Abstract
Purpose of review
Cancer is a common cause of morbidity and mortality in the USA. While the association between venous thrombosis and malignancy is well established, arterial thrombosis has more recently been recognized as a serious complication of cancer and certain chemotherapeutic agents. This review aims to summarize the most recent literature regarding the incidence and risk factors for cancer-related arterial thrombosis, understand the pathophysiologic mechanisms of thrombosis, and highlight the specific diagnostic and treatment considerations relevant to cancer patients.
Recent findings
Based on a recent study looking at the Surveillance, Epidemiology, and End Results (SEER) database, the incidence of arterial thromboembolic events (ATEs) in patients with cancer at 6 months is 4.7%; the presence of an ATE is predictive of worse outcomes. Certain drugs such as platinum-based agents, vascular endothelial growth factor inhibitors, tyrosine kinase inhibitors, and taxanes have been associated with high rates of ATEs. Increased platelet reactivity appears crucial to development of arterial thrombosis in cancer patients.
Summary
Cancer patients have an increased risk of arterial thrombosis that is likely due to both a cancer-associated procoagulant state as well as the adverse effects of certain chemotherapeutic agents. Treatment of arterial thromboembolism in cancer patients typically requires a multidisciplinary approach in part due to high rates of thrombocytopenia and stent thrombosis in the setting of percutaneous interventions. More studies are needed to investigate optimal prophylaxis, surveillance strategies, and treatments of cancer-related arterial thromboembolic disease.
Keywords: Arterial thrombosis, Cancer, Chemotherapy
Introduction
Cancer is a leading cause of morbidity and mortality in the USA [1] afflicting approximately 40% of people at some point in their lifetime [2]. With newer and more effective treatments, many patients live for years or even decades after an initial cancer diagnosis. In fact, there are an estimated 15.5 million children and adult cancer survivors in the USA as of 2016 [3], and this number is expected to increase to 20.3 million by year 2026 [3].
Patients with cancer experience a high burden of thromboembolic disease [4]. Traditionally, the main consideration has been given to venous thrombotic events in this population; however, in 2011, Blann et al. [5] outlined that approximately 25% of all PubMed citations on thrombosis are on arterial thrombosis. In one prospective study of cancer patients receiving outpatient chemotherapy, arterial thrombosis accounted for 5.6% of deaths [4]. Here, we review the incidence and risk factors for cancer-related arterial thrombosis, the pathophysiologic mechanisms of thrombosis, and the specific diagnostic and treatment considerations relevant to cancer patients.
Epidemiology
Incidence of arterial thrombosis in cancer patients
Prior studies of hospitalized cancer patients have reported an incidence of arterial thrombosis of around 1.5–5.2% in this population [6, 7]. More recently, Navi et al. analyzed the Surveillance, Epidemiology, and End Results (SEER) database in order to characterize the incidence of ATE (arterial thromboembolic events, myocardial infarction or stroke) in cancer patients across the USA (Fig. 1) [8••]. The study included 279,719 pairs of patients with cancer and matched controls with a new diagnosis of cancer identified between 2002 and 2011. The cancer types included breast, lung, prostate, colorectal, bladder, pancreatic, gastric, and non-Hodgkin lymphoma. The incidence of ATE at 6 months was 4.7% in all cancer patients compared to 2.2% in the matched control cohort. Patients with lung, gastric, or pancreatic cancers had the highest rates of ATE (8.3, 6.5, and 5.9%, respectively). Ischemic stroke was less common in cancer patients than myocardial infarction (2.0% at 6 month follow-up versus 3.0% respectively). One-year after diagnosis, the risk for ATE was significantly attenuated in most cancer types. Advanced stage of cancer was associated with a significant increase in ATE (stage 0 2.3% incidence at 6 months compared to 7.7% for stage 4). ATE was associated with increased mortality even after matching for all factors and the stage of the cancer (hazard ratio 3.1, CI 3.0–3.1) [8••]. The 30-day cumulative incidence of death after ATE was 17.6 versus 11.6% in patients with cancer and controls, respectively [8••]. In the National Heart, Lung, and Blood Institute Dynamic Registry, a history of cancer was a significant predictor of 1-year death and myocardial infarction (MI) in patients who presented with an acute MI requiring percutaneous coronary intervention (PCI) [9]. A retrospective analysis of 261 patients with a history of cancer in the Mayo Clinic PCI Registry of patients who underwent PCI for an ST segment elevation MI were found to have a three times higher risk of acute in-hospital and long-term non-cardiac mortality risk, but no increased acute or long-term cardiac mortality risk with evidence-based cardiac treatment and care [10].
Fig. 1.
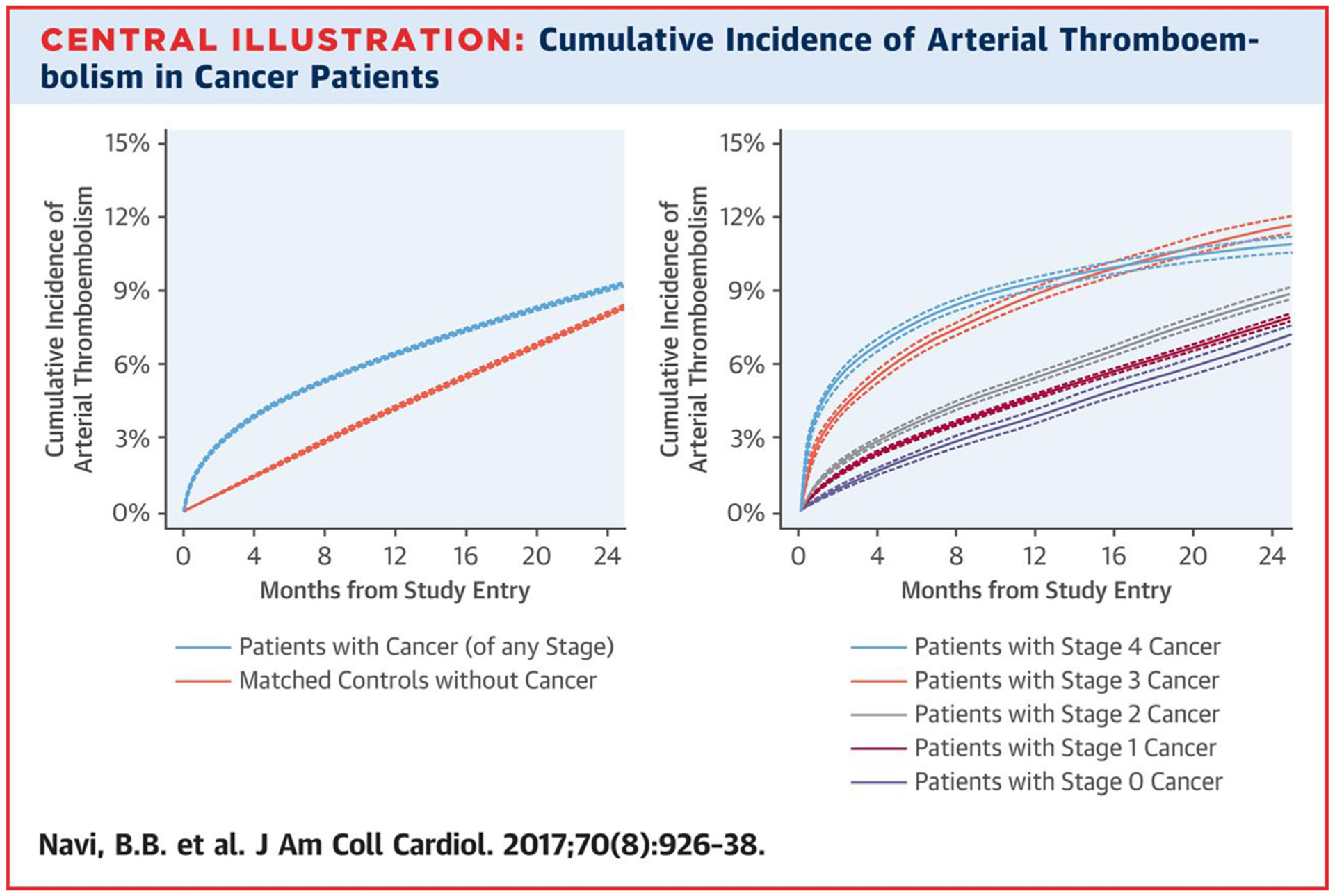
*Cumulative incidence of arterial thromboembolism (composite of myocardial infarction and ischemic stroke) in patients with cancer compared to matched control patients (left panel) and when stratified by cancer stage at the time of cancer diagnosis (right panel). Competing risk survival statistics were used to calculate incidence. Dashed lines are used to indicate 95% confidence intervals. Reprinted from Navi et al., J Am Coll Cardiol 2017;70(8):926–38, with permission from Elsevier.
The risk of recurrent ATE is not well described. One study evaluated cancer patients with acute ischemic stroke and showed that the rate of recurrent ATE in that population is 21, 31, and 37% at 1, 3, and 6 months, respectively. Among different cancer histologies, adenocarcinoma had the highest rates of recurrence (hazard ratio 1.65, CI 1.02–2.68) [11].
Malignancy appears to markedly increase the risk of in-stent thrombosis (IST) after percutaneous intervention. IST incidence in patients with known malignancies and bare metal stent(BMS) was reported at 5.56% (p < 0.000001, odds ratio 7.10, 95% confidence interval 2.70 to 17.61), with a median time to IST of 7 days. The majority of the patient were on dual anti-platelet therapy (DAPT, 83.3%) when the IST occurred [12].
Chemotherapy-related arterial thrombosis
Many chemotherapeutic agents are prothrombotic, and up until recently, the association between chemotherapy and arterial thrombosis was largely documented in case reports. Although it is difficult to precisely define the relative prothrombotic effects of the chemotherapy compared to the hypercoagulable state of malignancy, many chemotherapeutic agents have been associated with a high burden of ATE. The most common arterial thrombotic events include myocardial infarction (MI), cerebrovascular event (CVA), and peripheral artery disease (PAD). Platinum-based agents [13–15], vascular endothelial growth factor (VEGF) inhibitors [16–20], VEGF tyrosine kinase receptor inhibitors (TKI) [21–25], and Bcr-Abl TKI [26] have all been associated with increased rates of ATE. The reported incidence of ATE for different medications is shown in Table 1. Cisplatin (platinum-based agent) and ponatinib (Bcr-Abl TKI) have strikingly high rates of ATE (up to 8.3% [13] for cisplatin and 20% [26] for ponatinib). Of note, in 2013, the FDA requested a voluntary suspension of Ponatinib marketing until safety measures were implemented due to extremely high rates of vascular events during the post-marketing analysis. Patients with multiple myeloma who are treated with lenalidomide and dexamethasone also have a high rate of arterial thromboembolic events (MI 1.98% and CVA 3.4%) [55]. Medications such as bevacizumab (VEGF inhibitor), sorafenib/sunitinib/pazopanib (VEGF TKI), and tamoxifen (selective estrogen-receptor modulator) have more modest rates.
Table 1.
Arterial thromboembolic event rate for various chemotherapeutic agents
| Medication class | Medication name | Incidencea | Event type |
|---|---|---|---|
| Coronary artery events | |||
| Platinum-based agents [14, 27] | Cisplatin | 1.6–8.7% | Myocardial infarction |
| Platinum-based agents [28] | Cisplatin | 3.1-fold increase | Myocardial infarction |
| Immunomodulatory drugs [29] | Lenalidomide + dexamethasone | 1.98% | Myocardial infarction |
| VEGF inhibitor [16, 18, 22, 30] | Bevacizumab | 0.6–2% | Myocardial infarction/acute Coronary syndrome/cardiac ischemia |
| VEGF TKI [31] | Sunitinib | 11% | Heart failure or myocardial infarction |
| VEGF TKI [22] | Pazopanib | 2% | Myocardial infarction |
| VEGF TKI [32, 33] | Sorafenib | 3–4.9% | Cardiac ischemia or infarction |
| Bcr-Abl TKI [22] | Nilotinib | 7.50% | Acute coronary syndrome |
| Bcr-Abl TKI [26, 34] | Ponatinib | 1–12% | Myocardial infarction |
| Fluoropyrimidine [35, 36] | Capecitabine | 0.5–9% | Angina pectoris, myocardial ischemia, myocardial infarction, acute coronary syndrome |
| Fluoropyrimidine [37] | Capecitabine | 1.90% | Cardiotoxicity |
| Fluoropyrimidine [35, 38, 39] | 5-FU | 0.7–8% | Myocardial infarction, angina/cardiac chest pain |
| Fluoropyrimidine [37, 40–42] | 5-FU | 1.2–18% | Cardiotoxicity |
| Fluoropyrimidine [43] | Capecitabine +5-FU | 0–2% | Myocardial infarction/cardiogenic shock/cardiac arrest |
| Taxol [44, 45] | Paclitaxel | 0.26–5% | Myocardial infarction/myocardial ischemia |
| Taxol [46] | Paclitaxel | 5% | Arrhythmia, conduction blocks, cardiac ischemia |
| Aromatase inhibitors [47] | - | 4.20% | Cardiovascular event |
| Cerebrovascular accident | |||
| Platinum-based agents [27] | Cisplatin | 8.70% | Cerebrovascular accident |
| Platinum-based agents [14] | Cisplatin + gemcitabine | 0.80% | Cerebral ischemic stroke |
| Thalidomide analogue [29] | Lenalidomide + dexamethasone | 3.40% | Cerebrovascular accident |
| VEGF inhibitor [16,18, 22] | Bevacizumab | 1.1–1.9% | Ischemic stroke/cerebrovascular accident, transient ischemic attack |
| VEGFR inhibitors [33] | Sorafenib | 1.50% | Central nervous system ischemia |
| VEGFR inhibitors [48] | Pazopanib | 1% | stroke/transient ischemic attack |
| Bcr-Abl TKI [26] | Ponatinib | 6% | Cerebrovascular occlusion |
| Peripheral artery disease | |||
| Platinum-based agents [14] | Cisplatin + gemcitabine | 5.6% | Iliac artery embolism |
| Bcr-Abl TKI [22, 49–51] | Nilotinib | 1.1–25% | Peripheral artery (occlusive) disease, visceral arteries and limb ischemia |
| Bcr-Abl TKI [22, 26] | Ponatinib | 8–48% | Peripheral arterial occlusive events, visceral arteries and limb ischemia |
| All arterial thromboembolic events | |||
| Platinum-based agents [13–15] | Cisplatin | 1.4–8.3% | Arterial thromboembolic event |
| Immunomodulatory drugs [52, 53] | Thalidomide | 4.5–12.5% | Arterial thromboembolic event |
| VEGF inhibitor [16–19] | Bevacizumab | 1.7–3.8% | Arterial thromboembolic event |
| VEGF inhibitor [20] | Bevacizumab + chemotherapy | 5.5 events per 100 person-years | Arterial thromboembolic event |
| VEGF TKI [21] | Sorafenib | 1.7% | Arterial thromboembolic event |
| VEGF TKI [22] | Sorafenib (RR is compared to bevacizumab) | RR 2.3 | Arterial thromboembolic event |
| VEGF TKI [21, 23] | Sunitinib | 1.3–4.1% | Arterial thromboembolic event |
| VEGF TKI [22] | Sunitinib (RR is compared to bevacizumab) | RR 5.9 | Arterial thromboembolic event |
| VEGF TKI [24, 25] | Pazopanib | 3–4% | Arterial thromboembolic event |
| VEGF TKI [22] | Pazopanib (RR is compared to bevacizumab) | RR 4.6 | Arterial thromboembolic event |
| Bcr-Abl TKI [26] | Ponatinib | 20% | Arterial thromboembolic event |
| Selective estrogen-receptor modulator [54] | Tamoxifen + chemotherapy | 1.60% | Arterial thromboembolic event |
VEGF vascular endothelial growth factor, TKI tyrosine kinase inhibitor, FU fluorouracil, RR relative risk, HR hazard ratio, CI confidence interval
Unless otherwise noted
Cisplatin [14, 27, 28], nilotinib [22], ponatinib [26, 34], 5-FU [35, 37–42], and capecitabine [35–37] all have high incidence of coronary artery thrombotic events. However, there is some variation in the definition of cardiac events across studies and inclusion of non-thrombotic events in some studies may exaggerate some of the incidence rate. Chemotherapy-related CVA is overall less common than coronary artery thrombosis. Medications with the highest rates of CVA or transient ischemic attack (TIA) include cisplatin [14, 27], ponatinib [26], and lenalidomide [29]. PAD has been reported as a significant complication of only a few medications. The most significant medications include nilotinib [22, 49–51] and ponatinib [22, 26] with incidence of occlusive PAD up to 25 and 48%, respectively. The occlusive PAD associated with these Bcr-Abl TKIs can occur in patients without any traditional cardiovascular risk factors and may be severe and progressive despite cessation of therapy [51].
Risk factors for arterial thrombosis
Thrombosis can occur in any vessel leading to complications such as myocardial infarction, stroke, or limb ischemia as described above. The factors of Virchow’s triad, endothelial injury, stasis, and hypercoagulability, contribute to the development of both venous and arterial thrombi; however, platelet activation in the setting of pre-existing disease, such as atherosclerosis and vasculitis, appears to be crucial for the development of arterial thrombosis [56, 57]. Many risk factors, such as age, smoking, hypertension, and diabetes, are common to both venous and arterial thrombi [56, 57]. While thrombophilic disorders, such as factor V Leiden and prothrombin G20120A, are important risk factors for venous thrombosis, they carry a much lower risk for arterial thrombosis. Lupus anti-coagulant and hyperhomocysteinemia are notable exceptions that are well-known to cause arterial thrombi [57].
Cancer is a hypercoagulable state associated with a 7-fold increase in venous thromboembolism; however, the association with arterial thromboembolism (ATE) is less well established [58, 59]. Case reports of patients presenting with arterial thrombosis as the first sign of an occult malignancy or progression of an early-stage cancer [59, 60] suggest that cancer may be an independent risk factor for arterial thrombi. A case report of three patients with acute arterial thrombosis in the setting of a new malignancy additionally highlighted that the thrombotic events occurred with no signs of a non-neoplastic-associated hypercoagulable state or atherosclerosis [61]. Some malignancies, such as polycythemia vera and multiple myeloma, are commonly associated with arterial thrombosis [57, 62]. In a large population study in Sweden, patients with multiple myeloma were found to have an increased risk of ATE at 1, 5, and 10 years after the initial diagnosis with hazard ratios [95% confidence intervals] as follows: 1.9 [1.8–2.1)], 1.5 [1.4–1.6], and 1.5 [1.4–1.5], respectively [63].
Radiation is a common part of cancer treatment and an important modifier of arterial thrombosis risk. The earliest effects of radiation therapy are seen on the vascular endothelium [64], which is vulnerable to injury. Vascular fibrosis is a later findings that can affect any layer of the vessel wall [65]. Radiation vasculopathy is associated with accelerated atherosclerosis [66•]. For example, in a cohort of patients treated with radiation therapy for Hodgkin’s lymphoma, the prevalence of CAD based on CT scans was 39% two decades after treatment [67]. In a large population-based study of breast cancer patients in Sweden and Denmark, prior radiotherapy was associated with a significant increase in risk of major coronary events within a few years of treatment. This risk persisted for decades after radiotherapy and correlated linearly with the mean radiation dose (7.4% increase in risk per 1 Gray of radiation) [68]. Similarly, PAD is a risk for patients who undergo extracardiac radiation, although this has been less well studied compared to CAD. Radiation therapy is also associated with a higher risk of stroke. Dorresteijn et al. [69] reported a 12% 15-year cumulative risk of ischemic stroke following radiation for head and neck tumors. Plummer et al. reported that head and neck radiotherapy at least doubled the risk of transient ischemic attack/stroke [70, 71]. They suggest that both accelerated atherosclerosis and injury to the vasa vasorum contribute to carotid vasculopathy [70, 71].
Pathophysiologic mechanisms of arterial thrombosis in cancer
Virchow’s triad
The classical concept of vascular thrombosis is that of Virchow’s triad. Accordingly, a thrombus forms as a consequence of alterations of the blood contents (mainly platelets and coagulation factors), the vessel wall (mainly endothelium), and blood flow (mainly blood flow turbulence and stasis).
The first factor in Virchow’s triad appears critical to thrombogenesis in cancer patients. There is evidence that platelet reactivity is increased in cancer patients and that there are higher circulating levels of platelet-specific products such as soluble P-selectin, platelet factor 4, thrombospondin, and beta-thromboglobulin [5]. An emerging body of evidence suggests that there is a bidirectional interactions between platelets and cancer cells where paraneoplastic cells activate platelets, and conversely, platelets themselves play a role in cancer propagation and metastatic spread through a process described as “tumor education.” Direct interaction of platelets with tumor cells induces thrombocyte aggregation in experimental pancreatic, colorectal, and renal cell lines [72]. Our current understanding of the changes in platelet activation and coagulation factors in cancer is reviewed in the following section.
The second element of the Virchow’s triad, the vascular wall, is also an important contributor to thrombosis in cancer patients. Cancer therapies have a direct influence on the vascular wall, especially the endothelial monolayer. Increased circulating levels of von Willebrand factor (vWf) have been described in cancer patients and may not only be a marker of endothelial injury like soluble E-selectin, but also a contributor to thrombosis by promoting platelet-platelet and platelet-subendothelium interaction. Loss of expression of thrombomodulin on the endothelial surface reduces the capacity to activate the anti-coagulant protein C thereby adding to the prothrombotic state. These dysfunctional changes of the endothelium are the consequence of the production of inflammatory cytokines such as tumor necrosis factor and interleukin (IL)-1. The circulating levels of a number of different cytokines are elevated in cancer patients [73]. Vascular endothelial dysfunction in the setting of malignancy not only causes procoagulant activity and decreased fibrinolytic activity [74]. This abnormal condition of the vasculature furthermore fosters inflammation, proliferation, and vasoconstriction, all of which contribute to the development and clinical presentation of ischemic vascular disease [75].
The third factor in Virchow’s trial, blood flow, appears to play a minor role in the etiology of thrombosis in cancer patients [5]. It is rare that thrombosis would evolve as the consequence of external compression of a vessel due to an enlarging mass. Cancer patients do experience a decline in exercise tolerance and fatigue and assume a more sedentary lifestyle. Some patients may become quite bedridden which is a clear risk factor for venous thrombosis; however, this does not appear to play a critical role in arterial thrombosis.
Platelet activation and clotting factors
Cancer cells directly excrete a number of mediators of coagulation that likely significantly contribute to arterial thrombosis in cancer patients. Thrombin and other mediators excreted by cancer cells interact with platelet surface receptors via PAR-1 and PAR-4 receptors, P2Y12 receptor, and the thromboxane receptor. Secretion of matrix metalloproteinases (MMPs) and IL-6 have been shown to activate platelets directly [76, 77]. In breast cancer cell lines, secreted MMPs led to platelet activation, a change in shape to form pseudopodia, and an increase in the concentration of activated GPIIb/IIIa surface receptors, which subsequently bind with fibrinogen and form stable platelet aggregates [78]. A specific study of human small cell cancer cell lines revealed in vitro induction of platelet aggregation through direct cellular interactions observed under electron microscopy in small cell lung cancer and indirect cellular interactions via secreted thrombin and ADP mediators in non-small cell lung cancer [69]. In vitro, cancer cells can activate platelets by releasing stimulating factors such as ADP and thromboxane A2 [5].
Studies of patients with melanoma and mouse models of melanoma demonstrated that vWF plays a role in platelet aggregation and recruitment. Tumor-derived VEGF secretion mediates endothelial cell activation, promotes vWF expression in the tumor vessel lumen that then promotes platelet recruitment and atheroembolism [79]. A similar mouse xenograph of four different human pancreatic cell lines found two lines expressing known thrombogenic entities tissue factor (TF) and release TF-positive microparticles [80].
Other procoagulants produced by cancer cells that are of importance in shifting the balance between coagulation and fibrinolysis include fibrinogen and plasminogen activator inhibitor. Deficiencies in molecules such as anti-thrombin, proteins C and S, and tissue plasminogen activator also contributes to the development of arterial thrombosis [5].
Pathophysiologic mechanisms of treatment-related thrombosis
In addition to the milieu of the cancer environment, a number of chemotherapeutics have a detrimental effect on the function and viability of endothelial cells [81]. As mentioned above, there are many chemotherapeutic agents associated with thrombosis; however, the most classical illustration is found in the group of VEGF inhibitors [22].
VEGF is the central growth and survival factor for endothelial cells. Inhibition of the VEGF signaling pathway can therefore have profound cardiovascular effects including progression of atherosclerosis, provocation of ischemia, and thrombotic events. Binding of the VEGF receptor signals into the nitric oxide (NO) pathway, which is crucial for endothelial and vascular health. In cell culture experiments, pan-VEGF receptor inhibitor treatment reduced NO production in endothelial cells due to uncoupling of endothelial NO synthase, which was related to induction of mitochondrial oxidative stress [82]. Inhibition of the VEGF signaling pathway can leave an exposed endothelium due to impaired cell regeneration and may alter endothelial fluid shear stress sensing leading to vascular injury and thrombosis [83]. In addition to the effect on endothelial cell health, VEGF inhibitor-related hypertension likely further increases the risk of cardiovascular events [83].
VEGF receptor inhibitors decrease NO production, although with a mechanistic twist [82]. In an experimental model, pan-VEGF receptor inhibition significantly increased the extent of disease in mice prone to the development of atherosclerosis [82]. This was associated with a significant reduction in proliferating cell count but not with a reduction in capillary tube count or reduction in parameters of plaque vulnerability. Accordingly, VEGF inhibitor therapy may lead to a more gradual increase in atherosclerotic plaque burden, which may not become clinically evident for years, in contrast to thrombotic events that emerge acutely.
A second group of drugs that has recently been shown to increase the risk of vascular events are the Bcr-Abl inhibitors which are used in hematologic malignancies such as chronic myeloid leukemia [22]. The c-Abl signaling pathway operates in synergy with the VEGF signaling pathway and is important for the survival of endothelial cells. Inhibition of both pathways simultaneously should thus be greatly detrimental, as was seen with the ponatinib. In an unprecedented manner, ponatinib causes arterial more than venous vascular events including acute myocardial infarction, stroke, acute mesenteric ischemia, and limb ischemia. Some cases revealed such profoundly accelerated atherosclerosis, especially of the lower extremities, that a new term was coined: “progressive arterial occlusive disease.” The same phenomenon, but not as frequent, was also seen with nilotinib. This drug differs in its TKI target spectrum, and analyses had not shown an inhibition of VEGF receptor domains [84]. However, recent studies made the exciting discovery that nilotinib downregulates the expression of VEGF receptor 2 and behaves similarly to an angiogenesis inhibitor [85]. A meta-analysis from Sweden suggests that dasatinib also increases the risk of MI and stroke, pointing towards a class effect. How to best risk-assess and monitor these patients is currently unknown though proposals have been made.
Diagnosis of arterial thrombosis
Arterial thromboembolism often has an acute or subacute presentation with marked reduction in blood flow leading to critical ischemia and infarction of the supplied organ. The typical symptoms depend on the affected organ: chest pain for CAD, limb pain for PAD, or neurologic deficits for stroke. The overall diagnostic and treatment strategies center on the goal to restore blood flow as soon as possible. Invasive angiography is often pursued since it can quickly diagnose an occlusive thombus and enable revascularization. However, in non-emergent cases or when the diagnosis is unclear, noninvasive imaging such as CTA or MRA is often appropriate.
The classic definition of MI (type I) includes chest pain, ECG changes, and abnormal cardiac biomarkers caused by arterial thrombosis, usually in the setting of atherosclerotic disease. Type II MI is defined as myocardial infarction secondary to ischemia due to either increased oxygen demand or decreased supply such as coronary spasm, hypotension, anemia, and coronary embolism [86]. Type II MI represents a significant proportion of ACS events in cancer patients. It can be difficult to clinically distinguish between the two types of MI, and therefore noninvasive functional testing or invasive coronary angiography may be indicated in this scenario.
Treatment considerations
Anti-platelets and percutaneous vascular interventions
Anti-platelet agents are central to the treatment of arterial thrombosis in both patients with and without cancer. However, careful consideration about the bleeding risk is needed because thrombocytopenia is common in cancer patients [87]. In a study of patients presenting with acute coronary syndrome, baseline thrombocytopenia was associated with a higher rate of complications compared to patients without thrombocytopenia (30 day death rate 6.2 versus 2.1%, major bleeding 11.9 versus 7%, major cardiac events 9.6 versus 5.2%, major cardiac events plus major bleeding 18.5 versus 10.8%) [88]. A recently published expert consensus statement from the Society of Cardiovascular Angiography and Interventions recommends that aspirin can be given if the platelet count is > 10,000, and DAPT with aspirin and clopidogrel is reasonable for platelet counts between 30 and 50,000. Other newer P2Y12 receptor inhibitors such as ticagrelor and prasugrel which have a higher bleeding rate should generally be avoided below a platelet count of 50,000 [66•]. McCarthy et al. on the other hand, recommend a more conservative approach and advise against all anti-platelet agents and PCI in patients with a platelet count < 50,000 [89].
Revascularization is imperative in the setting of critical ischemia or infarction. Depending on the territory at risk, treatment options include thrombectomy (as in the case of PAD or stroke), percutaneous coronary intervention, bypass surgery, or percutaneous peripheral angioplasty. Based on the SCAI expert consensus, there is no platelet count limit for diagnostic left heart catheterization [66•, 90]. DAPT should be continued for the least safe time if platelet count is < 50,000 (meaning 4 weeks for BMS or 6 months for drug-eluting stents). In patients who have a platelet count < 30,000, a multidisciplinary discussion involving cardiology and oncology is recommended prior to pursing PCI [66•].
Coronary stent endothelialization
An important question is how chemotherapeutic agents may influence the endothelialization of stents and the risk of stent thrombosis. One study so far has found a 7-fold higher risk of stent thrombosis in cancer patients who had undergone BMS [12]. This observation and the un-derlying mechanisms remain to be confirmed. However, it is conceptually conceivable that cytostatic and cytotoxic chemotherapeutics impair stent endothelialization. This would affect the ingrowth of both surrounding endothelial cells and circulating progenitor cells [91]. Circulating progenitor cells are the main source for endothelialization of stents [92], and patients with stent thrombosis have lower endothelial progenitor colony forming unit capacity [93]. Endothelial and circulating progenitor cell levels are suppressed in cancer patients, especially in the acute treatment phase and remain lower in those receiving VEGF-directed therapies [94].
In a large animal model, VEGF significantly improved re-endothelialization, and the effect was even more pronounced under conditions of hypercholesterolemia [95]. Interestingly, VEGF also reduces excess neointima proliferation and restenosis [95]. Similar to the discussion above, VEGF is an important signaling factor for endothelial cell health in patients who undergo PCI. Cancer patients are at risk of arterial vascular events due to interference of this pathway during administration of drugs cytotoxic to endothelial cells.
Role of anti-platelet therapy in metastatic spread
The role of platelets in metastatic spread leads to the hypothesis that anti-platelet agents will decrease tumor progression [96, 97]. There is evidence that P2Y12 inhibitor ticagrelor reduced metastatic burden in murine models of melanoma and breast cancer [98]. In prostate cancer, aspirin use after diagnosis may only improve prostate cancer mortality in patients with high-risk cancer [99]. In a large population-based cohort study and a meta-analysis of multiple-cohort patients, starting low-dose aspirin therapy after diagnosis of colorectal cancer was not associated with a reduction in colorectal cancer-specific mortality [100, 101]. Overall, evidence suggests that a personalized approach should be used, rather than routine treatment with anti-platelet therapy, while the role of anti-platelet agents in slowing malignant progression remains unclear.
Future avenues
There are numerous opportunities for further investigation into preventive strategies for arterial thromboembolic disease in cancer patients. One important question that should be addressed is whether anti-platelet or anti-coagulation can be effective in the prevention of arterial thrombosis. Aspirin, for example, has been shown to decrease the rates of arterial thrombosis in polycythemia vera and multiple myeloma [102, 103]. However, whether we can prevent arterial thrombi in other cancers or prevent treatment-related thrombosis is unknown. Lipid-lowering and anti-inflammatory statin medications are protective against arterial thrombosis in the setting of atherosclerotic disease and have even been shown to decrease venous thromboembolism in patients with cancer [104]. Nonetheless, their efficacy in cancer-related arterial thrombosis is unknown. PET-CT scans, which are already done as part of cancer staging, may help identify at least some of the patients who should be started on a statin prior to chemotherapy based on the presence of coronary and vascular calcium, which may potentially be predictive of cardiac events [105]. However, the decision to initiate statin should also take into account the cancer type and the specific oncologic treatment [105]. Several recent/ongoing studies are starting to investigate the use of aspirin, statins, biomarkers, and novel anti-coagulants in the prevention and treatment of cancer patients with thromboembolic disease [106–110]. Optimal surveillance strategies for arterial thromboembolic disease remain unclear. There are many imaging modalities for identifying arterial disease; however, which patients should be screened and at what time interval is unknown and warrants further investigation. A personalized and multidisciplinary approach considering the patient’s traditional cardiovascular risk factors as well as the specific risks of the cancer type and oncologic treatment is recommended until more studies and guidelines are developed.
In regard to the potential mechanistic crossover of treatments that may contribute to the genesis of both cardiac and oncologic disease, interest has been generated recently with the CANTOS trial. The agent studied, canakinumab, is an anti-inflammatory, monoclonal anti-body targeting interleukin-1beta that has recently attracted attention within the cardiology community after demonstrating that it decreases the rate of myocardial infarction in patients with coronary artery disease. This effect occurred irrespective of the cholesterol level which helped prove the inflammatory hypothesis of atherothrombosis [111]. This discovery has opened the cardiology field to the pursuit of novel medications targeting inflammatory pathways. Cancer is a highly inflammatory condition; therefore, this or similar drugs may also prove to be beneficial in the prevention and treatment of cancer-related arterial thrombosis. Canakinumab may also be an appealing medication to study given the unexpected finding that canakinumab was associated with significantly lower rates of lung malignancy. This interesting observation requires further study but continues to demonstrate a possible commonality between the potential triggers of both cardiovascular disease and cancer [111].
Conclusion
In conclusion, malignancy-related arterial thrombosis results in an increased risk of morbidity and mortality. With cardiac events that may occur during cancer treatment, whether it is cancer or treatment induced, further research is needed to further delineate thrombosis risk by treatment agent and types of cancer. Collaboration between cardiology and oncology is essential in providing evidence-based and individualized care in treating these patients; this involves ongoing discussions of treatment strategies of such events, as well as the risks and benefits of continuing chemotherapies that may be contributing to the thrombotic events. National, multi-institutional registries tracking these events, as well as potential randomized controlled trials in looking at pharmacologic—anti-platelet and anti-coagulant—and invasive therapies, will advance our understanding in attenuating long-term thrombosis risk and potentially improve long-term outcomes in both cardiovascular and oncologic survival.
Footnotes
Conflict of Interest
Mirela Tuzovic, Cezar Iliescu, Kostas Marmagkiolis, Boback Ziaeian, and Eric H. Yang each declare no potential conflicts of interest.
Joerg Herrmann was a participant in the 2014 Ponatinib in CML Cardio-Oncology Advisory Board meeting organized by ARIAD Pharmaceuticals and the 2015 Advisory Board meeting of the Institute for Cardio-Oncology sponsored by Bristol-Myers Squibb.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Prevention C for DC and. Deaths and mortality. 2017. Available at: https://www.cdc.gov/nchs/fastats/deaths.htm. Accessed October 12, 2017.
- 2.Institute NC. Cancer statistics. Available at: https://www.cancer.gov/about-cancer/understanding/statistics. Accessed January 1, 2017.
- 3.American Cancer Society, Cancer Treatment & Survivorship: Facts &Figures, 2016-2017. https://www.cancer.org/content/dam/cancerorg/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-factsand-figures/cancer-treatment-and-survivorship-facts-and-figures-2016-2017.pdf. Accessed 16 Mar 2018.
- 4.Khorana AA, Francis CW, Culakova E, Kuderer NMLG. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–4. [DOI] [PubMed] [Google Scholar]
- 5.Blann AD DS. Arterial and venous thrombosis in cancer patients. Cardiol Res Pr 2011:394740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khorana AA, Francis CW, Blumberg N, Culakova E, Refaai MA, Lyman GH. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khorana AA, Francis CW, Culakova E, Fisher RI, Kuderer NMLG. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–90. [DOI] [PubMed] [Google Scholar]
- 8.••.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors describe the incidence of arterial thromboembolic events in cancer patients included in the SEER database. Incidence with respect to time from diagnosis, the type of cancer, and the cancer stage is also reported
- 9.Abbott JD, Ahmed HN, Vlachos HA, Selzer FWD. Comparison of outcome in patients with ST-elevation versus non-ST-elevation acute myocardial infarction treated with percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute dynamic registry). Am J Cardiol. 2007;100:190–5. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Gulati R, Lennon RJ, Lewis BR, Park J, Sandhu GS, et al. Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Mayo Clin Proc. 2016;91:1680–92. [DOI] [PubMed] [Google Scholar]
- 11.Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross CM, Posch MG, Geier C, Olthoff H, Krämer J, Dechend R, et al. Subacute coronary stent thrombosis in cancer patients. J Am Coll Cardiol. 2008;51:1232–3. [DOI] [PubMed] [Google Scholar]
- 13.Moore RA, Adel N, Riedel E, Bhutani M, Feldman DR, Tabbara NE, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barceló R, Muñoz AL-VG. Prospective evaluation of major vascular events in patients with nonsmall cell lung carcinoma treated with cisplatin and gemcitabine. Cancer. 2005;104:1110–1. [DOI] [PubMed] [Google Scholar]
- 15.Weijl NI, Rutten MF, Zwinderman AH, Keizer HJ, Nooy MA, Rosendaal FR, et al. Thromboembolic events during chemotherapy for germ cell cancer: a cohort study and review of the literature. J Clin Oncol. 2000;18:2169–78. [DOI] [PubMed] [Google Scholar]
- 16.Sugrue MM, Yi J, Purdie D, Dong W, Grothey A, Kozloff M Serious arterial thromboembolic events (sATE) in patients (pts) with metastatic colorectal cancer (mCRC) treated with bevacizumab (BV): results from the BRiTE registry. J Clin Oncol. [Google Scholar]
- 17.Schutz FA, Je Y, Azzi GR, Nguyen PLCT. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:14040–1412. [DOI] [PubMed] [Google Scholar]
- 18.Ranpura V, Hapani S, Chuang JWS. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol. 2010;49:287–97. [DOI] [PubMed] [Google Scholar]
- 19.Tebbutt NC, Murphy F, Zannino D, Wilson K, Cummins MM, Abdi E, et al. Risk of arterial thromboembolic events in patients with advanced colorectal cancer receiving bevacizumab. Ann Oncol. 2011;22:1834–8. [DOI] [PubMed] [Google Scholar]
- 20.Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–9. [DOI] [PubMed] [Google Scholar]
- 21.Choueiri TK, Schutz FA, Je Y, Rosenberg JEBJ. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–5. [DOI] [PubMed] [Google Scholar]
- 22.Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation. 2016;133:1272–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plana JC, Chair MG, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–39. [DOI] [PubMed] [Google Scholar]
- 24.McCormack P Pazopanib: a review of its use in the management of advanced renal cell carcinoma. Drugs. 2014;74:1111–25. [DOI] [PubMed] [Google Scholar]
- 25.Frampton J Pazopanib: a review in advanced renal cell carcinoma. Target Oncol. 2017;12:543–54. [DOI] [PubMed] [Google Scholar]
- 26.FDA. Ponatinib. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/203469s009lbl.pdf.
- 27.Doll DC, List AF, Greco FA, Hainsworth JD, Hande KRJD. Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med. 1986;105:48–51. [DOI] [PubMed] [Google Scholar]
- 28.Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. 2010;28:4649–57. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Cornell FR, Lenihan D, Slosky D, Jagasia M, Piazza G, et al. Cardiovascular complications of novel multiple myeloma treatments. Circulation. 2016;133:908–12. [DOI] [PubMed] [Google Scholar]
- 30.Chen XL, Lei YH, Liu CF, Yang QF, Zuo PY, Liu CY, et al. Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: a meta-analysis. PLoS One. 2013;8:e66721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. [DOI] [PubMed] [Google Scholar]
- 33.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Staehler M, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–8. [DOI] [PubMed] [Google Scholar]
- 34.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cutsem E, Hoff PM, Blum JL, Van Abt MOB. Incidence of cardiotoxicity with the oral fluoropyrimidine capecitabine is typical of that reported with 5-fluorouracil. Ann Oncol. 2002;13:484–5. [DOI] [PubMed] [Google Scholar]
- 36.Yeh ETBC. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47. [DOI] [PubMed] [Google Scholar]
- 37.Jensen SASJ. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol. 2006;58:487–93. [DOI] [PubMed] [Google Scholar]
- 38.Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2008;134:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Labianca R, Beretta G, Clerici M, Fraschini PLG. Cardiac toxicity of 5-fluorouracil: a study on 1083 patients. Tumori. 1982;68:505–10. [DOI] [PubMed] [Google Scholar]
- 40.Walko CMLC. Capecitabine: a review. Clin Ther. 2005;27:23–44. [DOI] [PubMed] [Google Scholar]
- 41.Meyer CC, Calis KA, Burke LB, Walawander CAGT. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy. 1997;17:729–36. [PubMed] [Google Scholar]
- 42.de Forni M, Malet-Martino MC, Jaillais P, Shubinski RE, Bachaud JM, Lemaire L, et al. Cardiotoxicity of high-dose continuous infusion fluorouracil: a prospective clinical study. J Clin Oncol. 1992;10:1795– 801. [DOI] [PubMed] [Google Scholar]
- 43.Polk A, Vaage-Nilsen M, Vistisen KND. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifesta-tions and predisposing factors. Cancer Treat Rev. 2013;39:974–84. [DOI] [PubMed] [Google Scholar]
- 44.Shah K, Gupta S, Ghosh J, Bajpai JMA. Acute non-ST elevation myocardial infarction following paclitaxel administration for ovarian carcinoma: a case report and review of literature. J Cancer Res Ther. 2012;8:442–4. [DOI] [PubMed] [Google Scholar]
- 45.Berardi R, Caramanti M, Savini A, Chiorrini S, Pierantoni C, Onofri A, et al. State of the art for cardiotoxicity due to chemotherapy and to targeted therapies: a literature review. Crit Rev Oncol Hematol. 2013;88:75–86. [DOI] [PubMed] [Google Scholar]
- 46.Rowinsky EK, McGuire WP, Guarnieri T, Fisherman JS, Christian MCDR. Cardiac disturbances during the administration of taxol. J Clin Oncol. 1991;9:1704–12. [DOI] [PubMed] [Google Scholar]
- 47.Amir E, Seruga B, Niraula S, Carlsson LOA. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2011;103:1299–309. [DOI] [PubMed] [Google Scholar]
- 48.Herrmann J Tyrosine kinase inhibitors and vascular toxicity: impetus for a classification system? Curr Oncol Rep. 2016;18:33. [DOI] [PubMed] [Google Scholar]
- 49.Coutre P, Rea D, Abruzzese E, Dombret H, Trawinska MM, Herndlhofer S, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103:1347–8. [DOI] [PubMed] [Google Scholar]
- 50.Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26:2197–203. [DOI] [PubMed] [Google Scholar]
- 51.Aichberger KJ, Herndlhofer S, Schernthaner GH, Schillinger M, Mitterbauer-Hohendanner G, Sillaber CVP. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86:533–9. [DOI] [PubMed] [Google Scholar]
- 52.Libourel EJ, Sonneveld P, van der Holt B, de Maat MPLF. High incidence of arterial thrombosis in young patients treated for multiple myeloma: results of a prospective cohort study. Blood. 2010;116:22–6. [DOI] [PubMed] [Google Scholar]
- 53.Alkindi S, Dennison DPA. Arterial and venous thrombotic complications with thalidomide in multiple myeloma. Arch Med Res. 2008;39:257–8. [DOI] [PubMed] [Google Scholar]
- 54.Saphner T, Tormey DCGR. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J Clin Oncol. 1991;9:286–94. [DOI] [PubMed] [Google Scholar]
- 55.Gov.uk, Drug Safety Update. Lenalidomide: risk of thrombosis and thromboembolism. https://www.gov.uk/drug-safety-update/lenalidomide-risk-ofthrombosis-and-thromboembolism. Accessed 16 Mar 2018. [Google Scholar]
- 56.Rumbaut RE, Thiagarajan P. Platelet-vessel wall interactions inHemostasis and thrombosis. Chapter 6: arterial, venous, and microvascular hemostasis/thrombosis. San Rafael: Morgan & Claypool Life Sciences; 2010. https://www.ncbi.nlm.nih.gov/books/NBK53450. Accessed 16 Mar 2018. [PubMed] [Google Scholar]
- 57.Lowe G Common risk factors for both arterial and venous thrombosis. Br J Haematol. 2008;140:488–95. [DOI] [PubMed] [Google Scholar]
- 58.Previtali E, Bucciarelli P, Passamonti SM, Martinelli I. Risk factors for venous and arterial thrombosis. Blood Transfus. 2011;9:120–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goto I, Okamoto R, Sawai T, Takasaki A, Takeuchi T, Matsuo H, et al. A case of aortic thrombosis and embolism preceding the progression of early esophageal cancer. J Cardiol Cases. 2013;7:e123–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vavlukis M, Kotlar I, Chaparoska E, Antova EKS. Diffuse arterial thrombosis as a first manifestation of occult malignancy. Case Rep Med. 2016;2016:1658392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rigdon E Trousseau’s syndrome and acute arterial thrombosis. Cardiovasc Surg. 2000;8:214–8. [DOI] [PubMed] [Google Scholar]
- 62.Li W, Garcia D, Cornell RF, Gailani D, Laubach J, Maglio ME, et al. Cardiovascular and thrombotic complications of novel multiple myeloma therapies: a review. JAMA Oncol. 2017;3:980–8. [DOI] [PubMed] [Google Scholar]
- 63.Kristinsson SY, Pfeiffer RM, Björkholm M, Goldin LR, Schulman S, Blimark C, et al. Arterial and venous thrombosis in monoclonal gammopathy of undeter-mined significance and multiple myeloma: a population-based study. Blood. 2010;115:4991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(80):293–7. [DOI] [PubMed] [Google Scholar]
- 65.Brosius FC 3rd, Waller BFRW. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med. 1981;70:519–30. [DOI] [PubMed] [Google Scholar]
- 66.•.Iliescu CA, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and sociedad Latino Americana de Cardiologıa interve). Catheter Cardiovasc Interv. 2016;87:E202–23. [DOI] [PubMed] [Google Scholar]; This article describes the current expert consensus regarding management of cancer patients requiring evaluation in the catheterization laboratory. Cancer associated complications such as thrombocytopenia require special attention when treating arterial thromboembolic disease
- 67.Mulrooney DA, Nunnery SE, Armstrong GT, Ness KK, Srivastava D, Donovan FD, et al. Coronary artery disease detected by coronary computed tomography angiography in adult survivors of childhood Hodgkin lymphoma. Cancer. 2014;120:3536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 69.Heinmöller E, Weinel RJ, Heidtmann HH, Salge U, Seitz R, Schmitz I, et al. Studies on tumor-cell-induced platelet aggregation in human lung cancer cell lines. J Cancer Res Clin Oncol. 1996;122:735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dorresteijn LD, Kappelle AC, Boogerd W, Klokman WJ, Balm AJ, Keus RB, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20:282–8. [DOI] [PubMed] [Google Scholar]
- 71.Plummer C, Henderson RD, O’Sullivan JDRS. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–8. [DOI] [PubMed] [Google Scholar]
- 72.Mezouar S, Frère C, Darbousset R, Mege D, Crescence L, Dignat-George F, et al. Role of platelets in cancer and cancer-associated thrombosis: experimental and clinical evidences. Thromb Res. 2016;139:65–76. [DOI] [PubMed] [Google Scholar]
- 73.Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–80. [DOI] [PubMed] [Google Scholar]
- 74.Herrmann JLA. The endothelium: dysfunction and be-yond. J Nucl Cardiol. 2001;8:197–206. [DOI] [PubMed] [Google Scholar]
- 75.Herrmann JLA. The endothelium—the cardiovascular health barometer. Herz. 2008;33:343–53. [DOI] [PubMed] [Google Scholar]
- 76.Seizer PMA. Platelets and matrix metalloproteinases. Thromb Haemost. 2013;110:903–9. [DOI] [PubMed] [Google Scholar]
- 77.Gremmel T, Perkmann T, Seidinger D, Koppensteiner R, Panzer S, Kopp CWSS. Differential impact of inflammation on six laboratory assays measuring residual arachidonic acid-inducible platelet reactivity during dual antiplatelet therapy. J Atheroscler Thromb. 2013;20:630–45. [DOI] [PubMed] [Google Scholar]
- 78.Alonso-Escolano D, Strongin AY, Chung AW, Deryugina EIRM. Membrane type-1 matrix metalloproteinase stimulates tumour cell-induced platelet aggregation: role of receptor glycoproteins. Br J Pharmacol. 2004;141:241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bauer AT, Suckau J, Frank K, Desch A, Goertz L, Wagner AH, et al. von Willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood. 2015;125:3153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang JG, Geddings JE, Aleman MM, et al. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood 2012;7:119(23):5543–52. 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soultati A, Mountzios G, Avgerinou C, Papaxoinis G, Pectasides D, Dimopoulos MAPC. Endothelial vascular toxicity from chemotherapeutic agents: preclinical evidence and clinical implications. Cancer Treat Rev. 2012;38:473–83. [DOI] [PubMed] [Google Scholar]
- 82.Winnik S, Lohmann C, Siciliani G, von Lukowicz T, Kuschnerus K, Kraenkel N, et al. Systemic VEGF inhibition accelerates experimental atherosclerosis and disrupts endothelial homeostasis—implications for cardiovascular safety. Int J Cardiol. 2013;168:2453–61. [DOI] [PubMed] [Google Scholar]
- 83.Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, et al. Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension. 2018;71:e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moslehi JJDM. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33:4210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hadzijusufovic E, Albrecht-Schgoer K, Huber K, Hoermann G, Grebien F, Eisenwort G, et al. Nilotinib-induced vasculopathy: identification of vascular endothelial cells as a primary target site. Leukemia. 2017;31:2388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thygesen Kristian, Alpert Joseph S., Jaffe Allan S., Simoons Maarten L., Bernard R. Chaitman HDW, infarction and the WG on behalf of the JETF for the UD of M. Third universal definition of myocardial infarction. Circulation 2012;126:2020–2035. [DOI] [PubMed] [Google Scholar]
- 87.Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137–46. [DOI] [PubMed] [Google Scholar]
- 88.Hakim DA, Dangas GD, Caixeta A, Nikolsky E, Lansky AJ, Moses JW, et al. Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: analysis from the harmonizing outcomes with revascularization and stents in acute myocardial infarction (HORIZONS). Am Heart J. 2011;161:391–6. [DOI] [PubMed] [Google Scholar]
- 89.McCarthy CP, Steg GBD. The management of anti-platelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur Heart J. 2017;38:3488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giza DE, Lopez-Mattei J, Vejpongsa P, Munoz E, Iliescu G, Kitkungvan D, et al. Stress-induced car-diomyopathy in cancer patients. Am J Cardiol. 2017;120:2284–8. [DOI] [PubMed] [Google Scholar]
- 91.Padfield GJ, Newby DEMN. Understanding the role of endothelial progenitor cells in percutaneous coronary intervention. J Am Coll Cardiol. 2010;55:1553–65. [DOI] [PubMed] [Google Scholar]
- 92.Douglas G, Van Kampen E, Hale AB, McNeill E, Patel J, Crabtree MJ, et al. Endothelial cell repopulation after stenting determines in-stent neointima formation: effects of bare-metal vs. drug-eluting stents and genetic endothelial cell modification. Eur Heart J. 2013;34:3378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lev EI, Leshem-Lev D, Mager A, Vaknin-Assa H, Harel N, Zimra Y, et al. Circulating endothelial progenitor cell levels and function in patients who experienced late coronary stent thrombosis. Eur Heart J. 2010;31:2625–32. [DOI] [PubMed] [Google Scholar]
- 94.Ramcharan KS, Lip GY, Stonelake PSBA. Effect of standard chemotherapy and antiangiogenic therapy on plasma markers and endothelial cells in colorectal cancer. Br J Cancer. 2014;111:1742–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirk-wood L, Stratford PW, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents: an alternative strategy for inhibition of restenosis. Circulation. 2004;110:36–45. [DOI] [PubMed] [Google Scholar]
- 96.Guillem-Llobat P, Dovizio M, Bruno A, Ricciotti E, Cufino V, Sacco A, et al. Aspirin prevents colorectal cancer metastasis in mice by splitting the crosstalk between platelets and tumor cells. Oncotarget. 2016;7:32462–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cooke NM, Spillane CD, Sheils O, O’Leary JKD. Aspirin and P2Y12 inhibition attenuate platelet-induced ovarian cancer cell invasion. BMC Cancer. 2015;627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gebremeskel S, LeVatte T, Liwski RS, Johnston BBM. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int J Cancer. 2015;136:234–40. [DOI] [PubMed] [Google Scholar]
- 99.Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci ELCJ. Aspirin use after a prostate cancer diagnosis and cancer survival in a prospective cohort. Cancer Prev Res. 2012;5:1223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cardwell CR, Kunzmann AT, Cantwell MM, Hughes C, Baron JA, Powe DGML. Low-dose aspirin use after diagnosis of colorectal cancer does not increase survival: a case-control analysis of a population-based cohort. Gastroenterology. 2014;146:700–8. [DOI] [PubMed] [Google Scholar]
- 101.Ye XF, Wang J, Shi WTHJ. Relationship between aspirin use after diagnosis of colorectal cancer and patient survival: a meta-analysis of observational studies. Br J Cancer. 2014;111:2172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Palumbo A, Cavo M, Bringhen S, Zamagni E, Romano A, Patriarca F, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011;29:986–93. [DOI] [PubMed] [Google Scholar]
- 103.Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G. Patrono C BT EC on L-DA in PVI. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114–24. [DOI] [PubMed] [Google Scholar]
- 104.Khemasuwan D, Divietro ML, Tangdhanakanond K, Pomerantz SCEG. Statins decrease the occurrence of venous thromboembolism in patients with cancer. Am J Med. 2010;123:60–5. [DOI] [PubMed] [Google Scholar]
- 105.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 106.A phase 2 pilot study of apixaban for the prevention of thromboembolic events in patients with advanced (metastatic) cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT00320255. Last Accessed February 10, 2018.
- 107.Biomarkers related to thrombosis in patients with newly diagnosed multiple myeloma receiving chemotherapy. Available at: https://clinicaltrials.gov/ct2/show/NCT01132833. Last Accessed February 10, 2018.
- 108.Anti-platelet and statin therapy to prevent cancer-associated thrombosis. https://clinicaltrials.gov/ct2/show/NCT02285738. Last Accessed February 10, 2018.
- 109.Apixaban for the prevention of venous thromboembolism in cancer patients (AVERT). https://clinicaltrials.gov/ct2/show/NCT02048865. Last Accessed February 10, 2018.
- 110.Enoxaparin Versus Aspirin in Patients with Cancer and Stroke. https://clinicaltrials.gov/ct2/show/NCT01763606. Accessed 16 Mar 2018.
- 111.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]


