem to using a home-built system consisting of components from various suppliers, as described previously.27 While the very beginning of the reaction provided interpretable Raman spectra of reactants, as soon as the product started building up, so did the fluorescence rise, which soon saturated the detector and rendered any in situ monitoring impossible. We found that using a Raman system from a single vendor, where the system was equipped with a PhAT large volumetric sampling probe, a fast (f/1.8) spectrometer, and an NIR-optimized CCD detector, enabled collecting quality Raman spectra throughout the reaction. With data collected by the PhAT-equipped Raman analyzer, we could completely visualize the transformation (Figure 4).
Figure 4.
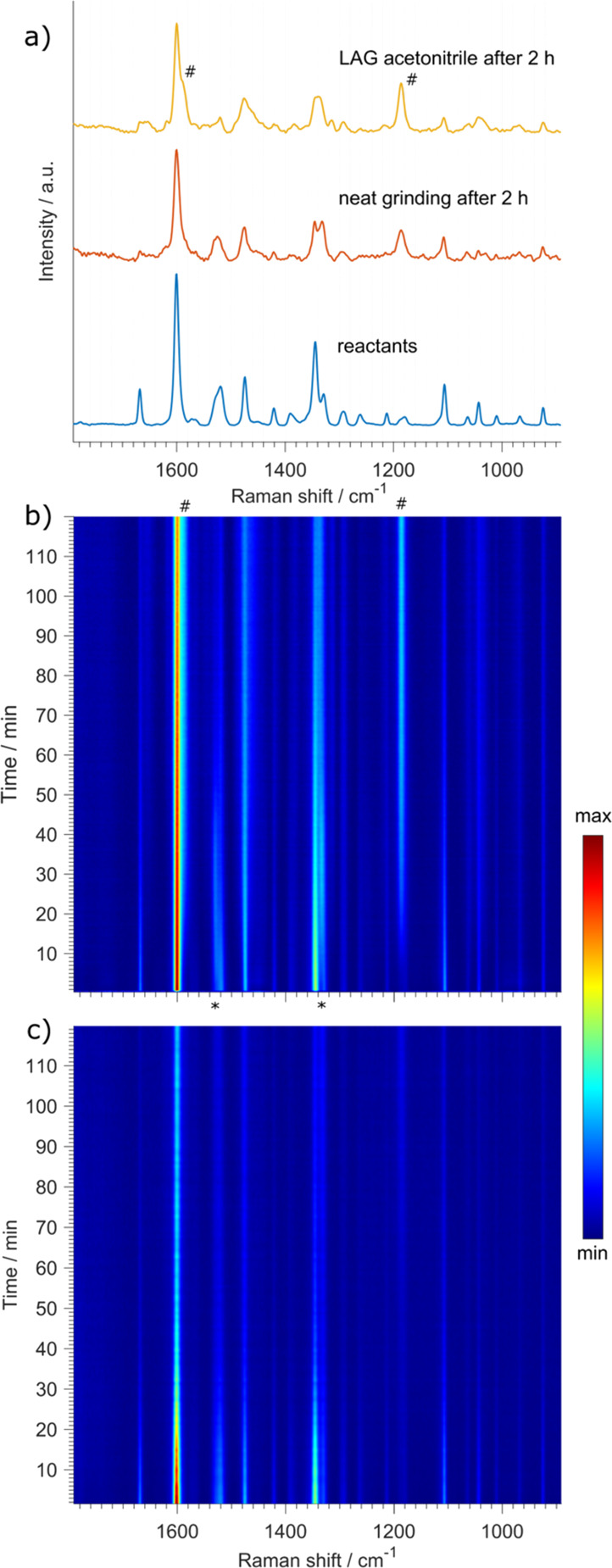
In situ Raman monitoring of dantrolene synthesis from 1-amino hydantoin hydrochloride and 5-(4-nitrophenyl)furfural. (a) Raman spectra of the 1:1 reactant mixture and final product dantrolene for NG and LAG reaction using acetonitrile. (b) Two-dimensional time-resolved plot of in situ-collected and baseline-subtracted Raman spectra for an LAG using acetonitrile (50 μL). An intermediate may be noticed by the bands appearing and disappearing denoted with “*”. The most characteristic bands of the product at 1186 and at 1590 cm–1 as a shoulder to the strongest band at 1600 cm–1 are denoted with “#”. (c) Two-dimensional time-resolved plot of in situ-collected and baseline-subtracted Raman spectra for a neat grinding reaction.
With the optimized Raman system, we were able to determine that dantrolene preparation in a neat grinding (NG) reaction proceeds at a very slow rate. Knowing that liquid-assisted grinding (LAG) may beneficially influence reaction kinetics,15,65 we switched from an NG approach to LAG using acetonitrile (50 μL) to observe a much faster reaction (Figure 4) where the formation of the product could be best observed by the emergence of characteristic bands. The characteristic bands for the hydrazone bond at 1186 and 1590 cm–1 belonged to the ν(N–N)66,67 and ν(C=N), respectively.67,68 At the same time, we observed a notable decrease in the bands at 1668 and 1115 cm–1 attributed to the ν(C=O) stretching of aldehyde and ν(N–N) stretching of the starting hydrazide in 1-amino hydantoin hydrochloride, respectively. The formation of an intermediate phase was also observed, but unfortunately the low quality of spectra did not allow us to identify the chemical nature of this intermediate.
A chemometric approach was applied to a series of spectra for the LAG dantrolene synthesis (Figure 5a) with the objective to determine the number of species present and their concentration profiles.
Figure 5.
(a) Background-subtracted Raman spectra of dantrolene synthesis as a function of reaction time. (b) Corresponding MCR decomposition into three reference spectra: spectrum in blue accounts for 23.3% of variations, spectrum in red accounts for 61.1% of variations, and the spectrum in green accounts for 11.5% of variations. (c) Concentration profile for each reference spectrum. Concentration C is dimensionless and expresses relative contributions of the three reference spectra in panel (b) to each spectrum displayed in panel (a).
Three main reference spectra explained more than 95% of all the in situ-collected Raman spectra (Figure 5b). Extracted concentration profiles for the reference spectra are presented in Figure 5c, assigning one reference spectrum jointly to the reactant mixture (blue line in Figure 5c), one to an intermediate species (green line in Figure 5c) and one to the product dantrolene (red line in Figure 5c). While visual inspection of the in situ-collected Raman spectra in Figure 4 may not have obviously revealed an intermediate, MCR-ALS analysis clearly evidenced the presence of a reaction intermediate. Moreover, it can be noticed from the MCR analysis that the concentration of the dantrolene product is not reaching a plateau at the end, which suggests that the reaction is not fully completed, as assessed also by the presence of the C=O bond contribution of the residual aldehyde (at 1668 cm–1). This observation is also in line with the final spectrum collected in situ during the reaction (Figure 4a, LAG reaction, spectrum in yellow) and with the previously reported results,51 indicating that full conversion of the reactants can be achieved only if more energetic milling regimes are applied.52,69 It can be also noticed that a small band at 1654 cm–1 appears on the spectral component of the LAG reaction product (Figure 5b, spectrum in red) attributed to the ν(C=O) and ν(C–N) stretching mode of the N-acylhydrazone.70
The identity of dantrolene obtained in LAG conditions was assessed by overlapping the 1H NMR spectrum of the crude mixture (Figure 6, red spectrum) with the spectrum of an authentic sample of dantrolene obtained by ball milling51 (Figure 6, black spectrum).
The final mixture, dissolved in DMSO-d6, was also analyzed by 1H NMR. Analysis of the 1H NMR spectra confirms the suggestion that the reaction is not fully completed (Figure 6). Beside the signals that correspond to the product, qNMR analyses show the presence of 4.6% of the starting material in the final mixture.
Data in Figure 5c are an excellent starting point to carry out a quantitative kinetic analysis. To this aim, the data have been normalized taking into account the larger experimental uncertainties affecting the estimation of the intermediate fraction. The obtained datasets are plotted in Figure 8 as a function of time t.
Figure 8.
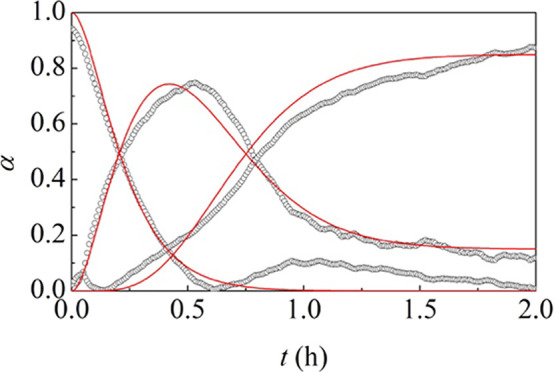
Reaction profile for the dantrolene formation. Best-fitted kinetic curves are shown.
We find that the variation of reactants αr , intermediate αi , and product αp fractions is best described with the equations
where αmax represents the maximum fraction of the intermediate that can be transformed in the final product. As discussed in detail elsewhere,53 the model equations indicate that the reactants need two critical compressions to form the intermediate, while the critical compressions for the intermediate to form the product are four. Although this is a phenomenological interpretation, it clearly indicates that the reactants are definitely more prone to transformation than the intermediate. The model equations best fit the experimental data with a αmax value of about 0.85 and a rate constant k of about 0.14 min–1. A single rate constant value suffices to describe satisfactorily the two reaction steps. It follows that the amount of effectively processed material in a single compression does not change during the transformation.
Quite interestingly, the 0.14 min–1 value is not far from those of 0.19 and 0.17 min–1 obtained for the silver sulfadiazine formation. We ascribe the result to the similar mechanical processing conditions used in the silver sulfadiazine and dantrolene syntheses.
Conclusions
We showed that in situ reaction monitoring by Raman spectroscopy is applicable to the study of mechanochemical milling processing and preparation of two model active pharmaceutical ingredients. Raman spectra can be used to better understand such processing and can be exploited in their optimization. For the metallodrug silver sulfadiazine, while aqueous ammonia is necessary to achieve a chemical reaction, too much ammonia has a contrary effect and slows down the transformation, probably through stabilization of Ag+ species. Synthesis of dantrolene is very slow if conducted by neat grinding but is accelerated under LAG conditions. Using acetonitrile as the liquid additive, an intermediate is recognized before the formation of the target product.
Worth noting is the mechanochemical set up for in situ monitoring having a high-resolution Raman system combined with a unique suitable Raman PhAT probe to investigate mechanochemical transformations involving highly fluorescent compounds, which may often be presented as an insurmountable obstacle for in situ Raman spectra collection using other technologies. Indeed, this process-analytical tool is helpful to determine the end point of the reactions and could be valuable for process optimization and control at any scale. This approach is promising to broaden the scope of Raman in situ investigations that would otherwise be impossible and are highly important because Raman monitoring is not limited by potentially poor crystallinity of the reaction mixture.
In addition, a mathematical model has been applied to examine the kinetic features of mechanochemical transformations for the preparation of APIs, allowing deeper insight into the fundamental processes involved in the chemical changes induced by mechanical processing. This approach can eventually help to unveil the mechanisms responsible for the chemical changes induced by the mechanical activation, thus paving the way to a better understanding of the mechanochemical reaction kinetics in the scale-up procedures for API,71 including metallodrugs and metallopharmaceuticals.
Acknowledgments
This work is a contribution to the COST Action CA1811272−74 supported by COST (European Cooperation on Science and Technology).75 We are grateful to the Ruđer Bošković Institute for financial support. S.L. is supported by the Croatian Science Foundation. A.P. is grateful to MIUR (Italy, PRIN project: MultIFunctional poLymer cOmposites based on groWn matERials, n° 2017B7MMJ5_001). The manuscript was written during the worldwide lockdown due to COVID-19 pandemic disease.
The authors declare no competing financial interest.
References
- Do J.-L.; Friščić T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. 10.1021/acscentsci.6b00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. L.; Adams C. J.; Bolm C.; Braga D.; Collier P.; Friščić T.; Grepioni F.; Harris K. D. M.; Hyett G.; Jones W.; Krebs A.; Mack J.; Maini L.; Orpen A. G.; Parkin I. P.; Shearouse W. C.; Steed J. W.; Waddell D. C. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. 10.1039/C1CS15171A. [DOI] [PubMed] [Google Scholar]
- Takacs L. The historical development of mechanochemistry. Chem. Soc. Rev. 2013, 42, 7649–7659. and references cited therein 10.1039/c2cs35442j. [DOI] [PubMed] [Google Scholar]
- Šepelák V.; Düvel A.; Wilkening M.; Becker K.-D.; Heitjans P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. 10.1039/c2cs35462d. [DOI] [PubMed] [Google Scholar]
- Hernández J. G.; Bolm C. Altering Product Selectivity by Mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. 10.1021/acs.joc.6b02887. [DOI] [PubMed] [Google Scholar]
- Kaupp G. Organic Solid-State Reactions with 100% Yield. Top. Curr. Chem. 2005, 254, 95–183. 10.1007/b100997. [DOI] [Google Scholar]
- Stolle A.; Szuppa T.; Leonhardt S. E. S.; Ondruschka B. Ball milling in organic synthesis: solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. 10.1039/c0cs00195c. [DOI] [PubMed] [Google Scholar]
- Wang G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. 10.1039/c3cs35526h. [DOI] [PubMed] [Google Scholar]
- Hernández J. G. C–H Bond Functionalization by Mechanochemistry. Chem. – Eur. J. 2017, 23, 17157–17165. 10.1002/chem.201703605. [DOI] [PubMed] [Google Scholar]
- Juribašić M.; Užarević K.; Gracin D.; Ćurić M. Mechanochemical C-H bond activation: rapid and regioselective double cyclopalladation monitored by in situ Raman spectroscopy. Chem. Commun. 2014, 50, 10287–10290. 10.1039/C4CC04423A. [DOI] [PubMed] [Google Scholar]
- Rightmire N. R.; Hanusa T. P. Advances in organometallic synthesis with mechanochemical methods. Dalton Trans. 2016, 45, 2352–2362. 10.1039/C5DT03866A. [DOI] [PubMed] [Google Scholar]
- Pichon A.; Lazuen-Garay A.; James S. L. Solvent-free synthesis of a microporous metal-organic framework. CrystEngComm 2006, 8, 211–214. 10.1039/B513750K. [DOI] [Google Scholar]
- Stolar T.; Batzdorf L.; Lukin S.; Žilić D.; Motillo C.; Friščić T.; Emmerling F.; Halasz I.; Užarević K. In Situ Monitoring of the Mechanosynthesis of the Archetypal Metal–Organic Framework HKUST-1: Effect of Liquid Additives on the Milling Reactivity. Inorg. Chem. 2017, 56, 6599–6608. 10.1021/acs.inorgchem.7b00707. [DOI] [PubMed] [Google Scholar]
- Friščić T.; Jones W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. 10.1021/cg800764n. [DOI] [Google Scholar]
- Hasa D.; Jones W. Screening for new pharmaceutical solid forms using mechanochemistry: a practical guide. Adv. Drug Delivery Rev. 2017, 117, 147–161. 10.1016/j.addr.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Colacino E.; Porcheddu A.; Charnay C.; Delogu F. From enabling technologies to medicinal mechanochemistry: an eco-friendly access to hydantoin-based active pharmaceutical ingredients. Reac. Chem. Eng. 2019, 4, 1179–1188. 10.1039/C9RE00069K. [DOI] [Google Scholar]
- Charnay C.; Porcheddu A.; Delogu F.; Colacino E.. Green Synthetic Processes and Procedures. New and up-and-coming perspectives for an unconventional chemistry: from molecular synthesis to hybrid materials by mechanochemistry; in, Ed. Ballini R.; Royal Society of Chemistry: Ch. 9, pp. 192–215: 2019. [Google Scholar]
- Tan D.; Loots L.; Friščić T. Towards medicinal mechanochemistry: evolution of milling from pharmaceutical solid form screening to the synthesis of active pharmaceutical ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. 10.1039/C6CC02015A. [DOI] [PubMed] [Google Scholar]
- Colacino E.; Dayaker G.; Morère A.; Friščić T. Introducing Students to Mechanochemistry via Environmentally Friendly Organic Synthesis Using a Solvent-Free Mechanochemical Preparation of the Antidiabetic Drug Tolbutamide. J. Chem. Educ. 2019, 96, 766–771. 10.1021/acs.jchemed.8b00459. [DOI] [Google Scholar]
- Cinčić D.; Friščić T.; Jones W. A stepwise mechanism for the mechanochemical synthesis of halogen-bonded cocrystal architectures. J. Am. Chem. Soc. 2008, 130, 7524–7525. 10.1021/ja801164v. [DOI] [PubMed] [Google Scholar]
- Drebushchak T. N.; Ogienko A. A.; Boldyreva E. V. ‘Hedvall effect’ in cryogrinding of molecular crystals. A case study of a polymorphic transition in chlorpropamide. CrystEngComm 2011, 13, 4405–4410. 10.1039/c1ce05189j. [DOI] [Google Scholar]
- Ma X.; Yuan W.; Bell S. E. J.; James S. L. Better understanding of mechanochemical reactions: Raman monitoring reveals surprisingly simple ‘pseudo-fluid’ model for a ball milling reaction. Chem. Commun. 2014, 50, 1585–1587. 10.1039/c3cc47898j. [DOI] [PubMed] [Google Scholar]
- Tumanov I. A.; Achkasov A. F.; Boldyreva E. V.; Boldyrev V. V. About the possibilities to detect intermediate stages in mechanochemical synthesis of molecular complexes. Russ. J. Phys. Chem. A 2012, 86, 1014–1017. 10.1134/S003602441206026X. [DOI] [Google Scholar]
- Friščić T.; Halasz I.; Beldon P. J.; Belenguer A. M.; Adams F.; Kimber S. A. J.; Honkimäki V.; Dinnebier R. E. Real-time and in situ monitoring of mechanochemical milling reactions. Nat. Chem. 2013, 5, 66–73. 10.1038/nchem.1505. [DOI] [PubMed] [Google Scholar]
- Gracin D.; Štrukil V.; Friščić T.; Halasz I.; Užarević K. Laboratory real-time and in situ monitoring of mechanochemical milling reactions by Raman spectroscopy. Angew. Chem., Int. Ed. 2014, 53, 6193–6197. 10.1002/anie.201402334. [DOI] [PubMed] [Google Scholar]
- Batzdorf L.; Fischer F.; Wilke M.; Wenzel K.; Emmerling F. Direct In Situ Investigation of Milling Reactions Using Combined X-ray Diffraction and Raman Spectroscopy. Angew. Chem., Int. Ed. 2015, 54, 1799–1802. 10.1002/anie.201409834. [DOI] [PubMed] [Google Scholar]
- Lukin S.; Stolar T.; Tireli M.; Blanco M. V.; Babić D.; Friščić T.; Užarević K.; Halasz I. Tandem in situ monitoring for quantitative assessment of mechanochemical reactions involving structurally unknown phases. Chem. – Eur. J. 2017, 23, 13941–13949. 10.1002/chem.201702489. [DOI] [PubMed] [Google Scholar]
- Halasz I.; Friščić T.; Kimber S. A. J.; Užarević K.; Puškarić A.; Mottillo C.; Julien P.; Štrukil V.; Honkimäki V.; Dinnebier R. E. Quantitative in situ and real-time monitoring of mechanochemical reactions. Faraday Discuss. 2014, 170, 203–221. 10.1039/C4FD00013G. [DOI] [PubMed] [Google Scholar]
- Lukin S.; Lončarić I.; Tireli M.; Stolar T.; Blanco M. V.; Lazić P.; Užarević K.; Halasz I. Experimental and Theoretical Study of Selectivity in Mechanochemical Cocrystallization of Nicotinamide with Anthranilic and Salicylic Acid. Cryst. Growth Des. 2018, 18, 1539–1547. 10.1021/acs.cgd.7b01512. [DOI] [Google Scholar]
- Halasz I.; Kimber S. A. J.; Beldon P. J.; Belenguer A. M.; Adams F.; Honkimäki V.; Nightingale R. C.; Dinnebier R. E.; Friščić T. In situ and real-time monitoring of mechanochemical milling reactions using synchrotron x-ray diffraction. Nat. Protoc. 2013, 8, 1718–1729. 10.1038/nprot.2013.100. [DOI] [PubMed] [Google Scholar]
- Babu N. J.; Nangia A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. 10.1021/cg200492w. [DOI] [Google Scholar]
- Caira M. R.; Nassimbeni L. R.; Wildervanck A. F. Selective formation of hydrogen bonded cocrystals between a sulfonamide and aromatic carboxylic acids in the solid state. J. Chem. Soc., Perkin Trans. 2 1995, 12, 2213–2216. 10.1039/P29950002213. [DOI] [Google Scholar]
- Delori A.; Friščić T.; Jones W. The role of mechanochemistry and supramolecular design in the development of pharmaceutical materials. CrystEngComm 2012, 14, 2350–2362. 10.1039/C2CE06582G. [DOI] [Google Scholar]
- Friščić T.; Jones W. Benefits of cocrystallisation in pharmaceutical materials science: an update. J. Pharm. Pharmacol. 2010, 62, 1547–1559. 10.1111/j.2042-7158.2010.01133.x. [DOI] [PubMed] [Google Scholar]
- Halasz I.; Puškarić A.; Kimber S. A. J.; Beldon P. J.; Belenguer A. M.; Adams F.; Honkimäki V.; Dinnebier R. E.; Patel B.; Jones W.; Štrukil V.; Friščić T. Real-Time In Situ Powder X-ray Diffraction Monitoring of Mechanochemical Synthesis of Pharmaceutical Cocrystals. Angew. Chem. 2013, 52, 11538–11541. 10.1002/anie.201305928. [DOI] [PubMed] [Google Scholar]
- Newman A. Specialized Solid Form Screening Techniques. Org. Process Res. Dev. 2013, 17, 457–471. 10.1021/op300241f. [DOI] [Google Scholar]
- Sanphui P.; Kumar S. S.; Nangia A. Pharmaceutical cocrystals of niclosamide. Cryst. Growth Des. 2012, 12, 4588–4599. 10.1021/cg300784v. [DOI] [Google Scholar]
- Sun C. C. Cocrystallization for successful drug delivery. Expert Opin. Drug Delivery 2012, 10, 201–213. 10.1517/17425247.2013.747508. [DOI] [PubMed] [Google Scholar]
- Weyna D. R.; Shattock T.; Vishweshwar P.; Zaworotko M. J. Synthesis and Structural Characterization of Cocrystals and Pharmaceutical Cocrystals: Mechanochemistry vs Slow Evaporation from Solution. Cryst. Growth Des. 2009, 9, 1106–1123. 10.1021/cg800936d. [DOI] [Google Scholar]
- Tan D.; Štrukil V.; Mottillo C.; Friščić T. Mechanosynthesis of pharmaceutically relevant sulfonyl-(thio)ureas. Chem. Commun. 2014, 50, 5248–5250. 10.1039/C3CC47905F. [DOI] [PubMed] [Google Scholar]
- Pérez-Venegas M.; Juaristi E. Mechanochemical and Mechanoenzymatic Synthesis of Pharmacologically Active Compounds: A Green Perspective. ACS Sustainable Chem. Eng. 2020, 8881. 10.1021/acssuschemeng.0c01645. [DOI] [Google Scholar]
- Friščić T.; Halasz I.; Štrukil V.; Eckert-Maksić M.; Dinnebier R. E. Clean and Efficient Synthesis Using Mechanochemistry: Coordination Polymers, Metal-Organic Frameworks and Metallodrugs. Croat. Chem. Acta 2012, 85, 367–378. 10.5562/cca2014. [DOI] [Google Scholar]
- Quaresma S.; André V.; Fernandes A.; Duarte T. M. Mechanochemistry - a green synthetic methodology leading to metallodrugs, metallopharmaceuticals and bio-inspired metal-organic frameworks. Inorg. Chim. Acta 2016, 455, 309–318. 10.1016/j.ica.2016.09.033. [DOI] [Google Scholar]
- André V.; Hardeman A.; Halasz I.; Stein R. S.; Jackson G. J.; Reid D. G.; Duer M. J.; Curfs C.; Duarte M. T.; Friščić T. Mechanosynthesis of the Metallodrug Bismuth Subsalicylate from Bi2O3 and Structure of Bismuth Salicylate without Auxiliary Organic Ligands. Angew. Chem., Int. Ed. 2011, 50, 7858–7861. 10.1002/anie.201103171. [DOI] [PubMed] [Google Scholar]
- Braga D.; Grepioni F.; André V.; Duarte M. T. Drug-containing coordination and hydrogen bonding networks obtained mechanochemically. CrystEngComm 2009, 11, 2618–2621. 10.1039/b913433f. [DOI] [Google Scholar]
- Braga D.; Grepioni F.; Maini L.; Brescello R.; Cotarca L. Simple and quantitative mechanochemical preparation of the first zinc and copper complexes of the neuroleptic drug gabapentin. CrystEngComm 2008, 10, 469–471. 10.1039/b719451j. [DOI] [Google Scholar]
- Friščić T.; Halasz I.; Strobridge F. C.; Dinnebier R. E.; Stein R. S.; Fábián L.; Curfs C. A rational approach to screen for hydrated forms of the pharmaceutical derivative magnesium naproxen using liquid-assisted grinding. CrystEngComm 2011, 13, 3125–3129. 10.1039/c0ce00894j. [DOI] [Google Scholar]
- Konnert L.; Reneaud B.; de Figueiredo R. M.; Campagne J.-M.; Lamaty F.; Martinez J.; Colacino E. Mechanochemical Preparation of Hydantoins from Amino Esters: Application to the Synthesis of the Antiepileptic Drug Phenytoin. J. Org. Chem. 2014, 79, 10132–10142. 10.1021/jo5017629. [DOI] [PubMed] [Google Scholar]
- Konnert L.; Dimassi M.; Gonnet L.; Lamaty F.; Martinez J.; Colacino E. Poly(ethylene) glycols and mechanochemistry for the preparation of bioactive 3,5-disubstituted hydantoins. RSC Adv. 2016, 6, 36978–36986. 10.1039/C6RA03222B. [DOI] [Google Scholar]
- Porcheddu A.; Delogu F.; De Luca L.; Colacino E. From Lossen Transposition to Solventless Medicinal Mechanochemistry. ACS Sustainable Chem. Eng. 2019, 7, 12044–12051. 10.1021/acssuschemeng.9b00709. [DOI] [Google Scholar]
- Colacino E.; Porcheddu A.; Halasz I.; Charnay C.; Delogu F.; Guerra R.; Fullenwarth J. Mechanochemistry for “no solvent, no base” preparation of hydantoin-based active pharmaceutical ingredients: nitrofurantoin and dantrolene. Green Chem. 2018, 20, 2973–2977. 10.1039/C8GC01345D. [DOI] [Google Scholar]
- Colacino E.; Carta M.; Pia G.; Porcheddu A.; Ricci P. C.; Delogu F. Processing and Investigation Methods in Mechanochemical Kinetics. ACS Omega 2018, 3, 9196–9209. 10.1021/acsomega.8b01431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M.; Colacino E.; Delogu F.; Porcheddu A. Kinetics of mechanochemical transformations. Phys. Chem. Chem. Phys. 2020, 22, 14489–14502. 10.1039/D0CP01658F. [DOI] [PubMed] [Google Scholar]
- Fox C. L. Jr. Silver sulfadiazine-a New Topical Therapy for Pseudomonas in Burns. Arch. Surg. 1968, 96, 184–188. 10.1001/archsurg.1968.01330200022004. [DOI] [PubMed] [Google Scholar]
- Fuller F. W.; Parrish M.; Nance F. C. A review of the dosimetry of 1% silver sulfadiazine cream in bum wound treatment. J. Burn Care Rehab. 1994, 15, 213–223. 10.1097/00004630-199405000-00003. [DOI] [PubMed] [Google Scholar]
- World Health Organization Model List of Essential Medicines, 21st List, 2019; World Health Organization: Geneva; 2019. [Google Scholar]
- Lansdown A. B. G. Silver in Health Care: Antimicrobial Effects and Safety in Use. Curr. Probl. Dermatol. 2006, 33, 17–34. 10.1159/000093928. [DOI] [PubMed] [Google Scholar]
- Chernousova S.; Epple M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem., Int. Ed. 2013, 52, 1636–1653. 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- Stober H.; DeWitte W. Sulfadiazine. Anal. Profiles Drug Subst. 1982, 11, 523–551. 10.1016/S0099-5428(08)60274-9. [DOI] [Google Scholar]
- Bult A.; Klasen H. B. Structures of silver sulfonamides. J. Pharm. Sci. 1978, 67, 284–287. 10.1002/jps.2600670249. [DOI] [PubMed] [Google Scholar]
- Narang K. K.; Gupta J. K. Silver (I) complexes of Sulfathiazole, Sulfadiazine, Sulfamerazine and Sulfamethazine. Curr. Sci. 1976, 45, 744–746. [Google Scholar]
- Cook D. S.; Turner M. F. Crystal and molecular structure of silver sulphadiazine (N1-pyrimidin-2-ylsulphanilamide). J. Chem. Soc., Perkin Trans. 2 1975, 10, 1021–1025. 10.1039/P29750001021. [DOI] [Google Scholar]
- Krause T.; Gerbershagen M. U.; Fiege M.; Weißhorn R.; Wappler F. Dantrolene - a review of its pharmacology, therapeutic use and new developments. Anaesthesia 2004, 59, 364–373. 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- Thota S.; Rodrigues D. A.; Pinheiro P. d. S. M.; Lima L. M.; Fraga C. A. M.; Barreiro E. J. N-Acylhydrazones as drugs. Bioorg. Med. Chem. Lett. 2018, 28, 2797–2806. 10.1016/j.bmcl.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Babu N. R.; Subashchandrabose S.; Padusha M. S. A.; Erdoǧdu H. S. Y. Synthesis and spectral characterization of hydrazone derivative of furfural using experimental and DFT methods. Spectrochim. Acta, Part A 2014, 120, 314–322. 10.1016/j.saa.2013.09.089. [DOI] [PubMed] [Google Scholar]
- González-Baró A. C.; Pis-Diez R.; Parajón-Costa B. S.; Rey N. A. Spectroscopic and theoretical study of the o-vanillin hydrazone of the mycobactericidal drug isoniazid. J. Mol. Struct. 2012, 1007, 95–101. 10.1016/j.molstruc.2011.10.026. [DOI] [Google Scholar]
- Saeed A.; Arshad M. I.; Bolte B.; Fantoni A. C.; Delgado Espinoza Z. Y.; Erben M. F. On the roles of close shell interactions in the structure of acyl-substituted hydrazones: An experimental and theoretical approach. Spectrochim. Acta, Part A 2016, 157, 138–145. 10.1016/j.saa.2015.12.026. [DOI] [PubMed] [Google Scholar]
- Pinheiro P. d. S. M.; Rodrigues D. A.; Alves M. A.; Tinoco L. W.; Ferreira G. B.; de Sant’Anna C. M. R.; Fraga C. A. M. Theoretical and Experimental Characterization of 1,4-N···S σ-hole Intramolecular Interactions in Bioactive N-Acylhydrazone Derivatives. New J. Chem. 2018, 42, 497–505. 10.1039/C7NJ03543H. [DOI] [Google Scholar]
- Full converion of the rectants was achieved with a planetary mill or a SPEX mixer-mill equipment, using zirconium oxide jars and balls as grinding media.
- Galić N.; Dijanošić A.; Kontrec D.; Miljanić S. Structural investigation of aroylhydrazones in dimethylsulphoxide/water mixtures. Spectrochim. Acta, Part A 2012, 95, 347–353. 10.1016/j.saa.2012.03.086. [DOI] [PubMed] [Google Scholar]
- Crawford D. E.; Porcheddu A.; McCalmont A. S.; Delogu F.; James S. L.; Colacino E. Solvent-free, continuous synthesis of hydrazone-based Active Pharmaceutical Ingredients by Twin-Screw Extrusion. ACS Sustainable Chem. Eng. 2020, 8, 12230–12238. 10.1021/acssuschemeng.0c03816. [DOI] [Google Scholar]
- COST Action CA18112 ’Mechanochemistry for Sustainable Industry’; http://www.mechsustind.eu/.
- Baláž M.; Vella-Zarb L.; Hernández J.; Halasz I.; Crawford D. E.; Krupička M.; André V.; Niidu A.; Garcia F.; Maini L.; Colacino E. Mechanochemistry: a disruptive innovation for the industry of the future. Chim. Oggi 2019, 37, 32–34. [Google Scholar]
- Hernández J. G.; Halasz I.; Crawford D. E.; Krupička M.; Baláž M.; André V.; Vella-Zarb L.; Niidu A.; García F.; Maini L.; Colacino E. European Research in Focus: Mechanochemistry for Sustainable Industry (MechSustInd). Eur. J. Org. Chem. 2020, 8–9. 10.1002/ejoc.201901718. [DOI] [Google Scholar]
- COST Actions; http://www.cost.eu/.