Abstract
As vectors of malaria, dengue, zika, and yellow fever, mosquitoes are considered one of the more severe worldwide health hazards. Widespread surveillance of mosquitoes is essential for understanding their complex ecology and behaviour, and also for predicting and formulating effective control strategies against mosquito-borne diseases. One technique involves using bioacoustics to automatically identify different species from their wing-beat sounds during flight. In this dataset, we collect sounds of three species of mosquitoes: Aedes Aegypti, Culex Quinquefasciatus & Pipiens, and Culiseta. These species were collected and reproduced in the laboratory of the Natural History Museum of Funchal, in Portugal, by entomologists trained to recognize and classify mosquitoes. For collecting the samples, we used a microcontroller and a mobile phone. The dataset presents audio samples collected with different sampling rates, where 34 audio features characterize each sound file, making it is possible to observe how mosquito populations vary heterogeneously. This dataset provides the basis for feature extraction and classification of flapping-wing flight sounds that could be used to identify different species.
Subject terms: Ecological epidemiology, Malaria
| Measurement(s) | Sound • audio feature |
| Technology Type(s) | bioacoustic sensing • audio board • Microphone Device • sensor • mobile phone |
| Factor Type(s) | species of mosquito • sampling rate |
| Sample Characteristic - Organism | Aedes aegypti • Culex pipiens x Culex quinquefasciatus • Culiseta |
| Sample Characteristic - Location | Madeira |
Machine-accessible metadata file describing the reported data: 10.6084/m9.figshare.13034597
Background & Summary
Mosquitoes (Culicidae) are a hazard for millions of people worldwide since they act as vectors for life-threatening pathogens and parasites. Monitoring and predicting mosquito abundance over time, identifying the main determinants of the mosquito population, and assessing control strategies are fundamental activities for implementing timely and effective policies to prevent the spread of vectors. The methods and tools for collecting ecological and behavioral data rely traditionally upon labour-intensive techniques such as sampling of immature stages from breeding sites, systematic search and collection of resting adults, or using sticky ovitraps that detect ovipositing females through their eggs. These methods are slow, often taking weeks to complete, and lack epidemiological sensitivity, in that they are unable to effectively detect host-seeking adults, which is the crucial indicator of risk for disease transmission.
With the widespread availability of low-cost IoT devices and developments in machine learning techniques, bioacoustics is becoming an up-and-coming technique for biodiversity monitoring. Recent work presented bioacoustic solutions based on mobile phones1 and low-cost IoT devices2 for detecting and classifying spatial and temporal metadata for species identification. The availability of training datasets is crucial for any effective acoustics-based technique to identify and classify wingbeat sounds automatically.
In this study, we present the work that motivated the creation of the bioacoustics dataset and some background on mosquitoes species3. We begin by presenting some classical approaches and methods to study mosquitoes, and then discuss the technical approaches for solving similar problems through bioacoustics sensing. Finally, we analyze how the data were evaluated in these preliminary studies, mostly based on the fundamental frequency.
Previous work recorded flight tones of Aedes Aegypti mosquitoes (31 males and 28 females) and concluded that the frequency of male wingbeats was higher than females (982 - 721 Hz vs 664 - 514 Hz)4,5. These frequency ranges can be influenced by body size, age and temperature6. According to a study by Unwin and Corbet in 19847, wingbeat frequency changes proportionally to temperature. Where they presents an analysis taking into account different capture angles in quiet environments. The main disadvantage of sex classification using the fundamental frequency arises when the mosquitoes are in the breeding phase. Here - the frequency of wingbeats of females is more similar to that of males8–10 and are thus very hard to distinguish based on that metric alone.
Another interesting approach to study the mosquitoes behavior by Batista et al. was to use an inexpensive optical sensor to classify insects throughout the day for three types of mosquitoes automatically. The prediction is based on the period of most significant daily activity, which was mapped from real distributions available in the literature based on the wingbeats frequency11, although this sensor has great accuracy, its range is reduced, being necessary to force the mosquito passing through the laser. The classification approach was successfully applied in the wild in2 to identify different species automatically.
More recently in12, the k-nearest neighbour criterion was used to compute the unknown fundamental frequency distance for each individual mosquito, and identify them using a Bayesian classifier13. The underlying dataset is presented in14. Others have also used machine learning methods15,16 with both temporal and spectral features. One crucial research question for mosquitoes bioacoustic identification is to what extent the changes induced by environmental conditions (location, temperature, time of day, humidity, air density, etc.) impact the pattern recognition algorithms. The availability of public datasets collected in different geographic and environmental conditions is critical to understanding how these issues affect recognition algorithms.
Finally, none of the published datasets includes environmental noise (e.g. wind or ambient noise), which is essential to fully characterize mosquitoes in real world scenarios. Here, we present a dataset with 34 features and different sampling rates that can be used to define a strategy to deal with different environmental factors, to find patterns, or to facilitate efficient audio fingerprinting for each species.
The mosquitoes were caught in the field, and all features have been collected in a laboratory, in the Madeira archipelago, located around 1,000 km from mainland Portugal and around 500 km from the north African coast. The island’s Mediterranean climate exhibits little temperature variation throughout the year.
Methods
We conducted a laboratory study in the facilities provided by the Natural History Museum of Funchal (Mosquito Lab). Three species of mosquitoes were recorded to determine their dominant frequencies and spectral behaviors. The species used for this collection and study were A. Aegypti, C. Quinquefasciatus & Pipiens and Culiseta, which came from a lab colony established from captures collected in Funchal city in 2019.
The mosquitoes were kept in an environmental room simulating natural conditions, with 60 ± 10% relative humidity and temperature of 20–25 °C. Mosquitoes were housed individuals in boxes (25 × 25 × 25 cm) covered with a mesh cap. They were fed with 20% sucrose solution supplemented with 1 g aquarium fish food mixed daily from the brand “Sera Guppy Gran”. The duration of the study was approximately 48 days. All mosquitoes used in these experiments were 7–25 days old. For the recording process, sensors were incorporated into the boxes and the tests conducted on 12–18 specimens for Aedes Aegypti, 7–12 specimens for Culex and 4 specimens for Culiseta. The duration of the extracted sequences ranged from 0 to 300 ms. To generate samples closer to real-world acquisition conditions we added environmental noise in some mosquito samples.
Uncompressed audio of real sound waves was converted to digital format without any further processing. This means that recordings are exact copies of the source audio, recorded in WAV files.
The acoustic sensor uses a low-noise omnidirectional microphone capsule2. The microphone converts sound into electrical signals with a specific signal to noise ratio (80 dB), self-noise, and residual noise. All these parameters influence the quality of the acquired sound.
Noise can be a significant problem when acquiring physical signals as voltages. Signal smoothing attempts to capture the essential information in the signal while leaving out the noise. This is done by interpolating the raw signal to estimate the original one17.
To collect samples, we used three devices: one of them was our prototype comprising a Teensy 3.2 audio board, microphone and environmental sensor for 44.1 kHz sampling rate. The other two were general-purpose smartphones (Huawei P20 Lite and IPhone 4) used to record samples with a 8 and 48 kHz sampling rate, respectively.
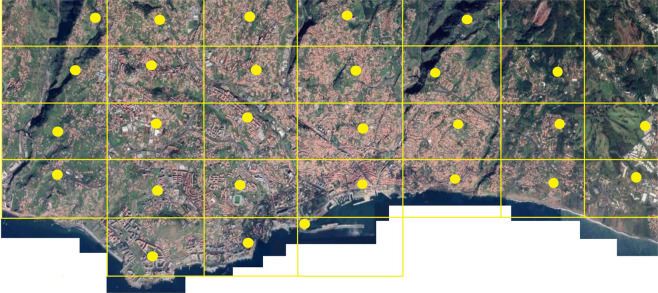
To start a colony for our experience, we installed traps and buckets of water to catch eggs and adult mosquitoes. The female Aedes mosquitoes require a blood meal before each egg-laying18. The eggs are deposited individually on the inner walls of any container capable of storing water. This work was conducted jointly with the Natural History Museum of Madeira and IASaude (the regional health authority of Madeira islands) as part of a plan to control the spread of mosquitoes in the city of Funchal (Fig. 1).
Fig. 1.
Location and number of traps in the city of Funchal, Madeira, Portugal.
A. Aegypti mosquitoes, lay the most eggs in the velcro tape, while Culex and Culiseta prefer to lay directly in rafts on still water or in other substances19. Traps with a ventilation system were also used to capture adult mosquitoes, especially Culex and Culiseta.
Figure 2 shows the procedure from egg collection to mosquito germination, and also the boxes that are used for further acquisition of sound samples. It is noteworthy that after 25–30 days the mosquitoes die due to the conditions imposed in the study.
Fig. 2.
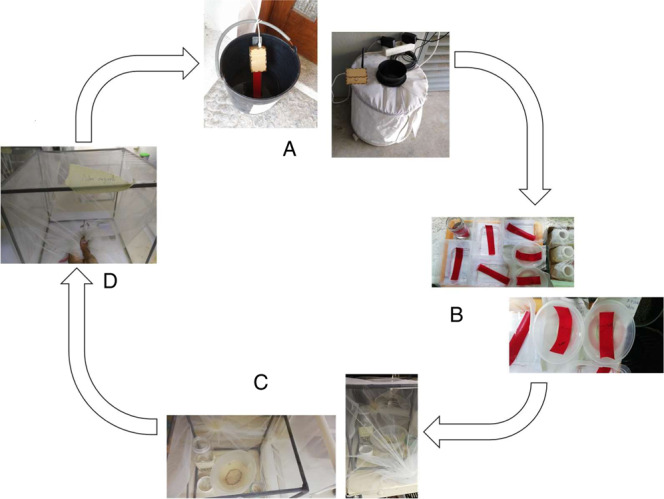
Procedure for collecting audio samples for different species.
Step A comprises the gathering of eggs and mosquitoes. The figures show a bucket inside which mosquitoes lay eggs on a velcro tape, and also a trap. These traditional methods allow a fine assessment of the distribution of mosquito populations over time and space (periodically summarized in epidemiological bulletins). In step B the collected eggs are germinated to create a colony. Then, (step C) mosquitoes are placed in boxes and fed with a sugar solution and fish food20. Finally, in step D, audio samples are collected by the devices: mobile phones and low-cost IoT. This procedure is repeated when the colony dies after 25 days, starting from step B.
Audio was recorded inside boxes (25 × 25 × 25 cm) where the mosquitoes were located at a maximum distance of 27 cm from the microphone placed in the center of the box. The signal amplitude fluctuates significantly over time as the mosquitoes in free flight approach the microphone or move away.
Continuous recordings were then split into 300 millisecond (ms) snippets. Since mosquitoes have a very short flight, it was necessary to apply a slight stimulus on the wall of the boxes (covered by a net) to force them to fly.
To analyze each mosquito recording, 34 features were extracted taking into account several parameters of the signal belonging to three different domains: time (1–3), frequency (4–8, 22–34) and cepstrum (9–21), analyzed below in the Technical Validation section21,22.
These features are often used for speech signal classification, but are useful when handling non-speech signals as well. They enable a comprehensive analysis of the mosquito sounds in terms of amplitude, energy, zero crossing rate, power, frequency variation in the audio file, tonality, loudness, etc. The features are included in the dataset23 and their computation is demonstrated in the Code Availability section.
Data Records
The files are organized by folders, where the main folder is the name of the mosquito species, with sub-folders organized by sample rate. Inside the sub-folders, and for different sampling rates (8, 44.1 and 48 kHz) of each species, are the associated 300 ms sound snippets and a CSV file identifying each snippet file and its 34 features mentioned above. The diagram presented in Fig. 3 exemplifies how the data are distributed and organized.
Fig. 3.
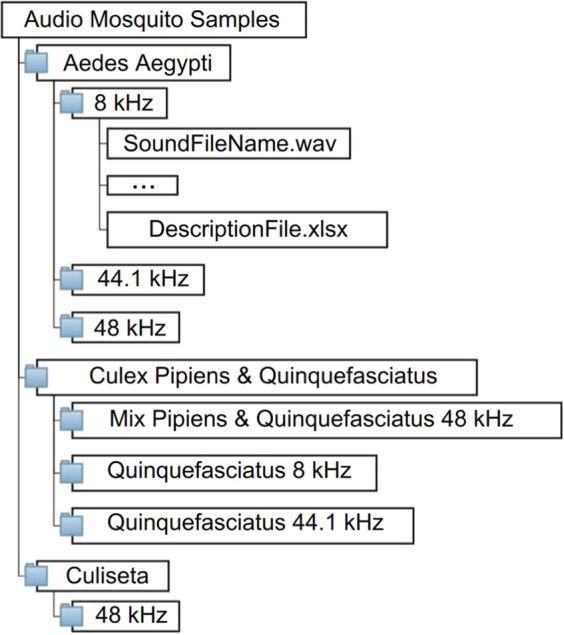
File organization of the dataset.
The diagram provides an overview of the data files contained in each folder and their formats. The dataset is in the figshare repository, where it is possible to find the insect statistics, bioacoustic recordings and the wing-beat frequency23. Table 1, indicates the number of samples for each species.
Table 1.
Number of features for each species and corresponding sampling rate!.
| Aedes Aegypti | Mix Pipiens & Quinquefasciatus | Culex Quinquefasciatus | Culiseta | |
|---|---|---|---|---|
| 8 kHz | 1 697 | — | 867 | — |
| 44.1 kHz | 89 | — | 17 | — |
| 48 kHz | 90 | 156 | — | 72 |
Technical Validation
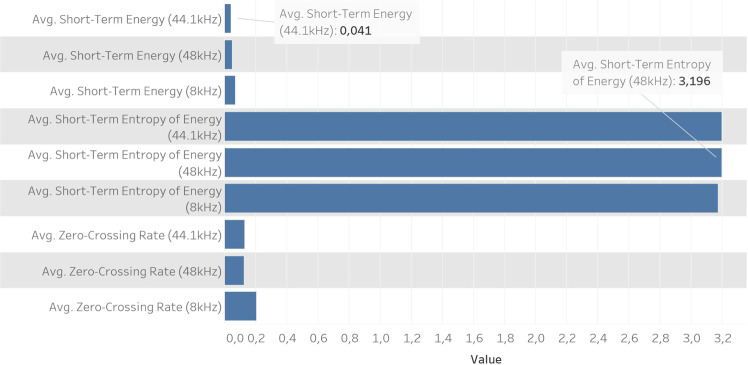
In this section, we present a structural analysis to support the interpretation of the dataset. This analysis is conducted through 4 fields, based on metrics derived in the time-domain, frequency-domain, cepstral-domain/MFCCs, and Chroma Vector. Numerical results are given for Aedes Aegypti only, as the statistics are similar for the remaining species. However, each species exhibits a distinctive pattern in terms of frequency and spectrum. Figure 4 shows the average for the three time-domain features, all having a weak dependence on the sampling rate. The zero-crossing rate represents the number of sign-changes of the signal for one complete frame; the energy is the sum of squares of the signal normalized by its length and the Entropy of Energy can be interpreted as a measure of abrupt changes. Using a sampling rate of 8 kHz we have a higher average for Short-Term Energy and Zero-Crossing Rate, however for the Short-Term Entropy of Energy the highest value is attained at 48 kHz.
Fig. 4.
Comparison between time-domain features for 8 kHz, 44.1 kHz & 48 Khz sampling rates.
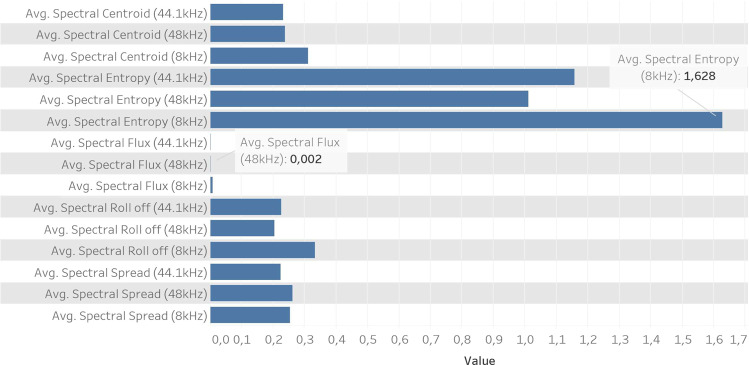
For the second set of features, computed in the frequency domain, we have a different behavior, which is strongly influenced by the sampling rate. The first feature is the center of gravity, which is related to the impression of brightness of the sound, and the second feature captures the peakiness of a distribution (normalised spectral energy for a set of sub-frames). The Roll Off feature quantifies the frequency below which 90% of the magnitude is concentrated. The last feature quantifies the average spread of the spectrum relative to its centroid. A high spectral spread represents a noisy sound, making it challenging to extract useful information. Figure 5 outlines the spectral features, where spectral entropy and Roll Off present a higher average for 8 kHz sampling rate. The average of Spectral Flux attains the lowest value of these features and represents the square difference between the normalized magnitudes of the spectrum for two consecutive frames.
Fig. 5.
Comparison between 5 spectral features for 8 kHz, 44.1 kHz & 48 kHz sampling rates.
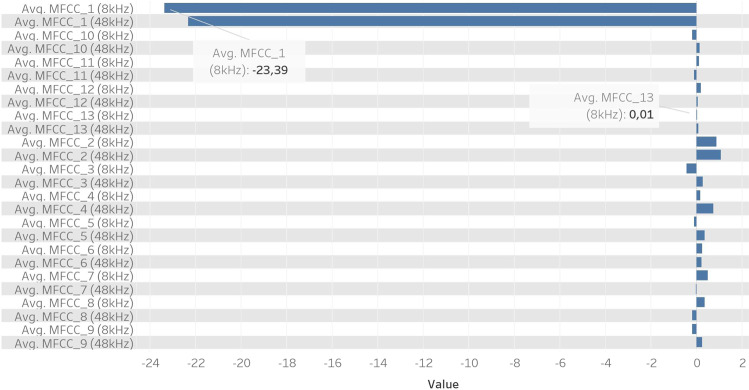
Figure 6 presents the analyzed Mel Frequency Cepstral Coefficients (MFCC) over a set of frequency bands that are distributed non-linearly according to the Mel scale. Note the strong general dependence of these average coefficients on the sampling frequency, with several algebraic sign reversals between 8 kHz and 48 kHz. Results for 44.1 kHz are omitted in the interest of space, as they are almost identical to those for 48 kHz. The highest absolute value is attained for MFCC_1 (8 kHz) and the lowest one for MFCC_13 (8 kHz).
Fig. 6.
Comparison between 13 MFCCs features for 8 kHz & 48 kHz sampling rates.
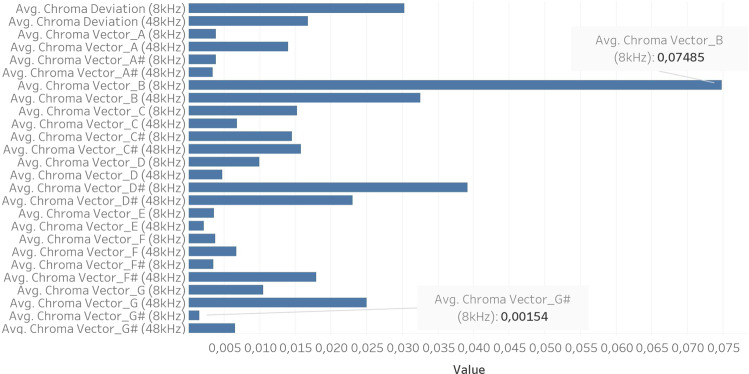
The last group of features analyzed are the Chroma Vectors. They have 12 elements that represent the harmonic and melodic content of the sound. These have the most discrepant values for different sampling rates, as shown in Fig. 7. Chroma Vectors A, C#, F, F#, G and G# attain the highest values for a sampling rate of 48 kHz. The most distinctive feature is Chroma Vector B at 8 kHz, with a value of 0.07485. The standard deviations of the Chroma vectors for 8 kHz are almost doubled when compared to those for 48 kHz.
Fig. 7.
Comparison between 12 Chroma Vectors and Chroma Deviation features for 8 kHz & 48 kHz sampling rates.
Usage Notes
The dataset can be publicly accessed through a free and open-source platform for broad dissemination and use23, where the data are organized in folders as described in the Data Records section.
Researchers can reuse the data downloading it as a zip archive. In addition to the audio files, a CSV database is associated with it, to make it possible to check the characteristics of each sample for each type of mosquito using temporal, frequency, spectral and MCFFs features.
To reproduce the representative features computed from WAV files, readers can use and replicate the algorithm listed in the code availability section. For that they need to install the open-source Anaconda Distribution platform with python 2.7 or 3 version and the following packages: numpy, matplotlib, scipy, sklearn, hmmlearn, simplejson, eyed3, pydub and glob.
Box 1: Algorithm for supervised segmentation using Ipython.
-
from pydub import AudioSegment
2:
newAudio = AudioSegment.from_wav(“Uninterrupted_Filename.wav”)
▷ ms
time_slip = 300
all_time = len(newAudio)
-
number_of_files = len(newAudio)/time_slip
8:
number_file = 0
for i in range(number_of_files + 1):
t1 = i*time_slip
t2 = (i + 1)*time_slip
-
new Audio = newAudio[t1:t2]
14:
▷ Exports to a wav file in the current path
newAudio.export(“filename_“ + str(number_file) + “.wav” format = “wav”)
-
number_file = number_file + 1
18:
Box 2: Algorithm to reproduce the features and csv file using Ipython.
import numpy, glob, csv
-
from pyAudioAnalysis import audioFeatureExtraction as aF
3:
-
▷ Extract the features and species name
5:
[features, classNames, fileNames] = aF.dirsWavFeatureExtraction([’Folders of each species’], 0.3, 0.15, aT.shortTermWindow, aT.shortTermStep, computeBEAT = False)
▷ Deletion of files that have NaN features
for f in features:
fTemp = []
for i in range(f.shape[0]):
temp = f[i,:]
if (not numpy.isnan(temp).any()) and (not numpy.isinf(temp).any()):
fTemp.append(temp.tolist())
else:
print “NaN Found! Feature vector not used for training”
features2.append(numpy.array(fTemp))
-
features = features2
18:
print’Number of species:’ + str(len(features))
print’Number of files of each species:’ + str(len(features[“id of each species”]))
▷ Features
namFeatures = [’zero crossing rate’,’short-term energy’,’short-term entropy of energy’,’spectral centroid’,’spectral spread’,’spectral entropy’,’spectral flux’,’spectral rolloff’,’MFCCs_1’,’MFCCs_2’,’MFCCs_3’,’MFCCs_4’,’MFCCs_5’,’MFCCs_6’,’MFCCs_7’,’MFCCs_8’,’MFCCs_9’,’MFCCs_10’,’MFCCs_11’,’MFCCs_12’,’MFCCs_13’,’Chroma Vector_A’,’Chroma Vector_A#’,’Chroma Vector_B’,’Chroma Vector_C’,’Chroma Vector_C#’,’Chroma Vector_D’,’Chroma Vector_D#’,’Chroma Vector_E’,’Chroma Vector_F’,’Chroma Vector_F#’,’Chroma Vector_G’,’Chroma Vector_G#’,’Chroma Deviation’]
▷ Print all the wave files
print(glob.glob(“Folders of each species/*.wav”))
▷ Creating csv file
with open(’Specie.csv’, mode = ’w’) as csv_file:
writer = csv.DictWriter(csv_file, fieldnames = namFeatures)
writer.writeheader()
csv_writer = csv.writer(csv_file, delimiter = ’,’, quotechar = ’“’, quoting = csv.QUOTE_MINIMAL)
for i in range(34):
csv_writer.writerow(features[0][0:len(features[0]),i])
Acknowledgements
We wish to acknowledge the Natural History Museum of the Municipality of Funchal and IASaude, the regional health authority of Madeira Islands, for facilitating their infrastructures and materials to gather this data. This research was funded by PTDC/CCI-CIF/32474/2017- LARGESCALE.
Author contributions
D.V. conducted the annotation, data preparation, upload, conducted baseline experiments, conception of the study and wrote the manuscript. N.N. Critical review, design of data sharing plan, supervised the annotation, advised on technical aspects of the project and wrote the manuscript. J.G. annotated, reviewed the manuscript and advised on technical aspects of the project. All authors read and approved the final manuscript for publication.
Code availability
Box 1 describes the algorithm to segment the audio (WAV file) into snippets of 300 ms. Supervised segmentation is a critical process for most of the audio analysis applications, its purpose being to split an audio stream into homogeneous segments.
We used the pyaudioanalysis library to generate and extract 34 features, represented in Box 2. This is an open python library that provides audio related functionalities such as: audio features, visualization, classification and segmentation22.
Box 2 shows the function to create, extract and manipulate the 34 features. Both algorithms can be found in the Github repository24.
We used the library audio feature extraction from pyaudioanalysis using the method dirWavFeatureExtraction() to extract the short-term feature sequences of WAV files (one audio signal per specimen), using a sliding window with a time overlap of 50% (frame size of 300 ms and frame step of 150 ms). The resulting 34-element feature vector for each audio file is extracted by mid-term averaging the short-term features.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dinarte Vasconcelos, Email: dinarte.vasconcelos@tecnico.ulisboa.pt.
Nuno Jardim Nunes, Email: nunojnunes@tecnico.ulisboa.pt.
João Gomes, Email: jpg@isr.tecnico.ulisboa.pt.
References
- 1.Mukundarajan H, Hol FJH, Castillo EA, Newby C, Prakash M. Using mobile phones as acoustic sensors for high-throughput mosquito surveillance. Elife. 2017;6:e27854. doi: 10.7554/eLife.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasconcelos, D., Nunes, N., Ribeiro, M., Prandi, C. & Rogers, A. Locomobis: a low-cost acoustic-based sensing system to monitor and classify mosquitoes 16thAnnu. Consum. Comm. Network. Conf. 1–6 (2019).
- 3.Gillott, C. Entomology (Springer Science & Business Media, 2005).
- 4.Arthur BJ, Emr KS, Wyttenbach RA, Hoy RR. Mosquito (aedes aegypti) flight tones: Frequency, harmonicity, spherical spreading, and phase relationships. J. Acoust. Soc. Am. 2014;135:933–941. doi: 10.1121/1.4861233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su MP, Andrés M, Boyd-Gibbins N, Somers J, Albert JT. Sex and species specific hearing mechanisms in mosquito flagellar ears. Nat. Commun. 2018;9:3911. doi: 10.1038/s41467-018-06388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villarreal SM, Winokur O, Harrington L. The impact of temperature and body size on fundamental flight tone variation in the mosquito vector Aedes aegypti (Diptera: Culicidae): implications for acoustic lures. J. Med. Entomol. 2017;54:1116–1121. doi: 10.1093/jme/tjx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unwin D, Corbet SA. Wingbeat frequency, temperature and body size in bees and flies. Physiol. Entomol. 1984;9:115–121. doi: 10.1111/j.1365-3032.1984.tb00687.x. [DOI] [Google Scholar]
- 8.Downes JA. The swarming and mating flight of Diptera. Annu. Rev. Entomol. 1969;14:271–298. doi: 10.1146/annurev.en.14.010169.001415. [DOI] [Google Scholar]
- 9.Patil PB, et al. Mating competitiveness and life-table comparisons between transgenic and Indian wild-type Aedes aegypti L. Pest Manag. Sci. 2015;71:957–965. doi: 10.1002/ps.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements, A. N. et al. The biology of mosquitoes. Volume 2: sensory reception and behaviour (CABI publishing, 1999).
- 11.Batista GE, Hao Y, Keogh E, Mafra-Neto A. Towards automatic classification on flying insects using inexpensive sensors. 10th Int. Conf. Mach. Learn. Applicat. 2011;1:364–369. [Google Scholar]
- 12.Ravi, P., Syam, U. & Kapre, N. Preventive detection of mosquito populations using embedded machine learning on low power IoT platforms. Proc. 7th Annu. Symp. Comput. Dev. 3 (2016).
- 13.Evangelista, I. Bayesian Wingbeat Frequency Classification and Monitoring of Flying Insects Using Wireless Sensor Networks. Reg. 10 Conf. 2403–2407 (2018).
- 14.Batista, G. E., Keogh, E. J., Mafra-Neto, A. & Rowton, E. SIGKDD demo: sensors and software to allow computational entomology, an emerging application of data mining. Proc. 17th Int. Conf. Knowl. Discovery Data Min. 761–764 (2011).
- 15.Rahuman AA, Veerappan DJ. Flying Insect Identification Based on Wing-beat Frequency using Modified SVM Classifier. Int. J. Res. Anal. Rev. 2018;5:337–342. [Google Scholar]
- 16.Lu L, Zhang H-J, Li SZ. Content-based audio classification and segmentation by using support vector machines. Multimed. Syst. 2003;8:482–492. doi: 10.1007/s00530-002-0065-0. [DOI] [Google Scholar]
- 17.Savitzky A, Golay MJ. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964;36:1627–1639. doi: 10.1021/ac60214a047. [DOI] [Google Scholar]
- 18.Kim KS, Tsuda Y, Sasaki T, Kobayashi M, Hirota Y. Mosquito blood-meal analysis for avian malaria study in wild bird communities: laboratory verification and application to Culex sasai (Diptera: Culicidae) collected in Tokyo, Japan. Int. J. Res. Anal. Rev. 2009;105:1351. doi: 10.1007/s00436-009-1568-9. [DOI] [PubMed] [Google Scholar]
- 19.Barbosa RM, Regis L, Vasconcelos R, Leal WS. Culex mosquitoes (Diptera: Culicidae) egg laying in traps loaded with Bacillus thuringiensis variety israelensis and baited with skatole. J. Med. Entomol. 2010;47:345–348. doi: 10.1603/ME09241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall-Mendelin S, et al. Exploiting mosquito sugar feeding to detect mosquitoborne pathogens. Proc. Natl. Acad. Sci. 2010;107:11255–11259. doi: 10.1073/pnas.1002040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitrovic D, Zeppelzauer M, Breiteneder C. Features for content-based audio retrieval. Adv. Comput. 2010;78:71–150. doi: 10.1016/S0065-2458(10)78003-7. [DOI] [Google Scholar]
- 22.Giannakopoulos, T. pyAudioAnalysis: An Open-Source Python Library for Audio Signal Analysis. PloS one10 (2015). [DOI] [PMC free article] [PubMed]
- 23.Vasconcelos D, Gomes JP, Nunes NJ. 2020. Mosquitoes Bioacoustic Features - A Public Dataset. figshare. [DOI]
- 24.Vasconcelos, D. Supervised Segmentation. GitHubhttps://github.com/DinarteVasconcelos/Supervised-Segmentation (2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Vasconcelos D, Gomes JP, Nunes NJ. 2020. Mosquitoes Bioacoustic Features - A Public Dataset. figshare. [DOI]
Data Availability Statement
Box 1 describes the algorithm to segment the audio (WAV file) into snippets of 300 ms. Supervised segmentation is a critical process for most of the audio analysis applications, its purpose being to split an audio stream into homogeneous segments.
We used the pyaudioanalysis library to generate and extract 34 features, represented in Box 2. This is an open python library that provides audio related functionalities such as: audio features, visualization, classification and segmentation22.
Box 2 shows the function to create, extract and manipulate the 34 features. Both algorithms can be found in the Github repository24.
We used the library audio feature extraction from pyaudioanalysis using the method dirWavFeatureExtraction() to extract the short-term feature sequences of WAV files (one audio signal per specimen), using a sliding window with a time overlap of 50% (frame size of 300 ms and frame step of 150 ms). The resulting 34-element feature vector for each audio file is extracted by mid-term averaging the short-term features.