Abstract
Children at risk for anxiety display elevated threat sensitivity and may inaccurately classify safe stimuli as threatening, a process known as overgeneralization. Little is known about whether such overgeneralization might stem from altered sensory representations of stimuli resembling threat, especially in youth. Here we implement representational similarity analysis of fMRI data to examine the similarity of neural representations of threat versus ambiguous or safe stimuli in threat and perceptual neurocircuitry among children at varying levels of anxiety traits. Three weeks after completing threat conditioning and extinction, children underwent an fMRI extinction recall task, during which they viewed the extinguished threat cue (CS+), safety cue (CS−) and generalization stimuli (GS) consisting of CS−/CS+ blends. Multivoxel BOLD signal patterns were measured in seven regions of interest: four affective areas (ventromedial prefrontal cortex (vmPFC), anterior insular cortex (AIC), dorsomedial prefrontal cortex (dmPFC), and amygdala) and three perceptual areas (inferior temporal cortex (ITC) and visual areas V1 and V4). Compared to low anxious children, children with high trait anxiety evidenced less neural pattern differentiation between the CS+ and similar GS, particularly in the vmPFC. Together, these results demonstrate the utility of multivariate neuroimaging approaches in arbitrating the relative contributions of perceptual versus affective sources to threat generalization.
Keywords: Anxiety, Threat overgeneralization, Middle childhood, fMRI, Representational similarity analysis (RSA)
1. Introduction
Because threat can manifest in different forms, it is adaptive for an observer to be vigilant around exemplars that may similarly predict an aversive outcome. Threat generalization – a learning mechanism whereby threat responses extend to a range of stimuli resembling a past threat – enables a rapid response to novel and potentially dangerous stimuli (Armony et al., 1997; Lissek et al., 2008; Vansteenwegen et al., 2005). It has been proposed that developmental changes in threat learning and generalization may contribute to the emergence of anxiety disorders in late childhood and early adolescence (Britton et al., 2013; Lau et al., 2011). Several threat generalization studies in humans have considered how varying degrees of perceptual resemblance to a threat stimulus elicit graded univariate responses in emotion neurocircuitry (e.g. insula, ventromedial prefrontal cortex; Britton et al., 2013; Dunsmoor et al., 2011; Greenberg et al., 2013a, 2013b; Lissek et al., 2014; Michalska et al., 2016, 2019; for a review see Dymond et al., 2015). However, far fewer studies have examined whether ambiguous stimuli are similarly represented as threatening in object identification areas earlier in the visual processing stream. Further, limited work has examined threat generalization processes in children and adolescents, perhaps reflecting the challenge of finding a potent and biologically-relevant unconditioned stimulus as well as ethical considerations with threat induction in youth (see Shechner et al., 2014 for a review of key developmental considerations in threat conditioning). As a consequence, the neural mechanisms that mediate affective versus sensory-perceptual aspects of threat generalization and their relation to childhood anxiety traits remain unclear. The present study addresses this gap by integrating functional magnetic resonance imaging (fMRI) with multivariate analytical techniques to test how neural representation of perceptually similar stimuli contributes to threat generalization in children on a range of anxiety symptoms. Since perceptual networks have a less protracted developmental course than cognitive networks (Gogtay et al., 2004; Sowell et al., 2003), identifying perceptual contributions to threat overgeneralization at a relatively early developmental stage would bear implications for treatment of anxiety disorders.
Some stimuli are classified as dangerous based on inherent stimulus features, whereas other stimuli are classified as dangerous through effects of learning. Generalizability can be examined with either inherent or learned dangerous stimuli. Nevertheless, experimental work has focused more on entirely learned threats, as examined in Pavlovian conditioning and extinction paradigms, rather than inherently dangerous stimuli. In these Pavlovian conditioning paradigms, a neutral stimulus (conditioned stimulus, CS+) is paired with a threat (unconditioned stimulus, UCS) (Britton et al., 2013; Dunsmoor and Paz, 2015; Dymond et al., 2015; Milad et al., 2007; Quirk and Mueller, 2008), to examine the generalization of threat responses to generalization stimuli (GS): stimuli resembling the CS+ that have never themselves predicted an aversive experience (Dunsmoor and LaBar, 2013; Glenn et al., 2012; Michalska et al., 2016, 2019; Shechner et al., 2018). Differential conditioning paradigms also incorporate a second, unreinforced stimulus (CS−) to act as a safety cue. (Dunsmoor et al., 2009; Honig and Urcuioli, 1981; Lissek et al., 2008, 2010; Michalska et al., 2016, 2017, 2019; Shechner et al., 2018). Threat generalization can be tested by presenting individuals with the CS and GS either immediately after conditioning and extinction or even days or weeks afterwards. This process is known as extinction recall. Much of our current understanding of threat representations is grounded in computational models of learning during threat conditioning and extinction (Li et al., 2011; Pearce and Hall, 1980; Rescorla and Wagner, 1972), and findings from extinction recall may thus be more difficult to contextualize within this literature. Given our incomplete understanding of how threat generalization is instantiated in multivariate brain patterns during extinction recall, our study therefore represents a promising new research direction.
The neural sources of threat generalization, in both children and adults, remain poorly understood, though several brain networks have been reliably implicated in these processes. Among healthy adults, as stimuli become more similar to CS+, the amygdala, anterior insula, and dorsomedial prefrontal cortex (dmPFC) are recruited in forming threat associations and producing threat-conditioned behaviors (Davis, 1992; Dunsmoor et al., 2011; Resnik and Paz, 2015). Activation of the ventromedial prefrontal cortex (vmPFC), on the other hand, decreases as stimuli more closely resemble the CS+ and increases as stimuli more closely resemble the CS− (Lissek et al., 2014; Schiller et al., 2008). A failure to recruit vmPFC in response to safe stimuli has been associated with deficiencies in threat generalization (Cha et al., 2014; Greenberg et al., 2013b; Holt et al., 2012). In comparison to healthy individuals, children and adults with anxiety display hyperactivity in the amygdala and insula as well as aberrant engagement of the vmPFC upon viewing learned threat stimuli (Britton et al., 2013; Dunsmoor and Paz, 2015; Indovina et al., 2011; Milad et al., 2009). However, it is not known whether perturbations in these affective areas parallel neural response profiles in perceptual areas, or whether the generalization gradients in affective areas are decoupled from perceptual representations.
Two competing accounts frame the current investigation of threat generalization, the tendency to view neutral or ambiguous stimuli as threatening. Under the perceptual account, threat overgeneralization – the exaggerated tendency to view neutral or ambiguous stimuli as threatening – stems from a disordered ability to differentiate between threatening and ambiguous non-threatening stimuli at the perceptual level, possibly from a dysfunction that also manifests during threat learning (Dunsmoor and LaBar, 2013; Dymond et al., 2015; Lashley and Wade, 1946; Lim and Pessoa, 2008). This account generates the hypothesis of impaired perceptual discrimination of stimuli. If overgeneralization manifests in the context of intact perceptual discrimination, then generalization does not result from impaired perceptual discrimination. Research supporting the perceptual account finds that threat conditioning induces a wider generalization gradient through altering perceptual thresholds (Dunsmoor and LaBar, 2013; Dymond et al., 2015; Lim and Pessoa, 2008; Resnik et al., 2011; Resnik and Paz, 2015). Under the conceptual account, overgeneralization instead reflects abnormalities in non-perceptual, affective processes, including threat learning and memory mechanisms, separate from perceptual discrimination (Greenberg et al., 2013a; Kindt, 2014; Lissek et al., 2014; Shepard, 1987; Soeter and Kindt, 2015). Under this view, threat generalization is an active process leading to the generalization of threat even in the presence of accurate discrimination of the CS. In other words, the organism elicits behaviors to the GS appropriate for a CS, not from a failure to perceptually differentiate the GS from the CS but rather because they are functionally similar even if perceptually distinct. Thus, the classification would be based on psychological rather than physical similarity (Shepard, 1987). Primate research finds a shift in the tuning curves of neurons in the basolateral amygdala after conditioning (Resnik and Paz, 2015), but this study did not reveal whether these changes occurred at sensory encoding. The lack of studies probing generalization gradients across perceptual and affective brain regions simultaneously precludes strong inferences regarding sources of individual differences in threat generalization related to anxiety. Here we do not explicitly disambiguate between the two accounts; rather, we contribute to the ongoing debate by characterizing profiles of stimulus-evoked neural representations in both perceptual and affective neural regions following a threat conditioning and extinction procedure during late middle childhood, a period of developmental plasticity during which the risk for internalizing disorders is heightened (Kessler et al., 2005) and brain regions underlying threat learning are still developing (Gee et al., 2013; Gogtay et al., 2004).
Research studying the neural underpinnings of threat generalization has failed to delineate whether perceptual mechanisms play a role in threat generalization in part because univariate analytic methods are not well suited for addressing the question as to where in the cortical hierarchy ambiguous stimuli are encoded as threatening. However, recent advances in analytic methods have facilitated innovations in cognitive neuroscience, which may accelerate discoveries in the etiology of threat overgeneralization. One particularly promising approach is representational similarity analysis (RSA; Kriegeskorte et al., 2008), which leverages information contained in the patterns of activity across multiple voxels to characterize the unique neural representation of a stimulus within a given brain region (Davis and Poldrack, 2013; Haxby, 2012; Mahmoudi et al., 2012; see Ritchie et al., 2019 for counterpoint) rather than averaging the response across multiple voxels, as is standard in univariate techniques. Thus, whereas univariate methods compare a voxel’s or region’s signal strength between conditions, RSA recognizes the unique contribution of multiple voxels within a population. One application of RSA involves comparing or classifying the neural representations of different stimuli via examining their multivoxel patterns: the dissimilarity of patterns is taken to identify which representations of stimuli are alike and which diverge. When leveraged with other indices of threat responding, multivariate methods offer increased sensitivity and strengthen inferences that are not readily gleaned from univariate strategies alone, hence improving traction in the basic science of overgeneralization. However, these methods have rarely been applied in studies of threat overgeneralization in youth, leaving a significant gap in our understanding.
Here, in a comprehensive and targeted approach, we study a sample of youth in late middle childhood/early adolescence on a range of anxiety traits across visits in the psychophysiology laboratory and neuroimaging environment. We tested (1) Whether multivariate neural responses exhibited similar threat-tuning profiles across brain regions implicated in affective versus perceptual processes during extinction recall, and (2) Whether multivoxel patterns of neural response across both sets of regions varied as a function of children’s anxiety symptoms. Based on prior work examining univariate responses in affective and cognitive regions using this dataset (Michalska et al., 2019), we hypothesized that high anxious children would show more similar representations between a threat cue and a novel, perceptually similar stimulus across emotional areas than do low anxious children, particularly in the vmPFC and anterior insula. Due to mixed evidence with regards to perceptual circuitry in the adult literature (Åhs et al., 2013; Dunsmoor et al., 2012; Dunsmoor and Murphy, 2015) and given the lack of empirical work on neural representation in inferior temporal and visual cortices in children, we remained agnostic about the direction of associations between pattern differentiation in perceptual regions and anxiety.
2. Materials and methods
2.1. Participants
The current report represents a re-analysis of data reported in Michalska et al. (2019). As in the original report, a total of 50 children and adolescents underwent a threat conditioning and extinction recall paradigm. One child discontinued participation during the scan due to anxiety. Data from 7 individuals were subsequently excluded due to excessive motion (n = 3), poor anatomical segmentation (n = 1), otherwise missing structural brain data (n = 2), or missing anxiety scores (n = 1), resulting in a final sample of n = 42 (17 females; ages 11–15 years, see Table 1). Participants were recruited from the community at 2 years of age and enrolled in an ongoing longitudinal study at the National Institute of Mental Health (NIMH) and the University of Maryland, College Park (see Michalska et al., 2019 for details about subject recruitment). Individuals in this sample served as age- and sex-matched control comparisons for another sample of children who were enrolled as infants on the basis of their expression of behaviorally inhibited temperament (Jarcho et al., 2016; Michalska et al., 2019). Children in this unselected sample were assessed on socially reticent behaviors at 2, 3, 4, 5, and 7 years. However, longitudinal measures of social reticence are not a focus of the present study. Participants were eligible to participate in the current study at 11 years of age if they were medication-free, had an IQ > 70 based on the Vocabulary and Matrix Reasoning subscales of the Wechsler Abbreviated Scale of Intelligence (Weschler, 1999), reported no contraindications for neuroimaging, and were free from psychopathology requiring immediate treatment. Ten children met diagnostic criteria for an affective disorder (Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version; see Supplement for a breakdown of diagnoses). Participants who verbally assented and whose primary caregivers gave written consent were enrolled. Procedures were approved by the University of Maryland, College Park and the National Institute of Mental Health (NIMH) Institutional Review Boards. Previous findings using this dataset have been reported by Michalska et al. (2019). The prior report did not examine multivariate pattern analyses and perceptual brain regions and all brain imaging analyses presented in this paper are novel. Moreover, the prior report focused on early-childhood social reticence. The current report focuses more narrowly on anxiety symptoms present concurrent with the acquisition of imaging data.
Table 1.
Sample demographic characteristics.
| N | Female (%) | Age in years (M, SD) | FSIQ-2 (M, SD) | SCARED composite (M, SD) | Hispanic (%) | Caucasian (%) | African- American (%) | (%) | Other (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Low anxiety | 21 | 23.8 | 13.36(0.61) | 116.3 (13.8) | 5 3(2 3) | 9.5 | 52.4 | 23.8 | 4.8 | 19.0 |
| High anxiety | 21 | 57.1 | 13.42 (0.65) | 114.5(12 41) | 14 7 (4 9) | 0.0 | 52.4 | 33.3 | 0.0 | 14.3 |
| Total | 42 | 40.5 | 13 39(0.63) | 115 4 (13 0) | 10 0(6.1) | 4 8 | 52.4 | 28.6 | 2.4 | 16.6 |
Note: SCARED = Screen for Child Anxiety Related Emotional Disorders. WASI = Wechsler Abbreviated Scale of Intelligence: Matrix Reasoning and Vocabulary subscales.
2.2. Procedure
The study paradigm consisted of two visits. In Visit 1, children participated in the threat conditioning and extinction phase in the psychophysiology clinic (described in detail below). Three weeks later, at Visit 2, children completed the extinction recall phase in the MRI scanner.
2.2.1. Measures
2.2.1.1. Anxiety symptoms.
Screen for Child Anxiety Related Emotional Disorders (SCARED).
Children’s anxiety symptomatology was a composite of maternal-report and child self-report on the Screen for Child Anxiety Related Disorders (SCARED) collected within six months of Visit 1 (mean = 9.26 days ± 34.69 days). The SCARED is a child- and parent-report measure comprising 41-items assessing recent anxiety symptoms (past 3 months) rated on a 3-point Likert scale (Birmaher et al., 1999, 1997). Item scores are summed to a total score (range: 0–82). The SCARED possesses strong psychometric properties (Birmaher et al., 1999, 1997). To minimize informant discrepancies, child- and parent-report scores were averaged as we find that the two scores load on the same factor in latent variable approaches, suggesting that they are indicators of the same factor (Behrens et al., 2019; Bowers et al., 2019; Kircanski et al., 2017). Moreover, combining the two scores reduces the number of statistical tests, and we thus regularly use this approach when examining cognitive neuroscience constructs (Guyer et al., 2008; Michalska et al., 2017, 2018; Shechner et al., 2017). SCARED scores for children missing either parent- or self-report were comprised of one complete informant report (n = 5).
Children with scores above (>50th percentile) or below (≤50th percentile) the median anxiety score were categorized as high or low anxiety. Groups did not differ by age, race, ethnicity, or IQ (χ2 tests, all ps > .15). Consistent with the literature on sex differences in anxiety at this age (Blumenthal et al., 2011; Lewinsohn et al., 1998), the high anxiety group had a higher proportion of females than the low anxiety group (χ2(42) = 4.842, p = .028). Sex was therefore used as a nuisance covariate in subsequent analyses which included anxiety.
2.2.1.2. Threat conditioning task.
We used an uninstructed child-appropriate threat-learning task that was previously found effective (i. e., produced threat conditioning while maintaining an acceptable dropout rate) among healthy and trait anxious participants from both pediatric and adult populations (Michalska et al., 2016, 2019; Shechner et al., 2015). In the task, yellow and blue colored cartoon bells served as the conditioned stimuli (CS+ and CS−). The unconditioned stimulus (UCS) was a 1s presentation of a red bell, with a brief 95 dB alarm sound delivered via headphones.
A schematic representation of the threat conditioning and extinction task is provided in Fig. 1. The task consisted of a pre-acquisition phase, an acquisition phase, and an extinction phase (Michalska et al., 2016, 2019; Shechner et al., 2015). During the pre-acquisition phase, each CS was presented four times to allow physiological responses to the novel stimuli to habituate. During the acquisition phase, each CS was presented 10 times, and the CS+ was followed by the UCS with an 80% reinforcement schedule. Participants were instructed that they could learn to predict when the UCS would occur but were not explicitly informed of this contingency. During the extinction phase, the CSs were each presented eight times in the absence of the UCS. In all phases, the CS+ and CS− were presented for 7–8 s, followed by an inter-trial-interval of a gray screen presented for 8–21 s (mean = 15s). The CS+ and CS− assignment was pseudorandomized (two different orders counterbalanced across participants). The task was programmed and administered using PsyLab psychophysiological recording system (PsyLab SAM System Contact Precision Instruments, London).
Fig. 1. Schematic representation of the conditioning and extinction recall paradigm.

Top: During acquisition, one bell (CS+) was repeatedly paired with a red bell and loud alarm sound (UCS); the other bell (CS−) was never paired with the UCS. During extinction, both bells were presented in the absence of the UCS. Bottom: the generalization stimuli and attention states (threat appraisal and explicit memory) during the extinction recall task. Note: CS = conditioned stimulus; UCS = unconditioned stimulus. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2.1.3. Extinction recall task.
Participants returned three weeks after threat conditioning and extinction (mean = 22.86 ± 8.89 days) to complete an in-scanner extinction recall task (Michalska et al., 2019) where they viewed the CS+ and CS− and four generalization stimuli (GS) that were morphed blends of the CS− and CS+ (GS20 (20% CS+), GS40 (40% CS+), GS60 (60% CS+), GS80 (80% CS+); Fig. 1). Stimuli were presented in blocks and task instructions varied across blocks. At the start of each block, participants were instructed to use an MRI-compatible button box to answer one of two questions in two attention states: 1) How afraid are you of this bell now? (threat appraisal); 2) How likely was the bell to ring in the past? (explicit memory). Stimuli, task instructions, and response scales ranging from 0 to 6 were then presented simultaneously for 4000 ms followed by a 200–1000 ms jittered inter-stimulus interval (mean 5s; Fig. 2). The task consisted of two runs, each with 6 blocks of 12 trials each. Subjects viewed 12 presentations of each GS for each attention state (threat appraisal, explicit memory), totaling 144 trials. All participants completed data collection runs in both conditions while BOLD response and skin conductance response (SCR) were collected. SCR was collected from the index and middle fingers of the non-dominant hand using an MRI-compatible MP-150 system (BIOPAC Systems, Inc., CA, USA) at a sampling rate of 1000 Hz. The task was programmed in E-prime (PST Inc., Pittsburgh, PA).
Fig. 2.
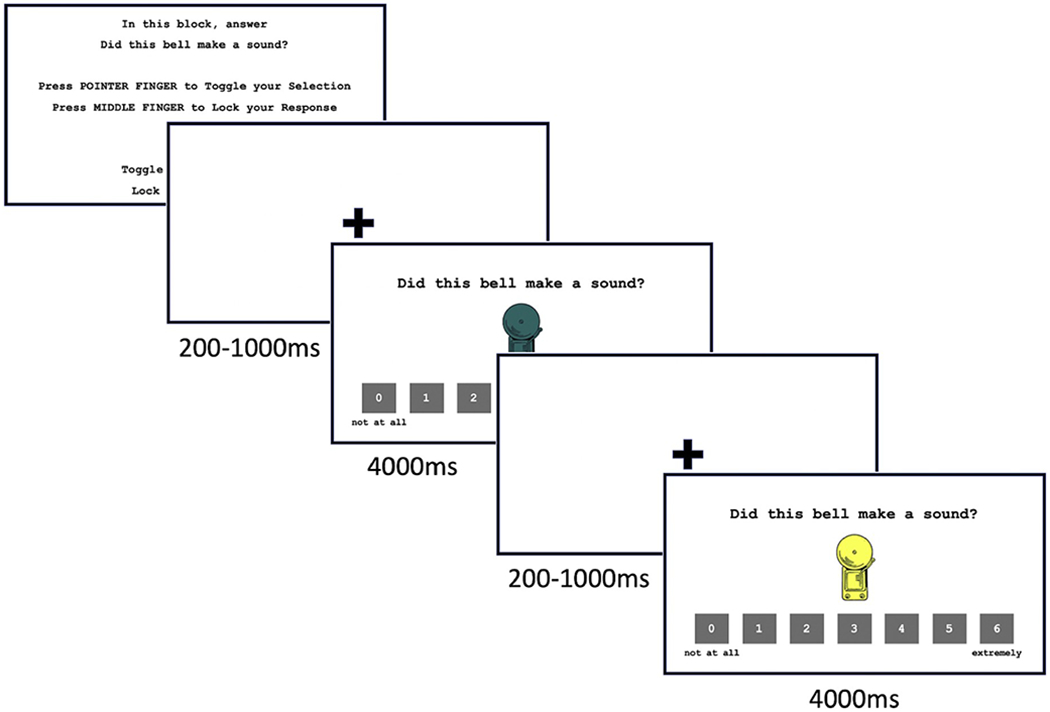
Extinction recall task in the explicit memory attention state. During extinction recall, participants they viewed the CS−, CS+, and four generalization stimuli (GS) that were morphed blends of the CS− and CS+. Task instructions were blocked corresponding to one of two attention states (threat appraisal, explicit memory). Stimuli, task instructions, and response scales were presented simultaneously for 4000 ms with a 200–10000 ms inter-stimulus interval. Note: CS− = non-threat-associated conditioned stimulus; CS+ = threat-associated conditioned stimulus; GS = generalization stimulus.
2.3. Imaging data
2.3.1. Acquisition parameters and preprocessing
All participants underwent MRI scanning at the NIMH Functional Magnetic Resonance Imaging Core Facility. Whole-brain neuroimaging data were collected using a 3 T General Electric 750 scanner and 32-channel head coil. During 2 runs of 13 min 9 s each, 343 functional image volumes, with 47 contiguous interleaved axial slices (in-plane resolution 2.5 mm, 3 mm slice thickness) were obtained with a T2*-weighted echo-planar sequence (TR = 2300 ms; TE = 25 ms; flip angle = 50; Field of View (FOV) = 240 mm; matrix = 96 × 96). Functional data were anatomically localized and coregistered to a high-resolution T1-weighted volumetric scan of the whole brain, using a magnetization prepared gradient echo sequence (MPRAGE) (TE = min full; TI = 425 ms; flip angle = 7; FOV = 256 mm; matrix = 256 × 256; in plane resolution 1.0 mm).
Individual echo-planar data were preprocessed and analyzed using AFNI (Analysis of Functional NeuroImages [http://afni.nimh.nih.gov/afni/]). Preprocessing included slicetime correction, motion correction, and spatial normalization to the Talairach template. Smoothing was not used to avoid reducing the amount of fine-grained spatial detail (i.e. by blurring distinct voxel-level signals) required for RSA (Pereira et al., 2009). BOLD data were scaled at the voxel-wise time series by their temporal means so that the effect estimates could be interpreted as percent signal change relative to the mean. Pairs of successive TRs for which more than 10% of voxels were outliers and where head displacement exceeded 1 mm were excluded. Subjects were excluded for excessive motion if more than 15% of TRs in one condition were censored for motion/outliers (n = 3).
2.3.2. Region of interest selection
We utilized RSA (Kriegeskorte et al., 2008) to explore threat representations in seven bilateral anatomically-defined regions of interest (ROIs): (1) amygdala, (2) anterior insular cortex (AIC), (3) ventromedial prefrontal cortex (vmPFC), (4) dorsomedial prefrontal cortex (dmPFC), (5) inferior temporal cortex (ITC), and visual areas (6) primary visual cortex (V1) and (7) visual area V4. ROIs were anatomically defined using the Desikan-Killiany atlas (Desikan et al., 2006) for bilateral amygdala (label: amygdala), ITC (label: inferior temporal cortex), vmPFC (label: medial orbitofrontal cortex), and dmPFC (label: superior frontal cortex), the Destrieux atlas (Destrieux et al., 2010) for the left and right anterior circular sulci of the insula (label: anterior circular sulcus of the insula), and using probabilistic maps of visual topography for V1 and V4 (Wang et al., 2015).
Cortical and subcortical segmentations were performed on T1-weighted whole-brain volumetric scans using FreeSurfer’s segmentation and surface-based reconstruction software (recon-all; v 6.0; http://surfer.nmr.mgh.harvard.edu; Fischl et al., 2002, 2004). This automated pipeline included motion correction, skull-stripping, B1 bias field correction, gray and white matter segmentation, and non-linear registration to Talairach space. Using AFNI (Cox, 1996), amygdala, AIC, vmPFC, dmPFC, and ITC segmentations were subsequently converted to volumetric data (@SUMA_Make_Spec_FS of SUMA), aligned to subjects’ BOLD space (@Align_centers), and transformed to Talairach space using AFNI’s non-linear warping tool, 3dNwarpApply. For visual areas V1 and V4, surface maximum probability map data from the probabilistic maps of visual topography (Wang et al., 2015) were converted to subjects’ SUMA volume data (@surf_to_vol_spackle) prior to alignment and transformation. Mask fit was visually inspected two times in AFNI: first by overlaying Freesurfer segmentations on the T1 images, and again, after alignment and transformation, masks were overlaid on the final anatomical dataset. Prior to group assignment, one participant was excluded due to poor anatomical segmentation.
2.4. Data analysis
2.4.1. SCR and behavioral data analysis
A mixed model regression analysis was used to analyze SCR elicited by each CS and GS during extinction recall. Linear and quadratic trends of SCR elicited by the 6 bell stimuli (CS−, GS20, GS40, GS60, GS80, CS+) were examined along with the interaction between linear and quadratic trends and anxiety group (high anxiety, low anxiety). Self-report measures obtained during extinction recall were analyzed using similar analyses.
2.4.2. Representational similarity analysis
Prior to performing RSA, we conducted standard general linear model analysis that included 12 condition regressors for each participant (6 regressors for each bell stimulus (CS−, GS, CS+) in each of the two attention states. Third-order Legendere polynomials modeling baseline drift and 6 head motion parameters were also included to account for possible confounding effects. For each subject, this produced twelve whole-brain average voxel patterns , each of which contained the voxel pattern of hemodynamic response for a single combination of attention state (i∈{threat appraisal, explicit memory}) and bell morph (j∈{CS−,GS20,GS40,GS60,GS80,CS+}) averaged across trials in that condition, i.e. with t the number of trials in that condition.
We utilized representational similarity analysis (RSA) to calculate pairwise dissimilarities between multivoxel activation patterns elicited by CS−/GS and the CS+. To do this, we extracted average voxel-wise responses to each generalization stimulus type (j), in each attention state (i), and in each of the seven ROIs (r), for each participant (s), yielding . We attempted to disambiguate whether overgeneralization is unique to a specific learned threat-stimulus or whether it is an individual trait applied indiscriminately to all similar-looking stimuli after threat learning. To do so, we quantified generalization in neural patterns elicited by novel stimuli that vary along a continuum from a safe stimulus (CS−) to the stimulus that most resembles the conditioned threat stimulus (GS80). Degree of representational differentiation between neural patterns elicited by the CS+ versus the CS−, GS20, GS40, GS60, and GS80 was computed as one minus the Pearson correlation coefficient, i.e. (Kriegeskorte et al., 2008).
To address our two research questions, (1) Whether affective and perceptual brain areas demonstrate similar profiles of CS+ versus GS representation, and (2) Whether high anxious children exhibit different patterns of threat generalization in these brain regions, we performed two sets of statistical tests. To address our first question about affective versus perceptual contributions in the entire sample, the first set of tests evaluated whether the dissimilarity between the CS+ and the CS−/GS (CS−, GS20, GS40, GS60, and GS80, “GS-CS+ neural pattern differentiation”) was modulated by ROI and attention state with a 7 × 2 × 5 within-subjects analysis of variance, with ROI (7: amygdala, vmPFC, AIC, dmPFC, IT, V1, V4), attention state (2: threat appraisal, explicit memory), and GS (5: CS−/CS+, GS20/CS+, GS40/CS+, GS60/CS+, GS80/CS+) as within-subjects factors. To address our second question about the degree to which anxiety interacts with these effects, the second set of tests evaluated whether GS-CS+ neural pattern differentiation was modulated by the above factors as they interacted with anxiety group using a 2 × 7 × 2 × 5 mixed design analysis of covariance (ANCOVA), with anxiety group (2: high, low) as a between-subjects factor, ROI (7: amygdala, vmPFC, AIC, dmPFC, IT, V1, V4), attention state (2: threat appraisal, explicit memory), and GS (5: CS−/CS+, GS20/CS+, GS40/CS+, GS60/CS+, GS80/CS+) as within-subjects factors, and sex as a covariate of no interest to account for sex differences across anxiety groups. In both sets of tests, we also conducted a linear planned contrast of affective versus perceptual neural regions which compared the mean for perceptual (ITC, V1, V4) versus affective (AIC, amygdala, vmPFC, dmPFC) clusters of ROIs such that perceptual regions differentiate less than affective regions. Significant interactions were followed by step-down ANOVAs/ANCOVAs and t-tests as appropriate. All tests were two-sided and significance was set at ɑ < 0.05. Greenhouse-Geisser corrections for non-sphericity were performed where indicated, though uncorrected degrees of freedom are reported for ease of readability. To conservatively avoid undue influence of outliers, outliers were Winsorized by setting neural pattern similarity values greater or less than three standard deviations above or below the mean to three standard deviations away from the mean. For completeness, we report analyses on un-Winsorized data in the Supplementary Material; the Winsorization procedure did not alter the pattern of results.
3. Results
3.1. Visit 1: response to conditioned cues
Children’s SCR and self-reported fear during conditioning indicated successful conditioning followed by extinction across the whole sample with no interaction by anxiety group (see Supplementary Material for details).
3.2. Visit 2: extinction recall
3.2.1. SCR
No main effects of anxiety group or interactions with morph were observed during threat appraisal or explicit memory (all ps > .09). See Supplemental Material for details.
3.2.2. Self-report ratings
A significant quadratic but not linear pattern of self-reported fear as a function of stimulus was observed in the threat appraisal condition (Quadratic: B = 0.078, SE = 0.019, t(40) = 4.021, p < 001; Linear: B = 0.005, SE = 0.003, t(40.64) = 1.60, p = .12). Pairwise comparisons revealed that participants reported being more afraid to the CS+ compared to the CS− and all GS (all ps < .002). Greater fear to the GS80 compared to the GS40 and GS60 was also observed (all ps < .008). Similar results were observed in the explicit memory condition. A linear and quadratic pattern of self-reported rating emerged (linear: B = 0.014, SE = 0.004, t(38.67) = 3.49, p = .001; quadratic: B = 0.147, SE = 0.030, t(340) = 4.905, p < .001) where children were significantly more likely to report that the CS+ rang compared to the CS− and all GS (all ps < .001). Participants also reported a higher likelihood that the CS+ rang relative to the GS80 and all other GS (all ps < .02). No main effects or interactions with anxiety group were observed (all ps > .16).
3.3. Visit 2: neural pattern differentiation in perceptual versus affective regions at extinction recall
3.3.1. ROI differences: perceptual versus affective brain areas
To test our first hypothesis regarding differential contributions of perceptual versus affective brain areas to threat generalization across our entire sample, an ROI × GS × attention state ANOVA examined interactions with each of our seven a priori defined regions of interest. To evaluate whether perceptual and affective regions differ in their neural pattern differentiation profiles we also computed a linear planned contrast of “ROI cluster.” This contrast compared the mean neural pattern differentiation for perceptual (ITC, V1, V4) versus affective (AIC, amygdala, vmPFC, dmPFC) ROI clusters. We observed a main effect of ROI (F(6, 246) = 123.37, p < .001, , Greenhouse-Geisser corrected; Fig. 3) as well as a main effect of “cluster” in our perceptual versus affective contrast (F(1, 41) = 191.66, p < .001, ), with regions in the affective cluster exhibiting greater multivariate pattern differentiation across all generalization stimuli than those in the perceptual cluster (Fig. 3, blue shaded region versus purple shaded region). No main effects or interactions were observed with GS or attention state (ps > .05), although we did observe a trending main effect of attention state (p = .058). This pattern did not change when sex was included as a nuisance covariate (see Supplemental Material).
Fig. 3.
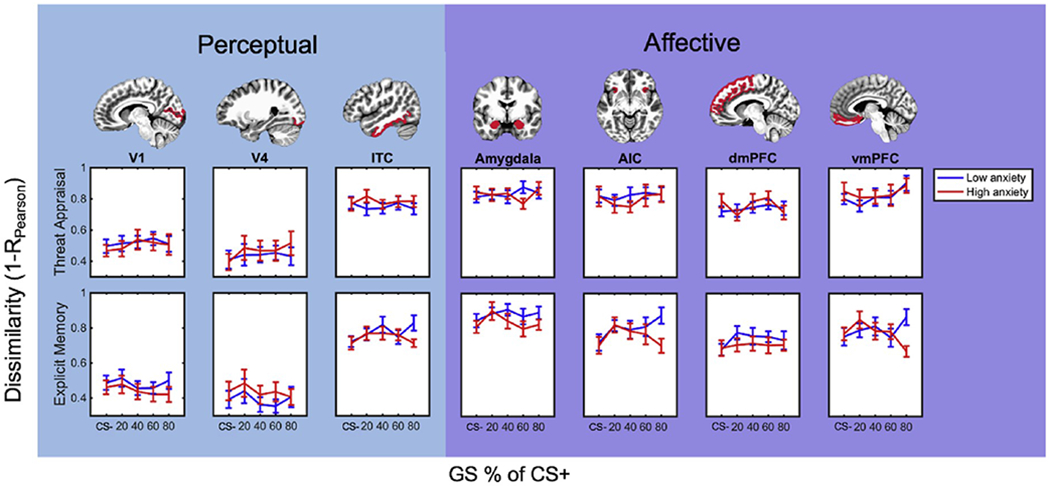
Neural pattern differentiation during threat appraisal and explicit memory. Anxiety group differences in neural pattern differentiation between CS−/GS versus CS+ during threat appraisal (top) and explicit memory (bottom) attention states. Neural pattern differentiation (1 - Pearson correlation coefficient) was measured in perceptual (V1, V4, ITC; columns 1–3) and affective brain regions (amygdala, AIC, dmPFC, and vmPFC; columns 4–7). Note: CS− = non-threat-associated conditioned stimulus; CS+ = threat-associated conditioned stimulus; GS = generalization stimulus; ITC = inferior temporal cortex AIC = anterior insular cortex; dmPFC = dorsomedial prefrontal cortex; vmPFC = ventromedial prefrontal cortex.
3.3.2. Anxiety moderates perceptual versus affective contributions
The impetus for the present study was to examine not only the relative contributions of perceptual versus affective representations to threat generalization overall, but also whether anxiety influences these representations in youth. To address this second question, we conducted a repeated-measures ANCOVA testing the effects of anxiety group, ROI, GS, and attention state (with sex as a covariate of no interest given unequal distribution of sex across anxiety groups; see Methods: Data analysis) on GS-CS+ neural pattern differentiation with the same planned perceptual versus affective “cluster” contrast described above. Similar to our first analysis, we observed a main effect of ROI (F(6, 234) = 9.61, p < .001, ) and cluster (F(1, 39) = 15.61, p < .001, ), with affective clusters overall showing more differentiation than perceptual clusters (Fig. 3, purple shaded region versus blue shaded region). We also found a marginal GS × cluster interaction (F(1, 39) = 3.99, p = .053, ), though exploratory step-down ANCOVAS (GS × ROI) within each ROI cluster revealed no interactions in affective or perceptual regions (ps > .45).
We also observed a three-way interaction among anxiety group (high, low), ROI cluster (perceptual, affective), and GS (CS−, GS20, GS40, GS60, GS80) (F(1, 39) = 6.52, p = .015, ). Step-down ANCOVAs within perceptual and affective clusters separately revealed an anxiety × GS × ROI interaction among regions comprised in affective clusters (F(12, 468) = 2.29, p = .024, , Greenhouse-Geisser corrected). No interactions with GS or anxiety emerged in perceptual clusters (ps > .31). Follow-up analyses within each of the regions in the affective ROI cluster yielded a significant anxiety × GS interaction in vmPFC (F(4, 156) = 4.08, p = .004, ), indicating diminished differentiation specifically between GS80 and the CS+ in vmPFC for high anxious children compared to those with low anxiety scores (t(40) = 2.57, p = .014). No group differences emerged in other affective regions (ps > .09) or for vmPFC pattern differentiation between the CS+ and other morphs (ps > .26). This pattern suggests that increased neural pattern similarity specifically between threat and highly similar generalization stimuli in the vmPFC, rather than in other affective areas or perceptual regions and rather than overall inability of the vmPFC to differentiate threat from dissimilar stimuli, may be related to anxiety symptoms.
4. Discussion
This study utilized representational similarity analysis to identify representations of threat generalization stimuli across perceptual and affective brain regions in youth exhibiting a range of anxiety symptoms. Two main findings emerged. First, across the entire sample, neural pattern differentiation of all stimuli was overall greater in regions typically considered to support affective as opposed to perceptual processes. However, perceptual and affective clusters of ROIs did not interact with GS type, indicating that both clusters may make parallel contributions to threat generalization. Second, children who exhibited more anxiety symptoms represented CS+ and GS80 more similarly in vmPFC, as compared to low anxious children, who represented these two stimuli more distinctly. Together, these results suggest that neural representations in both perceptual and affective areas contribute to threat generalization in children and that such representations are modulated by anxiety.
Our first aim was to capitalize on the precision of multivariate neuroimaging techniques to evaluate differential contributions of perceptual versus affective brain regions to threat generalization across our entire sample. We observed that across all generalization stimuli, affective brain regions displayed greater dissimilarity of neural patterns elicited by the CS+ versus various generalization stimuli as compared to perceptual regions. However, we did not find that brain region or ROI cluster significantly interacted with generalization stimuli. This suggests that, while affective and perceptual regions may differ in their processing of threat stimuli, the threat generalization gradient may be represented similarly in neuronal population codes across both affective and perceptual regions despite magnitude differences. This result provides a critical bridge between recent findings in adults that threat learning can lead to perceptual alterations (Dunsmoor and LaBar, 2013; Lim and Pessoa, 2008) and also subsequent engagement of affective circuitry during the retention of extinction (Dunsmoor et al., 2011; Lissek et al., 2013). Specifically, during processing of visual stimuli, input from visual areas informs interpretation in emotion-based areas; these emotion areas, in turn, send feedback to visual areas (Gayet et al., 2015; Phelps et al., 2006; Zadra and Clore, 2011). As a consequence, both affective and perceptual areas come to represent the similarity between a threatening stimulus and various ambiguous stimuli in a qualitatively similar pattern. Unfortunately, our design precludes an assessment of whether neural differentiation in perceptual regions causally determines the strength of the fear response or vice versa. Future studies should investigate whether and how representational overlap arises from feed-forward, feedback, or both directions of information flow.
Our second objective was to probe how perceptual and affective neural representations of generalization stimuli could contribute to threat overgeneralization in children with low versus high trait anxiety. We observed that anxiety moderates perceptual versus affective contributions to threat generalization as measured by neural pattern similarity. Anxiety symptom levels most clearly interacted with vmPFC pattern similarity. In other words, we demonstrate that for high anxious children, multivoxel patterns elicited by CS+ and GS80 stimuli overlap in vmPFC, an emotion-related neocortical region, more than they do for low anxious children. These results extend prior work on extinction recall using univariate methods showing that vmPFC engagement may facilitate the ability to distinguish dangerous from safe stimuli during the recall of extinguished threat (Milad et al., 2007), ostensibly via inhibition of amygdala response to safety cues (Maren and Quirk, 2004). It also builds on previous reports of vmPFC disruption in extinction recall in adult and adolescent anxiety (Britton et al., 2013). Because the vmPFC is essential to the identification of and response to extinguished threat, it is important for this region to have the capacity to precisely identify said threat. Although prior research suggests that anxious relative to nonanxious adults and adolescents show differential engagement in vmPFC during extinction recall, less is known about how threat- and safety-cues are represented differently among individuals with anxiety (Britton et al., 2013; Cha et al., 2014; Greenberg et al., 2013a; Indovina et al., 2011; Milad et al., 2009). Our results provide evidence that the neural representation of threat and highly similar GS overlap more in vmPFC in high versus low anxious children, thus potentially reducing its inhibitory influence on other nodes in fear circuitry. Notably, by extracting information from the patterns of brain activity, we were able to detect group differences even in a sample of children who did not all meet clinical diagnoses for anxiety. Our study thus speaks to the utility of RSA in studying how the brain categorizes ambiguous information during the recall of extinguished threat, a process that cannot be studied using traditional approaches based on activation magnitude alone.
Even though neural pattern differentiation values showed differences as a function of anxiety, especially in vmPFC, we did not observe a corresponding ability to explicitly distinguish among bell morphs along the generalization gradient at the behavioral level. One possibility for the discrepancy is that we did not probe perceptual thresholds behaviorally with sufficient granularity and only asked how likely it was that each bell rang in the past. Children reliably rated the CS+ as more likely to have rung than any of the GS and CS−, irrespective of anxiety levels. These findings suggest that the vmPFC may track the perceptual differentiation and salience of generalization stimuli independent of conscious awareness, a hypothesis supported by recent work tracking associative threat learning through patterns of neural representational similarity (Dunsmoor et al., 2014; Struyf et al., 2017; Visser et al., 2013). Future work might probe this relationship more directly. Similarly, in line with our findings, Struyf et al. (2017) found no effect of trait anxiety on the probability that a stimulus was perceived as either GS or CS+ nor on the overall generalization gradient as measured by behavioral responses. In that study, despite general proficiency in explicitly distinguishing stimuli along the generalization gradient, individual differences were evident in spatial representation of threat-associated stimuli. We hope that future studies can further investigate these ideas using methods that are appropriately rigorous.
Of note, neural pattern dissimilarity to the CS+ was not indicative of poor encoding of all generalization stimuli in high anxious youth, but was specific to stimuli that most resembled the CS+. That is, neural pattern differentiation (or lack thereof) was not indicative of a generalized propensity toward poor perceptual discrimination as a result of trait anxiety, but instead was relevant for explaining differences in subclinical anxiety levels under conditions of highest ambiguity following threat learning.
4.1. Limitations
Though we believe this RSA approach to be promising, we note several limitations of the current study and possible avenues for further research. As mentioned above, we do acknowledge that the findings reported here cannot provide insight into the directionality of affective versus perceptual contributions to threat generalization or overgeneralization. It may be that neural similarity in perceptual areas drives similarity in affective areas in a feed-forward fashion, or that feedback from higher-order cognitive and affective areas drives perceptual similarities – or a combination of these. Our findings support the theory that threat generalization reflects the integration of multiple bottom-up perceptual and top-down affective and regulatory psychological processes, but future work is needed to disentangle directional influences in vmPFC-perceptual circuitry during extinction recall. Relatedly, we did not evaluate perceptual thresholds with sufficient granularity to make strong causal inferences. To elucidate the potential causal role of threat learning on perceptual discrimination in anxious children, future research might build on promising work examining the role that emotional experiences play in inducing plasticity that prevents discrimination between highly similar stimuli (Lim and Pessoa, 2008; Resnik et al., 2011). For example, studies might probe how changes in perceptual thresholds following threat conditioning vary as a function of anxiety.
Another limitation of our approach is the use of a relatively small and mostly subclinical sample of children. Future studies should include clinical populations suffering from anxiety-related psychopathology so as to more robustly assess diminished neural pattern differentiation as a potential anxiety risk. However, even though most children in our sample did not meet clinical diagnostic criteria for anxiety, we nevertheless observed a clear if complex link between childhood anxiety traits and the distribution of neural representations of stimuli similar to learned threats across perceptual and affective brain regions.
Thus, this initial study in an existing dataset provides strong argument for pursuing careful and controlled studies examining how threat generalization in perceptual versus affective brain areas may emerge across development using multivariate neuroimaging approaches. Despite these limitations, the present results demonstrate the powerful utility of multivariate neuroimaging approaches in revealing the nature of perceptual contributions to threat generalization in health and overgeneralization in anxiety.
4.2. Broader impacts
Finally, our findings may also have important implications for the treatment of childhood anxiety. We observed that children high on trait anxiety showed more neural pattern similarity between threat and a highly similar generalization stimulus (GS80), especially in the vmPFC, suggesting reduced neural discrimination between these stimuli as a potential cause of overgeneralization. Though we found that affective and perceptual regions differ in their overall CS−/GS-CS+ differentiation, our findings do not preclude the possibility that they contribute similarly to threat generalization. Interventions that improve children’s perceptual discrimination during periods of elevated threat responses might therefore help attenuate anxiety-related states, especially if such increased perceptual differentiation were to improve affective differentiation in a feed-forward fashion. In support of this possibility, Ginat-Frolich et al. (2017) implemented a perceptual discrimination training task between threat conditioning and extinction recall among healthy adults and found that improved perceptual discrimination was related to less threat generalization to intermediate threat morphs. These findings may be especially significant in the treatment of childhood anxiety. Since perceptual networks have a shorter developmental course than cognitive networks (Gogtay et al., 2004; Sowell et al., 2003), improving perceptual discrimination may allow for earlier treatment and result in better outcomes. Future studies could therefore use the present multivariate approach to examine how perceptual training alters neural pattern differentiation in vmPFC, and how targeting this relationship may improve clinically relevant outcomes for anxious individuals.
Acknowledgements
We thank the participants and families, as well as the staff of the Intramural Research Program of the National Institute of Mental Health (IRP, NIMH), National Institutes of Health. We also thank Brenda Benson, Gang Chen, Julia Feldman, Daniel Glen, Jeffrey Knotts, Seth Margolis, Daniel Ozer, Richard Reynolds, and Vincent Taschereau-Dumouchel for their valuable contributions. This research was supported by the National Institute of Mental Health Intramural Research Program Project (ZIAMH00278), National Institute of Child Health and Human Development (R37HD17899), National Institute of Mental Health (R01MH093349) and a collaborative seed research grant to KJM and MAKP from the University of California Riverside Office of Research and Economic Development.
Footnotes
Declaration of competing interest
All authors report no biomedical financial interests or potential conflicts of interest.
References
- Åhs F, Miller SS, Gordon AR, Lundström JN, 2013. Aversive learning increases sensory detection sensitivity. Biol. Psychol. 92 (2), 135–141. 10.1016/j.biopsycho.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Armony JL, Servan-Schreiber D, Romanski LM, Cohen JD, LeDoux JE, 1997. Stimulus generalization of fear responses: effects of auditory cortex lesions in a computational model and in rats. Cerebr. Cortex 7 (2), 157–165. 10.1093/cercor/7.2.157. [DOI] [PubMed] [Google Scholar]
- Behrens B, Swetlitz C, Pine DS, Pagliaccio D, 2019. The screen for child anxiety related emotional disorders (SCARED): informant discrepancy, measurement invariance, and test-retest reliability. Child Psychiatr. Hum. Dev 50 (3), 473–482. 10.1007/s10578-018-0854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M, 1999. Psychometric properties of the screen for child anxiety related emotional disorders (SCARED): a replication study. J. Am. Acad. Child Adolescent Psychiatry 38 (10), 1230–1236. 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM, 1997. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolescent Psychiatry 36 (4), 545–553. 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Babson KA, Gahr JL, Trainor CD, Frala JL, 2011. Elevated social anxiety among early maturing girls. Dev. Psychol 47 (4), 1133–1140. 10.1037/a0024008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers ME, Reider LB, Morales S, Buzzell GA, Miller N, Troller-Renfree SV, et al. , 2019. Differences in parent and child report on the screen for child anxiety-related emotional disorders (SCARED): implications for investigations of social anxiety in adolescents. J. Abnorm. Child Psychol 1–11. 10.1007/s10802-019-00609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, Ernst M, Nelson EE, Leibenluft E, Shechner T, Pine DS, 2013. Response to learned threat: an FMRI study in adolescent and adult anxiety. Am. J. Psychiatr 170 (10), 1195–1204. 10.1176/appi.ajp.2013.12050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Greenberg T, Carlson JM, Dedora DJ, Hajcak G, Mujica-Parodi LR, 2014. Circuit-wide structural and functional measures predict ventromedial prefrontal cortex fear generalization: implications for generalized anxiety disorder. J. Neurosci.: Off. J. Soc. Neurosci 34 (11), 4043–4053. 10.1523/JNEUROSCI.3372-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29 (3), 162–173. 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davis M, 1992. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci 15 (1), 353–375. 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis T, Poldrack RA, 2013. Measuring neural representations with fMRI: practices and pitfalls. Ann. N. Y. Acad. Sci. 1296, 108–134. 10.1111/nyas.12156. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31 (3), 968–980. 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E, 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53 (1), 1–15. 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Kragel PA, Martin A, LaBar KS, 2014. Aversive learning modulates cortical representations of object categories. Cerebr. Cortex 24 (11), 2859–2872. 10.1093/cercor/bhtl38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, LaBar KS, 2013. Effects of discrimination training on fear generalization gradients and perceptual classification in humans. In: Behav. Neurosci, 127, pp. 350–356. 10.1037/a0031933, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Martin A, LaBar KS, 2012. Role of conceptual knowledge in learning and retention of conditioned fear. Biol. Psychol 89 (2), 300–305. 10.1016/j.biopsycho.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS, 2009. Generalization of conditioned fear along a dimension of increasing fear intensity. In: Learn. Mem, 16, pp. 460–469. 10.1101/lm.1431609, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Murphy GL, 2015. Categories, concepts, and conditioning: how humans generalize fear. Trends Cognit. Sci 19 (2), 73–77. 10.1016/j.tics.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Paz R, 2015. Fear generalization and anxiety : behavioral and neural mechanisms. Biol. Psychiatr 78 (5), 336–343. 10.1016/j.biopsych.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Prince SE, Murty VP, Kragel PA, Labar KS, 2011. Neurobehavioral mechanisms of human fear generalization. Neuroimage 55 (4), 1878–1888. 10.1016/j.neuroimage.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D, 2015. Fear generalization in humans: systematic review and implications for anxiety disorder research. Behav. Ther 46 (5), 561–582. https://doi.org/10.10l6/j.beth.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33 (3), 341–355. 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatically parcellating the human cerebral cortex. Cerebr. Cortex 14 (1), 11–22. 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gayet S, Paffen C, Belopolsky A, Theeuwes J, Van der Stigchel S, 2015. Fear conditioned visual information is prioritized for visual awareness. In: J. Vis, 15, p. 384 10.1167/15.12.384, Issue 12. [DOI] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer E, Shapiro M, Hare T, Bookheimer S, Tottenham N, 2013. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 33 (10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginat-Frolich R, Klein Z, Katz O, Shechner T, 2017. A novel perceptual discrimination training task: reducing fear overgeneralization in the context of fear learning. Behav. Res. Ther. 93, 29–37. 10.1016/j.brat.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G, 2012. The development of fear learning and generalization in 8–13 year-olds. Dev. Psychobiol. 54 (7), 675–684. 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U.S.A. 101 (21), 8174–8179. 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR, 2013a. Neural reactivity tracks fear generalization gradients. In: Biol. Psychol, 92, pp. 2–8. 10.1016/j.biopsycho.2011.12.007, 1. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR, 2013b. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress. Anxiety 30 (3), 242–250. 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJR, Leibenluft E, Fox NA, Pine DS, Nelson EE, et al. , 2008. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch. Gen. Psychiatr 65 (11), 1303–1312. 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, 2012. Multivariate pattern analysis of fMRI: the early beginnings. Neuroimage 62 (2), 852–855. 10.1016/j.neuroimage.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR, 2012. Failure of neural responses to safety cues in schizophrenia. Arch. Gen. Psychiatr. 69 (9), 893–903. 10.1001/archgenpsychiatry.2011.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig WK, Urcuioli PJ, 1981. The legacy of Guttman and Kalish (1956): twenty-five years of research on stimulus generalization. In: J. Exp. Anal. Behav, 36, pp. 405–445. 10.1901/jeab.1981.36-405, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indovina I, Robbins TW, Nfrnez-Elizalde AO, Dunn BD, Bishop SJ, 2011. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron 69 (3), 563–571. 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Davis MM, Shechner T, Degnan KA, Henderson HA, Stoddard J, Fox NA, Leibenluft E, Pine DS, Nelson EE, 2016. Early-childhood social reticence predicts brain function in preadolescent youths during distinct forms of peer evaluation. Psychol. Sci. 27 (6), 821–835. 10.1177/0956797616638319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, 2005. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatr. 62 (6), 593–602. 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kindt M, 2014. A behavioural neuroscience perspective on the aetiology and treatment of anxiety disorders. Behav. Res. Ther 62, 24–36. 10.1016/j.brat.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Zhang S, Stringaris A, Wiggins JL, Towbin KE, Pine DS, Leibenluft E, Brotman MA, 2017. Empirically derived patterns of psychiatric symptoms in youth: a latent profile analysis. J. Affect. Disord. 216, 109–116. 10.1016/j.jad.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P, 2008. Representational similarity analysis - connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 4 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashley KS, Wade M, 1946. The Pavlovian theory of generalization. Psychol. Rev. 53, 72–87. 10.1037/h0059999. [DOI] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin N, Pine DS, 2011. Distinct neural signatures of threat learning in adolescents and adults. Proc. Natl. Acad. Sci. U.S.A. 108 (11), 4500–4505. 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB, 1998. Gender differences in anxiety disorders and anxiety symptoms in adolescents. Journal of Abnormal Psychology 107 (1), 109. [DOI] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND, 2011. Differential roles of human striatum and amygdala in associative learning. Nat. Neurosci. 14 (10), 1250–1252. 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-L, Pessoa L, 2008. Affective learning increases sensitivity to graded emotional faces. Emotion 8 (1), 96–103. 10.1037/1528-3542.8.1.96. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C, 2008. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav. Res. Ther. 46 (5), 678–687. 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Bradford DE, Alvarez RP, Burton P, Espensen-sturges T, Reynolds RC, Grillon C, 2014. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc. Cognit. Affect. Neurosci 9 (8), 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, 2013. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol. Psychiatr. 75 (11), 909–915. 10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C, 2010. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am. J. Psychiatr 167 (1), 47–55. 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi A, Takerkart S, Regragui F, Boussaoud D, Brovelli A, 2012. Multi voxel pattern analysis for FMRI data: a review. Comput. Math. Methods Med. 2012, 961257 10.1155/2012/961257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ, 2004. Neuronal signalling of fear memory. Nat. Rev. Neurosci. 5 (11), 844–852. 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Feldman JS, Abend R, Gold AL, Dildine TC, Palacios-Barrios EE, Atlas LY, 2018. Anticipatory effects on perceived pain: Associations with development and anxiety. Psychosom. Med. 80 (9), 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Feldman JS, Ivie EJ, Shechner T, Sequeira S, Averbeck B, Pine DS, 2019. Early-childhood social reticence predicts SCR-BOLD coupling during fear extinction recall in preadolescent youth. Developmental Cognitive Neuroscience 36, 100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Machlin L, Moroney E, Lowet DS, Hettema JM, Roberson-Nay R, Averbeck BB, Brotman MA, Nelson EE, Leibenluft E, Pine DS, 2017. Anxiety symptoms and children’s eye gaze during fear learning. J. Child Psychol. Psychiatry 58 (11), 1276–1286. 10.1111/jcpp.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Shechner T, Hong M, Britton JC, Leibenluft E, Pine DS, Fox NA, 2016. A developmental analysis of threat/safety learning and extinction recall during middle childhood. J. Exp. Child Psychol. 146, 95–105. 10.1016/j.jecp.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL, 2009. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatr 66 (12), 1075–1082. 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL, 2007. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatr. 62 (5), 446–454. 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G, 1980. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol. Rev. 87 (6), 532–552. 10.1037/0033-295X.87.6.532. [DOI] [PubMed] [Google Scholar]
- Pereira F, Mitchell T, Botvinick M, 2009. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage 45 (1 Suppl. 1), S199–S209. 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M, 2006. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science 17 (4), 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D, 2008. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33 (1), 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR, 1972. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. Class. Condition. II: Curr. Res. Theory 2, 64–99. [Google Scholar]
- Resnik J, Paz R, 2015. Fear generalization in the primate amygdala. Nat. Neurosci 18 (2), 188–190. 10.1038/nn.3900. [DOI] [PubMed] [Google Scholar]
- Resnik J, Sobel N, Paz R, 2011. Auditory aversive learning increases discrimination thresholds. Nat. Neurosci 14 (6), 791–796. 10.1038/nn.2802. [DOI] [PubMed] [Google Scholar]
- Ritchie JB, Kaplan DM, Klein C, 2019. Decoding the brain: neural representation and the limits of multivariate pattern analysis in cognitive neuroscience. Br. J. Philos. Sci 70 (2), 581–607. 10.1093/bjps/axx023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA, 2008. From fear to safety and back: reversal of fear in the human brain. Journal of Neuroscience 28 (45), 11517–11525. 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Ronkin EG, Jarcho JM, Mash JA, Michalska KJ, Leibenluft E, Pine DS, 2015. Fear conditioning and extinction in anxious and nonanxious youth and adults: examining a novel develop mentally appropriate fear-conditioning task. Depress. Anxiety 32 (4), 277–288. 10.1002/da.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Fox NA, Mash JA, Jarcho JM, Chen G, Leibenluft E, Britton JC, 2018. Differences in neural response to extinction recall in young adults with or without history of behavioral inhibition. Dev. Psychopathol 30 (1), 179–189. [DOI] [PubMed] [Google Scholar]
- Shechner T, Hong M, Britton JC, Pine DS, Fox NA, 2014. Fear conditioning and extinction across development: evidence from human studies and animal models. Biol. Psychol 100, 1–12. 10.1016/j.biopsycho.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Jarcho JM, Wong S, Liebenluft E, Pine DS, Nelson EE, 2017. Threats, rewards, and attention deployment in anxious youth and adults: an eye tracking study. Biol. Psychol. 122, 121–129. https://doi.org/10.10l6/j.biopsycho.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard RN, 1987. Toward a universal law of generalization for psychological science. Science 237 (4820), 1317–1323. 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M, 2015. Retrieval cues that trigger reconsolidation of associative fear memory are not necessarily an exact replica of the original learning experience. Front. Behav. Neurosci 9, 122 10.3389/fnbeh.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW, 2003. Mapping cortical change across the human life span. Nat. Neurosci 6 (3), 309–315. 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Struyf D, Zaman J, Hermans D, Vervliet B, 2017. Gradients of fear: how perception influences fear generalization. Behav. Res. Ther 93, 116–122. 10.1016/j.brat.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, Eelen P, 2005. Return of fear in a human differential conditioning paradigm caused by a return to the original acquistion context. Behav. Res. Ther 43 (3), 323–336. 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Visser RM, Scholte HS, Beemsterboer T, Kindt M, 2013. Neural pattern similarity predicts long-term fear memory. Nat. Neurosci 16 (4), 388–390. 10.1038/nn.3345. [DOI] [PubMed] [Google Scholar]
- Wang L, Mruczek REB, Arcaro MJ, Kastner S, 2015. Probabilistic maps of visual topography in human cortex. Cerebr. Cortex 25 (10), 3911–3931. 10.1093/cercor/bhu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D, 1999. Weschler Abbreviated Scale of Intelligence (WASI). Psychological Corporation, London. [Google Scholar]
- Zadra JR, Clore GL, 2011. Emotion and perception: the role of affective information. Wiley Interdisciplinary reviews: Cognit. Sci 2 (6), 676–685. 10.1002/wcs.147. [DOI] [PMC free article] [PubMed] [Google Scholar]


