Abstract
The high mobility group box (HMGB) protein family consists of four members: HMGB1, 2, 3, and 4. They share similar amino acid sequences and identical functional regions, especially HMGB1, 2, and 3. The homology in structure may lead to similarity in function. In fact, though their targets may be different, they all possess the fundamental function of binding and distorting target DNAs. However, further research confirmed they are distributed differently in tissues and involved in various distinct physiological and pathological cellular processes, including cell proliferation, division, migration, and differentiation. Recently, the roles of HMGB family members in carcinogenesis has been widely investigated; however, systematic discussion on their functions and clinical values in malignant tumors is limited. In this review, we mainly review and summarize recent advances in knowledge of HMGB family members in terms of structure, distribution, biochemical cascades, and specific mechanisms regarding tumor progression. Importantly, the diagnostic, prognostic, and therapeutic value of these proteins in cancers is discussed. Finally, we envisage the orientation and challenges of this field in further studies.
Keywords: biochemical cascades, biological function, carcinogenesis, HMGB, prognosis
Introduction
HMGB protein family members, which are less than 30 kDa in length, include HMGB1, 2, 3, and 4. They can bind to DNA in a structure-dependent manner, regardless of specific sequences.1 HMGB1, 2, and 3, which have similar biochemical structures and share semblable biochemical properties, comprise two HMG-box domains and an acidic tail.2 However, HMGB4 is less conserved at the nucleotide sequence level and is specific to mammals. In addition, HMGB4 contains two HMG-boxes but lacks the acid tail.3,4 Since research into HMGB4 is limited, we thus mainly focus on recent advances in knowledge of HMGB1, 2, and 3 in human cancers.
Despite the high level of similarity in the amino acid sequences of HMGB1, 2, and 3, they share diverse expression patterns in human tissues. HMGB1 and 2 are expressed ubiquitously at high levels during embryonic development,5,6 while HMGB3 is expressed mainly during embryogenesis.7 HMGB4 can be detected only in the developing pancreas and brain.8,9 In adult tissues, HMGB1 is universally regarded as a housekeeping gene for its ubiquitous high expression in whole tissue extract assays.9,10 However, it is reported that HMGB1 is expressed more highly in testis and lymphoid tissues, and lower in the liver and brain, suggesting a role in the differentiation of various tissues.10 HMGB2 is expressed mainly in the thymus and testis.6 HMGB3 is expressed mainly in hematopoietic stem cells,11 and HMGB4 localizes in neural cells and testis.12
These HMGB family members also share similar functions, especially in the nucleus.13 Specifically, box A identifies and binds to non-B-type DNA structures, and then box B distorts target DNAs by lending, looping, or winding them.1 These members all play an important role in the construction of nucleoprotein complexes by changing chromatin structure, which leads to combinations of other factors and the modification of multiple DNA.14,15 Still, the difference in expression patterns of HMGB family members indicates their possible different roles in various biological processes. For example, HMGB1 participates widely in both embryonic development and adult tissue formation, while other members play a relatively redundant role.4
HMGB family members can participate in different pathological progressions, including inflammation,16 disorders of immunological system,17 and carcinogenesis.18 Accumulating studies have reported their significant roles in various cancers, including hepatocellular cancer (HCC),19 colorectal cancer (CRC),18 and lung cancer.20 Evidence has also revealed that HMGB family members are widely involved in cellular processes of tumor cells, including proliferation,21 metastasis,22 autophagy,23 apoptosis,18 and drug resistance.20 In this review, we provide a comprehensive overview of the recent advances in our knowledge of HMGB1, 2, and 3 in human cancers. Particularly, we focus on the potential value of HMGB family members as markers and therapeutic targets in human cancers. We also envisage the challenges and future orientation of this field.
Characteristics of the HMGB family members
Structure
As described above, HMGB1, 2, and 3 are all composed of three structurally and functionally conserved regions.3 The N-terminal A box is a DNA-binding region, which identifies and binds targeted genes in a structure-dependent but sequence-independent manner.1 In detail, the A box identifies and binds to the AT-rich DNA fragment.11 The Central B box can modulate targeted DNAs by bending them and altering their structures.24 The C-terminal tail contains abundant glutamic and aspartic acid residues and it is relatively less investigated.25 However, the diversity in sequence and length of the tail may influence or even determine the distinctive functions of these three members, which remains to be investigated.26 Another research study highlighted the role of the C-terminal acidic tail in the antibacterial activity.27 In a word, these three members are more than 80% identical in amino acid sequence, but their diversity in key sites may lead to different chemical and biological properties.
In terms of primary structure, human HMGB1 has 215 amino acid residues.28 The DNA binding regions (A and B boxes) contain nuclear-emigration signals (NES), while the steady domain is located in the nucleus with the assistance of two nuclear-localization signals (NLS1 and NLS2). Residues 7–74 and 89–108 are reported to be associated with the combination of TLR4 and p53 transactivating domains, while residues 150–183 are responsible for RAGE binding.29 In secondary structure, the two HMGB boxes are composed of three alpha-helices and two loops, which subsequently form an “L” shape whose arms form an angle of 80°.30 The short arm is made up of helix I and helix II, whereas the long arm is constructed by helix III as well as an N-terminal unstructured segment that is in parallel with helix III. The A box has higher alpha helix content and is more positively charged than the B box.31 In tertiary structure, HMGB1 has three cysteine residues at positions C23, C45, and C106, respectively, that are sensitive to the oxidative situation. Their oxidation–reduction status partly determines their characteristics in biological development. Depending on their form, they are categorized as fully reduced HMGB1 (all-thiol), semi-oxidized HMGB1 (disulfide), or terminally oxidized HMGB1.32 In addition to the redox state, different posttranslational modifications (PTMs) can also play important roles in the alteration of chemical structure and biochemical properties. These PTMs include methylation, acetylation, and phosphorylation.32 In the quaternary structure, native HMGB1 exists in homodimer and oligomer form, while acid-extracted HMGB1 does not.33 Indeed, the structure of HMGB1 can be altered when the extracting methods are different.33 In addition, exogenous HMGB1 can bind to other chemicals in the forms of dimer, trimer, tetramer, or oligomer.34
Regarding the structures of HMGB2 and 3, it is universally accepted that they share 80% identity with HMGB1 at the amino acid level and they are each composed of the three special functional regions. HMGB2 is also revealed to be modified by PTMs including phosphorylation by case kinase 2 and acetylation by CREB-binding protein.35,36 Whether HMGB3 could be modified by PTMs is still little known. Unlike HMGB1, few research studies have reported the effects of oxidation–reduction conditions on the structure alteration of HMG2 and 3. Little information can be obtained from current research on the secondary, tertiary, and quaternary structure of HMGB2 and 3. Thus, identifying the specific structure of HMGB2 and HMGB3 is of primary importance for further investigations.
Distribution
The distribution of these three members from the macro perspective was described above. We also confirmed their expression levels in different tumors and corresponding normal tissues via the GEPIA database (http://gepia.cancer-pku.cn/).37 Compared with normal tissues, HMGB1 is highly expressed in CHCL, ESCA, LIHC, and LUSC (Figure S1), while the expression of HMGB2 is higher in BLCA, BRCA, CESC, COAD, ESCA, HNSC, KIRP, LUAD, LUSC, PCPG, READ, STAD, and UCES than in normal tissues (Figure S2). Higher expression of HMGB3 is observed in BLCA, BRCA, CESC, COAD, ESCA, HNSC, KIRP, LUAD, LUSC, READ, STAD, and UCEC compared with normal tissues (Figure S3). The distribution of these three members at the cellular level will be also discussed below.
HMGB1 is active both inside and outside cells.38 In the nucleus, it binds to DNA and serves as an architectural chromatin-binding factor.39 HMGB1 can also translocate to the cytoplasm and act as a cytokine.40 The lysine residues located in A box (aa28–44) and B box (aa179–185) interact together and form NLSs. The control of NLSs is the main mechanism for the translocation of HMGB1 from the nucleus to the cytoplasm. The monomethylation of lysine 42 can limit the HMGB1 from binding DNAs and set it free, resulting in its tendency to translocate into the cytoplasm.41 The acetylation of NLS is also pivotal in the nuclear-to-cytoplasmic translocation of HMGB1.42 As is shown by immunofluorescence, HMGB1 is concentrated in the cytoplasm after acetylation, while a mutant form of HMGB1 is still localized in the nucleus.42 In addition, phosphorylation of serine also plays a significant role in its shuttling from the nucleus to cytoplasm.43 Secretive HMGB1 can either be secreted actively by specific immune cells or released passively when a cell is dead or injured.39,44,45 HMGB1 lacks the leader sequence that plays an essential role in the classical secretion pathway in which endoplasmic reticulum (ER)-Golgi participates.46 In hematopoietic cells, secretory lysosomes are generated to assist the active secretion of HMGB1 previously localized and concentrated in the cytoplasm.28 In non-hematopoietic cells, HMGB1 follows the same rule when it trans-locates from the nucleus to the cytoplasm. Nevertheless, secretory lysosomes are not observed to be employed, and a concrete mechanism has not been elucidated. It is speculated that HMGB1 may cross the plasma membrane with the assistance of membrane transporters.17 The passive release of HMGB1 occurs in necrotic cells but not apoptotic cells. In addition, the engulfment of dying cells by immune cells, including macrophages, can induce the active secretion of HMGB1.47 This indicates the possible correlation and diversity between dying cells and immune cells in the aspect of HMGB1 release or secretion.23 On the one hand, dying cells can activate immune cells to secrete HMGB1. On the other hand, HMGB1 passively released from dying cells or actively secreted from immune cells comes in different modified forms and exerts different functions, which will be depicted in detail below.23,42
Similar to HMGB1, HMGB2 also distributes both intracellularly and extracellularly.48 However, the concrete mechanisms of its localization still need in-depth investigation. As for HMGB3, its extracellular localization and function have not been reported.
Role of HMGB family members in carcinogenesis
HMGB family members have been investigated broadly in the three decades since they were discovered. They are confirmed to take part in different diseases, including inflammatory conditions,4,16 autoimmune disease,17 and cancers.18,20 The roles and mechanisms of HMGB family members in different localizations may be various. HMGB1 can play pivotal roles in the nucleus, in the cytoplasm, on the membrane, and outside the cells.49 In the nucleus, HMGB1 takes part in nucleosome stability,50 nuclear catastrophe,51 nucleosome release,51 DNA bending,52 and gene transcription.53 Cytosolic HMGB1 is highly expressed and co-localized with lysosome protein in tissue types including colon, liver, gastric, and brain,49,54 indicating its significant roles in regulating autophagy in the cytoplasm. Membrane HMGB1 can facilitate cell maturation and repair, including neurite outgrowth,55 activation of platelets,56 and cell differentiation.57 Extracellular HMGB1 can bind to receptors such as RAGE and TLRs, which can activate downstream cascades and then induce inflammation,58 immune disorder,59 and malignant transformation.60–62
Similar to HMGB1, HMGB2 plays different roles according to its localization. For example, nuclear HMGB2 participates mainly in DNA bending and gene transcription.4,63 Extracellular HMGB2 can also bind to those receptors, but its affinity to target receptors and inflammation-inducing capability are relatively lower compared with HMGB1.64 In addition, HMGB2 is reported to facilitate efficient non-viral gene delivery, which is not widely investigated and not reported to occur in the other family members.63 The roles of HMGB2 in the cytoplasm and on the membrane are poorly elucidated.4
HMGB3 is merely confirmed to participate in bending targeted DNAs and assisting gene transcription in the nucleus. Its roles in the cytoplasm, on the membrane, and outside the cell are little known.49 Table 1 presents the roles of HMGB family members in carcinogenesis. An in-depth comprehension of the HMGB family may provide important clues for developing novel biomarkers and targets for cancer management.
Table 1.
Role of HMGB family members in carcinogenesis.
| Hallmarks | HMGB family | Key findings | References |
|---|---|---|---|
| Angiogenesis | HMGB 1 | HMGB1 can promote the expression of neuropilin-1, VEGFA, VEGF receptors -1 and -2 to induce angiogenesis. | van Beijnum et al.65 |
| HMGB1 can promote the expression of PDGF to induce angiogenesis. | van Beijnum et al.65 | ||
| HMGB1 binds to RAGE to activate NF-κB and then induces angiogenesis. | van Beijnum et al.66 | ||
| Metastasis | HMGB 1 | HMGB1 binds to RAGE and mediates EMT via MMP-7, phosphor-NF-kB and Snail. | Zhu et al.67 |
| HMGB1 binds to RAGE and mediates EMT via the production of NF-kB, p65, iNOS, MMP-9 and phosphorylation of Rac-1, ERK1/2 and AKT. | Kuniyasu et al.68 | ||
| HMGB1 binds to TLR4 and upregulates downstream MAPK and PI3K signaling pathways. | Sharma et al.69 | ||
| Secreted HMGB1 targets other stromal cells, whose released factors can cause ECM proteolytic degradation. | Gialeli et al.70 | ||
| miR-218 can inhibit the expression of HMGB1 and metastasis. | Zhang et al.71 | ||
| miR-325-3p can suppress the expression of HMGB1 and metastasis. | Yao et al.72 | ||
| miR-142-3p can inhibit the expression of HMGB1 and metastasis. | Xiao and Liu73 | ||
| HMGB 2 | HMGB2 correlates with OCT4 and retains the pluripotent gene expression signature. LncCRCMSL plays as a guide and directs the cytoplasmic maintenance of HMGB2. | Han et al.74 | |
| vIRF1 promotes cell migration by p53- and lnc-OIP5-AS1-mediated downregulation of miR-218-5p, leading to increased expression levels of HMGB2. | Li et al.75 | ||
| miR-329 downregulates HMGB2 via the β-catenin pathway, leading to invasion and metastasis in melanoma. | Mo et al.76 | ||
| HMGB 3 | miR-758 regulates HMGB3, leading to the inhibition of migration, and promotes apoptosis of NSCLC cells. | Zhou et al.22 | |
| miR-513b inhibits the invasion and promotes the apoptosis by negatively targeting HMGB3 and mTOR signaling in NSCLC cells. | Wang et al.77 | ||
| Overexpression of miR-200b targets HMGB3 protein and inhibits HCC cell migration. | Wang et al.19 | ||
| Overexpression of miR-205-5p leads to downregulation of HMGB3 and lower abilities in proliferation and metastasis. | Yamada et al.78 | ||
| miR-532-5p directly targets HMGB3 and downregulates Wnt/β-catenin signaling, inhibiting the proliferation and invasion of bladder cancer cells. | Xie et al.79 | ||
| In CRC, HMGB3 promotes growth and migration by regulating Wnt/β-catenin pathway via c-Myc and MMP-7. | Zhang et al.80 | ||
| Proliferation | HMGB 2 | HMGB2 upregulates the AR-YY1 mediated transcription and interacts with HOX10, contributing to the proliferation in prostate cancers. | Barreiro-Alonso et al.81 |
| HMGB2 transcriptionally regulates LDHB and FBP1 and then regulates the Warburg effect, promoting the proliferation and glycolysis of breast cancer. | Fu et al.21 | ||
| HMGB2 upregulates p53 or potentiates Wnt/β-catenin signaling. This can be suppressed by anti-human EGFR2 antibody via the AKT pathway. | Kwon et al.82 | ||
| HMGB2 activates AKT signaling pathway, resulting in the proliferation of cervical carcinoma. | Zhang et al.83 | ||
| Senescence | HMGB 2 | HMGB2-TOP1cc-cGAS axis regulates SASP and assists cytoplasmic chromatin recognition, enabling response to immune checkpoint blockade. | Zhao et al.84 |
| Biogenesis can stimulate HMGB1’s dsDNA sensing pathway, which can be repressed by p53. | Bianco and Mohr85 | ||
| HMGB2 binds to the SASP gene promoter area, preventing HP1α protein recruitment. | Völp et al.86 | ||
| Drug resistance | HMGB 2 | HMGB2 protein migrates from the cytosol to the nucleus, where it can bind to cis-Pt-DNA adducts in genomic DNA and activates repair system under cisplatin treatment. | van Beijnum et al.87 |
| A complex including HMGB2 and the coactivator SRC-1 binds to the promoter of DDX18, leading to the chemical resistance. | van Beijnum et al.65 | ||
| miR-23b-3p inhibits autophagy via regulating ATG12 and HMGB2 and sensitizes GC cells to chemotherapeutic treatment. | van Beijnum et al.66 | ||
| HMGB2 might be involved in the regulation of p53 and MMP-2/TIMP2 and results in the resistance to TMZ chemotherapy. | Lin et al.88 | ||
| HMGB 3 | HMGB3 deletion attenuates ATR/CHK1/p-CHK1 DNA damage signaling pathway and increases apoptosis and sensitivity to cisplatin. | Xiao and Liu73 | |
| EGb 761 can sensitize 5FUR CRC cells by inhibiting the EMT phenotype and suppressing HMGB3 expression via the Wnt/β-catenin pathway. | Gialeli et al.70 | ||
| Hypoxia | HMGB 3 | HOTTIP deficiency represses glycolysis under hypoxic conditions partly by targeting miR-615-3p/HMGB3 axis in NSCLC cells. | Abraham et al.89 |
| Silence of HMGB3 attenuates HIF1α and inhibits cell proliferation. HMGB3 silence also suppresses the expression of Nanog, SOX2 and OCT-4 genes/proteins. | Barreiro-Alonso et al.81 |
EMT, epithelial-mesenchymal transition; GC, gastric cancer; HMGB, high mobility group box; NSCLC, non small cell lung carcinoma; PDGF, platelet-derived growth factor; PI3K, phosphatidylinositol 3-kinase; TMZ, temozolomide.
HMGB1
HMGB1 is the most investigated member of the HMGB family.18 HMGB1 is involved in the pathogenesis of different tumors, including CRC,18 HCC,90 and lung cancer.20 Several biological and pathological processes toward malignant transformation are proved to be associated with HMGB1, including inflammation,17 dysregulation of immunological system,17 autophagy,23 and migration.67
Role of HMGB1 in inflammatory and immunological response
Multicellular animals can discriminate between self and non-self and distinguish between dead and live cells, keeping the body in homeostasis. Maintenance of the integrity status depends partly on the smooth functioning of the immune system.17 Damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) are the two concepts universally accepted to stimulate the innate and adaptive immune system.91,92 Dysregulation of the immune system will undermine homeostasis and result in the formation of illegal tissues, including tumors.
HMGB1 is one of the best characterized DAMPs.17 DAMPs are molecules that are released passively by dead or dying cells undergoing unscheduled death during cellular stress. They can be recognized by pattern recognition receptors (PPRs), such as RAGE and TLRs.93 The interaction between DAMPs and PPRs leads to the activation of downstream signaling pathways and the dysregulation of inflammatory and immunological responses.93 The negatively charged V-domain of RAGE and the positively charged C-terminal of HMGB1 form this ligand–receptor complex.94,95 Besides, RAGE was confirmed to form a complex with heparan sulfate, a cell-surface proteoglycan, before binding to HMGB1.96 The HMGB1–RAGE, as well as the downstream cascade, is associated closely with cell migratory responses.97 The physicochemical mechanism of the interaction between HMGB1 and TLRs (including TLR2, TLR4, and TLR9) is little known,98,99 although it is accepted that HMGB1 binding to TLR4 relies on its disulfide form.100 The disulfide form of HMGB1 can induce release of the cytokine after interacting with TLR4.100 Fully reduced HMGB1 can also function as a chemoattractant without binding to TLR4, but the concrete receptor remains for identification.101 In addition, HMGB1 can form complexes with abundant TLR ligands, including extracellular DNA material class A cytosine-guanine-rich DNA and the lipopolysaccharide, and these complexes can strengthen the inflammatory responses rather than HMGB1 alone.102 One of the possible reasons is the co-activation and co-operation of both TLR and RAGE.
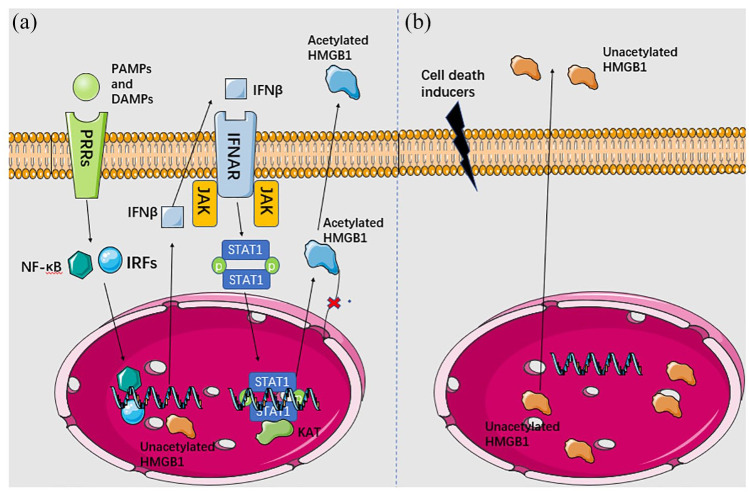
HMGB1 is also a kind of soluble proteins that acts as an alarmin and is actively secreted by immune cells such as macrophages.16 HMGB1’s active secretion or negative release is revealed in Figure 1. Active secretion occurs when cells suffer from severe cell stress, not limited to cell damage, such as ischemia or pathogen infection.42,103 Under these circumstances, macrophages detect the extracellular PAMPs or DAMPs via TLR4, after which NF-kB is activated and interferon regulatory factors (IRFs) are phosphorylated. They function as transcription factors to promote the transcription of interferon-beta (IFN-β) and assist its eventual secretion. Secreted IFNβ then binds to interferon receptors (IFNARs) located on the membranes of adjacent cells, resulting in the stimulation of associated Janus kinases (JAK) and phosphorylation of signal transducer and activator of transcription 1 (STAT1). Phospho-STAT1 dimers translocate into the nucleus and recruit histone acetylases, leading to acetylation of HMGB1.17,42 In this cascade, acetylation prevents HMGB1 from re-entering the nucleus and assists its secretion. This happens in a lysosome-dependent manner in hematopoietic cells or in a lysosome-independent way in non-hematopoietic cells,46 whereas the passively released HMGB1 functioning as DAMP leaks out from dead cells without acetylation or other modification.17
Figure 1.
HMGB1 is secreted actively (a) and passively (b) via different cascades. In active secretion, HMGB1 is acetylated or phosphorylated in the nucleus and then translocated into the cytoplasm, where it can then be secreted via lysosomes in hematopoietic cells or via not confirmed mechanisms in non-hematopoietic cells. In passive release, HMGB1 is released by dead cells confronting with tissue injury. Intracellular HMGB1 exists in the reduced form, while extracellular HMGB1 gradually transforms into fully reduced form. Thin black arrows indicate secretion or activation of the downstream targets.
DAMPs, damage-associated molecular patterns; HMGB, high mobility group box; IFNAR, interferon receptor; IFNβ, interferon β; IRFs, interferon regulatory factors; JAK, Janus kinase; PAMPS, pathogen-associated molecular patterns; PPRs, pattern recognition receptors.
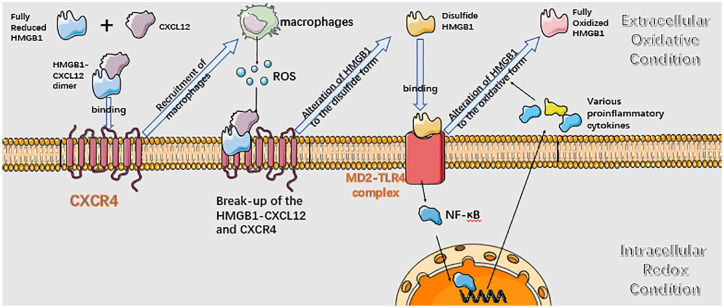
Figure 2 presents the role of HMGB1 in inflammation. Intracellular HMGB1 tends to exist in the reduced state due to the strongly negative reducing redox potential in the cytosol and the nucleus.104,105 The released fully reduced HMGB1 interacts with C–X–C motif chemokine ligand 12 (CXCL12) and forms a heterocomplex, which then binds C–X–C motif chemokine receptor 4 (CXCR4), one 7-transmembrane G-protein-coupled receptor (GPCR).106 CXCR4 can form dimers, multimers, or heterodimers with other GPCRs. The transformation is induced by different ligands, including the CXCL12, CXCL4, HMGB1-CXCL12 heterocomplex.107,108 HMGB1–CXCL12 can promote the recruitment of leukocytes after interacting with CXCR4.17 The reactive oxygen species (ROS) originating from infiltrating leukocytes and the oxidizing condition in the extracellular milieu assists the transformation of reduced HMGB1 to the disulfide form. The disulfide HMGB1 loses the ability to interact with CXCL12 and cannot stimulate CXCR4.105 Instead, it interacts with the lymphocyte antigen 96 (MD2)-TLR4 complex and stimulates activation of NF-kB, inducing the secretion of multiple proinflammatory cytokines and chemokines.100 The oxidizing environment eventually induces the terminal oxidation of HMGB1 to the sulfonyl form, which no longer promotes chemoattractant and proinflammatory activities. More HMGB1 are secreted by tissue-healing phenotype macrophages after inflammation resolution, which contributes to the repair of damaged tissues by stimulating stem cells and promoting angiogenesis.100
Figure 2.
HMGB1 participates in inflammatory processes. HMGB1 exists in redox form after initially being passively released or actively secreted from cells. The redox form of HMGB1 can form a heterocomplex with CXCL12 and this complex binds to CXCR4, and macrophages are recruited after that. Macrophages secrete ROS and transform HMGB1 to disulfide form. The disulfide form of HMGB1 cannot interact with CXCL12 and CXCR. The disulfide HMGB1 binds to MD2-TLR4 complex and activates the NF-kB signaling pathway, proinflammatory cytokines are transcriptionally activated and secreted extracellularly subsequently. The disulfide HMGB1 is eventually oxidized to fully oxidized form and loses inflammation inducing capability. Black arrows indicate secretion or activation of the downstream targets, while blue arrows represent the alterations of the next inflammatory stage.
HMGB, high mobility group box; ROS, reactive oxygen species.
Inflammation is a hallmark of carcinogenesis. It can induce alteration of the microenvironment, which influences the formation of carcinoma. HMGB1 – a protein central to inflammation and injury – is also involved in tumor biology.109 Mesothelioma is the most representative exemplary in which HMGB1-induced inflammation participates.110 Asbestos and other mineral fibers cause mesothelioma. The constant secretion of HMGB1 leads to recruitment of inflammatory cells, resulting in the carcinogenesis of mesothelial cells.111 The prolonged bio-persistence of the mineral fibers leads to the maintenance of HMGB1-induced inflammation condition.112 Although the concrete mechanism is still obscure, a possibility is that HMGB1-induced recruitment of tissue-healing phenotype macrophages assists the survival of cells with malignant transformation, which simply die out under normal conditions.113 The growth of colon cancer secondary lesions in the peritoneum, melanoma, and papilloma also result partly from the constant secreted HMGB1-induced inflammation, similar to the process in mesothelioma.114,115
In this process, necrotic cell death in a tumor will induce the release of HMGB1 and the recruitment of macrophages and neutrophils.116 HMGB1 binds to TLR4 and leads to malignant transformation by generating chronic inflammation. Macrophages and neutrophils assist the survival of these cells, which will be recognized and eliminated in normal tissues.117 From this aspect, HMGB1 is a typical oncogene; however, it can also exert anti-tumor function via the immune system.118 Immunogenic cell death (ICD) is a form of apoptotic death and it occurs when cancer cells are exposed to radiotherapy or chemotherapeutics.119 What triggers ICD is still obscure, but for sure ICD is characterized by the secretion of HMGB1, calreticulin, and ATP.120 Secreted HMGB1 from cells undergoing ICD stimulates the maturation of dendritic cells and the cross-presentation of neoantigens to lymphocytes. B- and T-cell responses are stimulated. The anti-tumor immunological memory is established after that.120 ROS triggered by ICD and TLR4 imply the significant role of disulfide HMGB1 in this anti-tumor process.121
HMGB1 plays a dual role in the inflammatory and immunological response, which can eventually result in tumor formation. The type of release may be one possible reason for its completely contradicting function.122 Chronic release can induce the recruitment of macrophages and alter the microenvironment to be suitable for the malignant transformation, while acute massive release can stimulate dendritic cell (DC) activation and induce ICD via HMGB1-TLR4 interaction, leading to the suppression of carcinogenesis.122 Based on this finding, the method to control its release type and direct its affinity to DC cells can be applied as a therapeutic method.
Role of HMGB1 in autophagy
Autophagy plays a pivotal role in promoting cell survival via aggregating proteins and eliminating damaged organelles by degeneration of the lysosome.9 Recent evidence has shed light on the role of autophagy in assisting the survival of cancer cells by mitigating genome damage and limiting inflammation and necrosis.18,123 HMGB1, widely distributed inside and outside the cell, can induce autophagy through various pathways.124 For example, HMGB1 can function as the transcription factor in the nucleus and enhance the expression of heat-shock protein 27 (HSP 27).125 HSP 27 is phosphorylated by MAPK activated protein kinase 2 (MAPKAPK2) and then is enabled to modulate mitophagy, a cargo-specific kind autophagy of mitochondria,126 through the Pink/Parkin pathway.127 In the cytoplasm, Beclin-1 is validated to participate in the initiation of autophagy.128 The formation of the Beclin-1/phosphatidylinositol 3-kinase-III (PI3K-III) complex by Beclin-1 and class III phosphatidylinositol-3 kinase can recruit Atg proteins to the phagophore.129 The Beclin-1/PI3K-III complex is reduced after binding between Beclin-1 and Bcl-2.130 HMGB1 can promote the phosphorylation of Bcl-2 via the extracellular signal-regulated kinase (ERK)/MAPK pathway, resulting in the dissociation of the Beclin-1/Bcl-2 complex.40 Conclusively, HMGB1 promotes autophagy by maintaining the formation of Beclin-1/PI3K-III complex in the cytoplasm. Extracellular HMGB1 binds to RAGE on adjacent cells and induces autophagy activity.131 It is reported Beclin-1-dependent autophagy is stimulated by the binding of HMGB1 and RAGE. The phosphorylation of ERK1/2 and activation of AMPK/mTOR signaling pathway are confirmed to be involved in the HMGB1/RAGE axis induced autophagy.132,133
Role of HMGB1 in apoptosis
Apoptosis – a programmed form of cell death – is always abrogated during carcinogenesis. Two pathways are clarified underlying apoptosis: the extrinsic pathway and the intrinsic pathway.18 Through the extrinsic pathway, death receptors are activated by external signals such as TNF-α. The adaptor proteins including Fas-associated death domain proteins are recruited by death receptors after that. Downstream activated caspase-8 promotes the expression of caspase-3/-7, therefore provoking the apoptotic cascade.134 While in the intrinsic pathway, the release of different proapoptotic proteins, including Bcl-2 associated agonist of cell death (BAD), Bcl-2 interacting killer (BIK), NOXA, and Bcl-2 modifying factor is triggered by cellular stress. These proteins can stimulate the assembly of the Bcl-2 antagonist/killer 1 (BAK)-Bcl-2 associated X (BAX) oligomers localized on the outer mitochondrial membrane.135 BAK-BAX oligomers will promote the efflux cytochrome c to the cytosol, which activates the recruitment of caspase-9 and then the expression of executioner caspase-3/-7 cascades.135
It is reported that HMGB1 may play a significant role in apoptosis by suppressing pro-apoptotic proteins such as caspase-3 and BAX or promoting anti-proapoptotic proteins including Bcl-2.136 Bcl-2 is responsible for the suppression of the efflux of cytochrome and the pro-apoptotic proteins. Its activity can also be inhibited by ABT-737, which is a negative target of HMGB1.137 Other studies show that HMGB1 can increase the activity of NF-kB and subsequently promote the expression of c-IAP2, another anti-apoptotic protein. In addition, HMGB1 may also suppress the expression of apoptosome caspase-9 and interfere with the apoptotic machinery.86
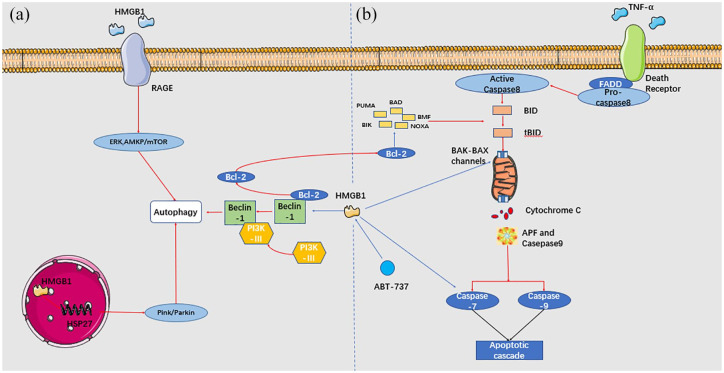
In fact, the roles of HMGB1 in the autophagy and anti-apoptosis are closely related. Figure 3 presents the roles of HMGB1 in autophagy and apoptosis. Disrupting the association between HMGB1 and Bcl-2 may be an effective manner to regulate autophagy and apoptosis, thus repressing the survival of cancer cells. In addition, the redox status of HMGB1 functions as a checkpoint between apoptosis and autophagy. Oxidized HMGB1 results in the apoptosis of cancer cells while reduced HMGB1 is responsible for the activation of autophagy.138 This also indicates another applicable therapeutic method to modify HMGB1 via the oxidation-reduction environment.
Figure 3.
HMGB1 and BCL-2 are involved in autophagy and apoptosis. a. In the nucleus, HMGB1 transcriptionally activates HSP27 and results in autophagy by the Pink/Parkin pathway. In the cytoplasm, HMGB1 leads to the dissociation of Beclin-1-BCL-2 complex. Beclin-1/PI3K-III is formed, which then contributes to the initiation of autophagy. Extracellularly, HMGB1 binds to RAGE and activates downstream ERK and AMPK/mTOR pathway, which induces autophagy. b. HMGB1 exert anti-apoptosis role via the intrinsic and extrinsic pathways. HMGB1 can directly targets BCL-2, BAX–BAK channels, caspase-9, and caspase-3. Besides, HMGB1 indirectly targets cytochrome and pro-apoptotic proteins via BCL-2. Red arrows represent activation of the downstream targets, while blue arrows represent suppression of the downstream targets.
HMGB, high mobility group box.
Role of HMGB1 in angiogenesis
Angiogenesis is another hallmark of cancer. Additional vasculature formation is required for the growth and development of tumors.139 Downregulation of HMGB1 by antibody results in the repression of endothelial cell migration and sprouting.140 Rapid tumor growth leads to low microvessel density and chronic hypoxia, which results in necrotic regions. These necrotic areas tend to overexpress angiogenic growth factors and attract macrophages recruitment and HMGB1 release.87 HMGB1 was found to promote the expression of neuropilin-1, endothelial growth factor A (VEGFA), and VEGF receptors 1 and 2 to induce angiogenesis.65 In addition, platelet-derived growth factor is another angiogenesis inducing factor regulated by HMGB1.65 Interaction between HMGB1 and RAGE leads to activation of NF-kB, thereby upregulating leukocyte adhesion molecules and the production of angiogenetic factors.66 TLR4 is also reported to be activated in HMGB1-mediated neovascularization; however, elucidating the exact mechanism requires further investigation.88
Role of HMGB1 in cancer metastasis
Tumor invasion and metastasis are the most significant hallmarks of malignant tumors. HMGB1, which is involved in various cascades, can also regulate metastasis.18 Epithelial-mesenchymal transition (EMT) is a cellular process in which epithelial cells lose cell–cell contacts and apical–basal polarity and acquire the mesenchymal phenotype. These alterations result in reduced interactions among cells and increased invasive capacities.141 As is reported, HMGB1 can induce EMT in a RAGE-dependent manner. HMGB1 interacting with RAGE can upregulate abundant proteins that exert functions as EMT-inducing molecules, including matrix metallopeptidase 7 (MMP-7), phosphor-NF-kB, and Snail.67 The production of NF-kB/p65, inducible nitric oxide synthase, matrix metallopeptidase 9 (MMP-9), and the phosphorylation of Rac family small GTPase 1 (Rac1), ERK 1/2, and AKT were also reported to be associated with HMGB1/RAGE axis and EMT.68 HMGB1 can also interact with the other main receptor TLR4 and enhance the expression of downstream signaling pathways, such as MAPK and PI3K pathways.69 In addition, certain microRNAs, including, but not limited to, miR-218,71 miR-325-3p,72 and miR-142-3p,73 can suppress the expression of HMGB1 and its metastasis-inducing ability by directly targeting the 3′ untranslated region of HMGB1 mRNA. Secreted HMGB1 can also target other stromal cells such as myofibroblasts within the microenvironment. Activated myofibroblasts subsequently release various matrix metalloproteinases and growth factors. These factors may cause extracellular matrix (ECM) proteolytic degradation and alter the interaction of cell–cell and cell–ECM, which can, in turn, promote cancer cell migration.70
As discussed, HMGB1 is involved in the initiation, development, and progression of tumors. This is consistent with our expectation that it exerts extensive function in the formation of cancer. Paying attention to the expression of HMGB1 and regulating it is advisable in any period during tumorigenesis. Focusing on the alteration of HMGB1 before the cancer is irreversible or untreatable is especially meaningful.
HMGB2
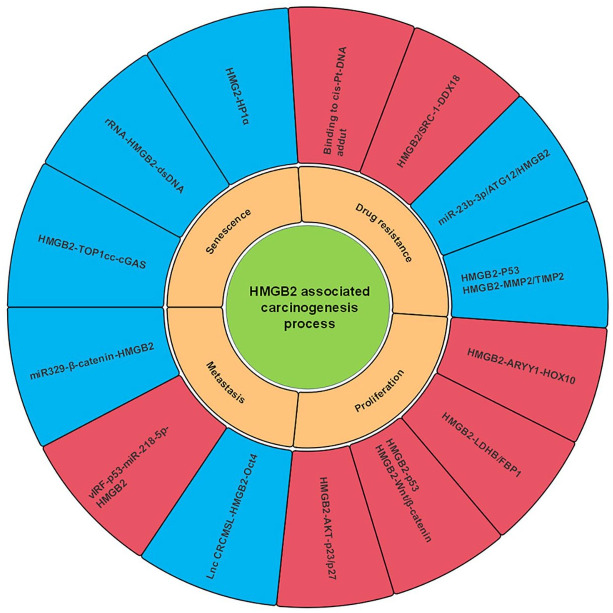
The roles of HMGB2 in carcinogenesis have been gradually elucidated in recent years. HMGB2 plays a role in the chromosomal distorting process in a structure-dependent manner intracellularly like HMGB1.89 Its extracellular effects, either triggering the immunological system or recruiting inflammatory factors are more limited than HMGB1, indicating its lower ability to promote the initiation of carcinogenesis.48 Its roles in carcinogenesis are presented in Figure 4. In addition to proliferation and metastasis, HMGB2 also participates in the senescence and chemotherapy resistance. This suggests that HMGB2 may be mainly involved in the development and progression of tumors.
Figure 4.
Role of HMGB2 in carcinogenesis. This figure reveals that HMGB2 participates in the processes of carcinogenesis via several signaling pathways. The yellow frameworks represent several cellular processes in carcinogenesis. The blue frameworks represent the negative effects of HMGB2 on the corresponding processes, while the red frameworks represent its positive effects.
HMGB, high mobility group box.
Role of HMGB2 in tumor proliferation and metastasis
HMGB2 is reported as an oncogene to induce malignant transformation in various tumors. It can promote the proliferation of different cancer cells, including prostate cancer,81 breast cancer,21 HCC,82 and cervical cancer.83 HMGB2 upregulates the androgen receptor (AR)-YY1-mediated transcription and interacts with HOX10 in prostate cancer and subsequently induces proliferation.81 In breast cancer, HMGB2 transcriptionally regulates glycolytic enzymes lactate dehydrogenase and fructose-bisphosphatase 1 (FBP1) and then regulates the Warburg effect, which provides essential energy for cancer cell’s proliferation.21 Furthermore, HMGB2 can promote p53 or potentiate Wnt/β-catenin pathway, which can be attenuated by anti-human epidermal growth factor receptor 2 antibody via the AKT pathway in HCC.82 HMGB2 can also activate the AKT pathway by phosphorylating AKT and suppress the expression of p21 and p27 in cervical cancer.83 This suggests that AKT and Wnt pathways are closely associated with HMGB2-mediated proliferation of cancer.
HMGB2 also plays a significant role in tumor invasion and migration. Noncoding RNAs including long noncoding RNA (LncRNA) and micro RNA (miRNA) are closely associated with HMGB2-mediated metastasis. For example, in CRC cells, LncCRCMSL mediates the cytoplasmic maintenance of HMGB2 and inhibits downstream EMT signaling.74 To put it another way, HMGB2-mediated EMT could lead to metastasis.74 In human immunodeficiency virus (HIV)-induced Kaposi’s sarcoma (KS), HMGB2 is upregulated by vIRF1/p53 and vIRF1/Lnc-OI5P-AS1 mediated miR-218-5p suppression.75 miR-329 suppresses the expression of HMGB2 via the β-catenin pathway in melanoma.76 miRNAs-mediated HMGB2 suppression is involved in these processes, which can eventually suppress metastasis. In addition to noncoding RNAs, CENPU is another co-expression gene of HMGB2, and together they contribute to the invasion and migration of ovarian cancer cells.142
Role of HMGB2 in senescence
Senescence is a pivotal carcinoma repression process, in which the propagation of cells suffering from insults is limited.143 The termed senescence-associated secretory phenotype (SASP), including different secreted chemokines, cytokines, proteases, and growth factors, is a hallmark of senescence.143 Cyclic GMP-AMP synthase (cGAS) senses cytosolic double-strand DNA (dsDNA) in a sequence-independent manner.144 The cGAS-dsDNA sensing pathway is critical in the regulation of SASP and senescence.145 Cytoplasmic chromatin fragments (CCF) resulted from nuclear membrane blebbing is the main factor to induce cGAS activation.145 DNA topoisomerase 1 (TOP1) forms a stable cleavage complex (TOP1cc) via its enzymatic effect.84 TOP1cc plays a significant role in regulating the recognition of CCF by cGAS. HMGB2 mechanistically stabilizes TOP1cc and subsequently strengthens the binding between cGAS and dsDNA.84 A HMGB2-dsDNA sensing pathway is also reported to be stimulated by rRNA biogenesis.85 The inhibition of rRNA biogenesis can stabilize p53 and suppress HMGB2 expression in a p53-dependent manner.85 HMGB2 is also reported to bind to the SASP gene promoter region directly and prevent heterochromatin protein 1α (HP1α) protein recruitment, subsequently regulating senescence. This effect can be inhibited by ATM-p53-p21 axis.146
Role of HMGB2 in drug resistance
Chemotherapy is always the first-line therapy for various advanced tumors.147 However, several anti-chemotherapy mechanisms, such as autophagy, anti-apoptosis, and immune defense, can compromise the effects of chemotherapy treatment.148 HMGB2 is critically involved in chemotherapy resistance. Under cisplatin treatment, HMGB2 can translate from the cytoplasm into the nucleus, where it binds to cis-Pt-DNA adducts and initiates the repair system, leading to chemoresistance to cisplatin.149 Low HMGB2 is reported as a significant predictor for long-term disease-free survival in breast cancer.150 In the presence of tamoxifen, HMGB2 combines with its coactivator steroid receptor coactivator-1 (SRC-1), and they then bind to the promoter of DEAD-box helicase 18 (DDX18), leading to resistance to tamoxifen.150 Previously, our laboratory found that miR-23b-3p can inhibit autophagy regulated by HMGB2 and sensitize gastric cancer (GC) cells to chemotherapy.151 Furthermore, HMGB2 can also sensitize glioblastoma cells to temozolomide (TMZ) therapy, partly by downregulation of p53 and matrix metalloproteinase 2 (MMP2). However, HMGB2 cannot induce the expression of O-6-methylguanine-DNA methyltransferase (MGMT) protein. Since MGMT is the major factor in the activation in TMZ therapy, this process suggests that HMGB2 may be another independent factor predicting the therapeutic effect of TMZ.65
HMGB3
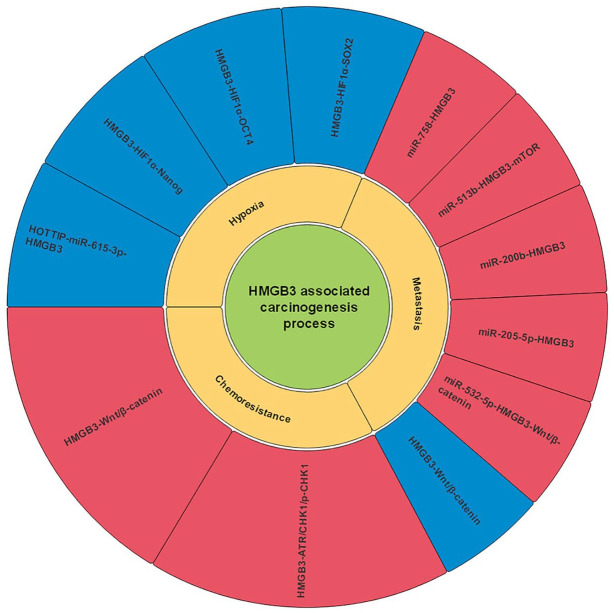
Structurally, HMGB3 shares more than 80% identical amino acids with HMGB1 and HMGB2.152 However, its distribution is limited in embryonic development and adult tissues.152 Like HMGB1 and HMGB2, HMGB3 is also involved in the development and progression of several tumors. Figure 5 presents the roles of HMGB3 in carcinogenesis.
Figure 5.
Role of HMGB3 in carcinogenesis. This figure shows that HMGB3 participates in the processes of carcinogenesis through several signaling pathways. The blue frameworks represent the negative effects of HMGB3 on the corresponding processes, while the red frameworks represent its positive effects.
HMGB, high mobility group box.
Role of HMGB3 in cancer metastasis
HMGB3 can also regulate cancer metastasis, and this process is often modulated by miRNAs. For example, miR-758 and miR-513b can inhibit non-small lung cancer metastasis by targeting HMGB3.22,77 HMGB3 induced metastasis is suppressed by miR-200b and miR-205-5p in HCC and prostate cancer, respectively19,78; while in bladder cancer, miR-532-5p and Wnt/β- catenin signaling pathway can modulate the expression of HMGB3.79 Besides, the Wnt/β-catenin signaling pathway can also suppress HMGB3 via c-Myc and MMP7 in a miRNA-independent manner in CRC metastasis.80
Role of HMGB3 in drug resistance
HMGB3 is also involved in chemotherapy resistance. HMGB3 can transcriptionally upregulate serine/threonine kinases ATR and CHK1 and decrease cell apoptosis, leading to cisplatin resistance via ATR/CHK1/p-CHK1 DNA damage signaling pathway.153 In addition, EGb 761 can suppress HMGB3 expression via the Wnt/β-catenin pathway and improve sensitivity of CRC cells to 5-fluorouracil.154
Role of HMGB3 in hypoxia
Hypoxia, a pivotal character of solid tumors, drives several malignant processes by mediating the metabolic reprograming out of oxidative stress.155 Under hypoxic conditions, HMGB3 is upregulated by HOXA transcript at the distal tip (HOTTIP), and glycolysis is subsequently promoted.155 It was reported that hypoxia-inducible factor 1α (HIF1α) could mediate the upregulation of Nanog, SRY-box transcription factor 2 (Sox2), and organic cation/carnitine transporter 4 (OCT-4).156 Later research revealed that this mediation could be strengthened by HMGB3.157
Clinical significance of HMGB family members in human cancers
HMGB family members are widely involved in carcinogenesis, increasing evidence also indicate that HMGB family members are promising targets for cancer treatment. Moreover, the diagnostic and prognostic roles of HMGB family members are gradually revealed.
Diagnostic and prognostic role of HMGB family members in human cancers
HMGB family members are investigated in numerous studies. The results indicate that its dysregulation is related to the development of several tumors, including CRC,74 HCC,158,159 gastric cancer,160 breast cancer,161 and nasopharyngeal carcinoma.162 In addition, the expression levels of HMGB family members are associated with the prognosis of human cancers. We analyzed the survival rate of patients with high and low expression of HMGB family members in several tumors using the Kaplan–Meier-plotter website (http://kmplot.com/analysis/).163 The results show that overexpression of HMGB1 is associated with poor prognosis in patients with ECA, HNSC, LUAD, PAAD, and SARC, while its overexpression is related to good prognosis in BLCA, SARC, THCA, and THYM (Figure S4). HMGB2 overexpression is significantly correlated with shorter overall survival (OS) in ECA, KIRC, KIRP, LIHC, LUAD, OV, PADD, and THYM, but its overexpression is an indicator for longer OS in BRCA, CESC, HNSC, LUSC, OV, READ, STAD, TGCT, and THCA (Figure S5). Moreover, patients with higher HMGB3 have shorter OS in BRCA, ECA, KIRC, LIHC, PCPG, SARC, THCA, and UCEC, while patients with higher HMGB3 show a longer OS in CESC, KIRP, LUAD, LUSC, OV, TGCT, and THCA (Figure S6).
HMGB1 plays a significant role in carcinogenesis. Evidence in case–control studies showed that the expression of HMGB1 is significantly higher in the patients with HCC,21,82,159 breast cancer,21,161 nasopharyngeal carcinoma,162 and squamous-cell carcinoma of the head and neck,164 indicating its potential in early cancer diagnosis and prognosis judgment. Specifically, higher expression of HMGB1 is observed in HCC patients with a higher rate of vascular invasion.159 In addition, the expression of HMGB1 is higher in breast cancer patients with prolonged metastasis-free survival.161 Moreover, HMGB1 is higher expressed in patients with a superior clinical stage in nasopharyngeal carcinoma and squamous-cell carcinoma of the head and neck.162,164 The expression level of HMGB2 is also reported to be higher expressed in patients with HCC,82 gastric cancer,165 and breast cancer.21 Researchers found that overexpression of HMGB2 is closely associated with shorter OS in HCC,82 advanced gastric cancer,165 and advanced breast cancer.21 HMGB3 is found to be significantly different between carcinoma and peritumoral tissues.160 The concentration of serum HMGB3 is higher in HCC, lower in liver cirrhosis, chronic hepatitis, and the healthy control.158 The positive expression rate of HMGB3 is higher in the gastric cancer than in the peritumoral tissue.160
Clearly, HMGB family members are promising diagnostic and prognostic markers in human cancers. Nevertheless, questions remain to be solved. Firstly, the diagnostic and prognostic role of HMGB family members needs to be investigated in larger-scale case-control or cohort studies. Secondly, the development of fast, effective, and convenient detection techniques and tools are necessary before it can be used in clinical settings.
Therapeutic potential of HMGB family members in human cancers
Many studies highlight the potential role of HMGB family members as novel therapeutic targets for cancer treatment. HMGB1 have been investigated extensively in the pre-clinical stage,166 although the therapeutic value of HMGB2 and HMGB3 is still obscure because their carcinogenic mechanisms have not been systematically elucidated. Here, we will mainly review and summarize the therapeutic potential of HMGB1 in cancers. In addition, we envisage the future investigation of therapeutic strategies regarding HMGB family members.
Given that HMGB1 can play a dual role in carcinogenesis, current fundamental therapeutic methods targeting HMGB1 are different. Repressing its oncogenic function and stimulating its tumor-repressing effect are both theoretically applicable. Basic research on this hypothesis has been performed extensively, while clinical studies are still limited.166 We can achieve the former strategy by reducing the release of HMGB1 or disrupting its connection with molecules or receptors that strengthen the process of carcinogenesis.166 Based on this consideration, sodium salicylate and ethyl pyruvate (EP) were confirmed to switch the glucose deprivation-induced necrosis to autophagy,166,167 resulting in the repression of HMGB1 release and prevention of tumor development. EP was also reported to suppress the release of HMGB1 via the NF-kB signaling pathway,168 and nicotine could also inhibit NF-kB signaling and HMGB1 release by binding to the α7-nicotine acetylcholine receptor (nAChR).46 Besides, antibodies to HMGB1 have been shown to block the interaction between HMGB1 and RAGE in animal models. Inflammation and carcinogenesis are repressed afterward.169 In addition, targeting RAGE is another strategy to prevent the HMGB1–RAGE interaction. This strategy includes the administration of extracellular, soluble domain of RAGE (sRAGE), which is ligand-binding, and preformation of transfected C6 glioma expressing sRAGE or RAGE mutant lacking the cytosolic tail.170
To activate the tumor-suppressing effect of HMGB1 is to strengthen its ICD-inducing capability. In fact, HMGB1 stimulating the immunological system is one of the main causes of radiotherapy and chemotherapy to inhibit the development of carcinoma.171,172 Chemotherapy drugs including oxaliplatin, mitoxantrone, shikonin, and bortezomib are all reported to induce ICD in an HMGB1-dependent way.173–176 However, their ICD-inducing capabilities vary, with the most likely reason being that they share different affinities in assisting HMGB1 binding to TLR4 on DC cells.
In conclusion, for HMGB1, the primary task is to translate the achievements of basic research to clinical application. To achieve this, clinical research must be carried out, and before that, the safety and effectiveness of the corresponding proposals must be confirmed in the preclinical stage. As for HMGB2 and 3, more extensive basic research is still needed, so that sufficient evidence can be employed to choose the most promising strategies.
Potential challenges in future researches regarding to the HMGB family members
There are still many challenges in translating the above findings into clinical uses. Initially, since HMGB family members can be secreted or released in various types that lead to different progression and outcomes,23 we should detect the main secretive methods in the target carcinoma before performing HMGB-targeted therapy. Different secretion mechanisms may guide us to use up-regulation or down-regulation of HMGB1 expression to achieve the purpose of tumor inhibition. Secondly, multiple receptors can bind to HMGB family members and induce the activation of distinctive downstream cascades.49 We can decide on whether to select HMGB as a target after identifying the affinity between HMGB and these receptors in different carcinomas. We can also alter the process toward what we expect partly by changing the affinity. Besides, we should exclude the involvement of other receptors or confirm cooperation of multiple receptors when validating each receptor’s downstream cascades and function. To take HMGB1 as an example, recent studies provide us with approaches to address this, one of which is to employ double knockout mice such as RAGE-TLR4 deficient mice, while another would be to conduct parallel studies that employ recombinant HMGB1 lacking other receptors’ binding domains so that HMGB1 can bind to specific target receptors.97 Finally, redox states and PTMs can influence, or even determine, the roles of HMGB family members in the process or stage in carcinogenesis.17 Determining the expression level of the main forms would be beneficial; however, current analysis and assay are not sensitive enough to meet our expectations. We should update existing detective means and tools as well as further investigate the roles of different modified forms of HMGB family members in carcinogenesis. If we could accurately assess the redox status and PTMs of HMGB1 in patients and confirm their roles, we may be more confident in predicting patient tumor stage and prognosis. In addition, alteration of such modified forms may be applicable and efficient as a therapeutic target.
Discussion
Our review has summarized the commonalities and disparities of the three HMGB protein family members in structure, distribution, carcinogenesis, and clinical potentials. We have concluded from current research and reviews that the similarity in their amino acids sequence and regions may indicate functional consistency. However, discrimination, either in structure or distribution, partly determines their distinctive roles in biochemical cascades and regulation of carcinogenesis. The broader distribution and higher expression level of HMGB1 than HMGB2 and 3, both in embryonic development and adult tissues, exposes its more extensive roles in biochemical and pathological procedures. This implication was validated by existing research, as reviewed herein.
From the above analysis, extensive and intensive research is necessary for the complete understanding of the HMGB protein family. Based on the confirmed functions of HMGB1, we can analogously investigate the undiscovered effects of the other two members. Initially and primarily, the specific structure and modifying mechanisms of HMGB2 and 3 should be elucidated, since structure plays a critical role in determining function. The comparison between their construction will provide deep insights into the comprehension of their features. Then, their distribution at the cellular level, and the translocation mechanisms of HMGB2 and 3 should be investigated, which will allow their localization to be understood. Furthermore, their involvement in biochemical cascades and carcinogenesis should be investigated at a larger scale and compared more rationally. In addition to focusing on the identity of their targets, we should also consider their varied affinity. Apart from looking for their connection, we ought to pay attention to how they antagonize each other as well. Their distinctive roles in tumorigenesis indicate that their subtle but critical distinction in conformation matters. We can place emphasis on this difference since directly modifying or even altering the main regions may be effective in their clinical application. Besides, regulating their expression by indirectly targeting other factors in the cascades, or alteration of the oxidation-reduction environment can be another feasible strategy, while existing therapeutic strategies still need to be verified in large-scale preclinical and clinical experiments.
Collectively, the last several decades have witnessed great progression in investigating this abundantly expressed protein family, while further researches are still urgently need. We firmly hold the opinion that this protein family is critically involved in tumorigenesis. In-depth studies can help us to get a better understanding of its roles in carcinogenesis and its clinical potential for cancer management.
Supplemental Material
Supplemental material, Supplements for Biological functions and theranostic potential of HMGB family members in human cancers by Liaoran Niu, Wanli Yang, Lili Duan, Xiaoqian Wang, Yiding Li, Chengchao Xu, Chao Liu, Yujie Zhang, Wei Zhou, Jinqiang Liu, Qingchuan Zhao, Yu Han, Liu Hong and Daiming Fan in Therapeutic Advances in Medical Oncology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported in part by grant from the Scientific Foundation of Shaanxi Province (No.2019ZDLSF01-02-01; No.2018SF-240), grant from the National Clinical Research Center for Digestive Diseases (No.2015BAI13B07) and grant from the State Key Laboratory of Cancer Biology (No. CBSKL2014Z13).
ORCID iD: Liu Hong  https://orcid.org/0000-0002-8276-2345
https://orcid.org/0000-0002-8276-2345
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Liaoran Niu, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Wanli Yang, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Lili Duan, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Xiaoqian Wang, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Yiding Li, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Chengchao Xu, 94719 Military Hospital, Ji’an, Jiangxi Province, China.
Chao Liu, School of Basic Medical Sciences, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Yujie Zhang, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Wei Zhou, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Jinqiang Liu, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Qingchuan Zhao, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
Yu Han, Department of Otolaryngology, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi Province, 710032, China.
Liu Hong, State Key Laboratory of Cancer Biology, National Clinical Research Center for Digestive Diseases, and Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Shaanxi Province, 710032, China.
Daiming Fan, State Key Laboratory of Cancer Biology and National Clinical Research Center for Digestive Diseases, Xijing Hospital of Digestive Diseases, Fourth Military Medical University, Xi’an, Shaanxi Province, China.
References
- 1. Stros M. HMGB proteins: interactions with DNA and chromatin. Biochim Biophys Acta 2010; 1799: 101–113. [DOI] [PubMed] [Google Scholar]
- 2. Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Mol Cell Biol 1999; 19: 5237–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Catena R, Escoffier E, Caron C, et al. HMGB4, a novel member of the HMGB family, is preferentially expressed in the mouse testis and localizes to the basal pole of elongating spermatids. Biol Reprod 2009; 80: 358–366. [DOI] [PubMed] [Google Scholar]
- 4. Taniguchi N, Kawakami Y, Maruyama I, et al. HMGB proteins and arthritis. Hum Cell 2018; 31: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pauken CM, Nagle DL, Bucan M, et al. Molecular cloning, expression analysis, and chromosomal localization of mouse Hmg1-containing sequences. Mamm Genome 1994; 5: 91–99. [DOI] [PubMed] [Google Scholar]
- 6. Ronfani L, Ferraguti M, Croci L, et al. Reduced fertility and spermatogenesis defects in mice lacking chromosomal protein Hmgb2. Development 2001; 128: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 7. Vaccari T, Beltrame M, Ferrari S, et al. Hmg4, a new member of the Hmg1/2 gene family. Genomics 1998; 49: 247–252. [DOI] [PubMed] [Google Scholar]
- 8. Zhou Q, Law AC, Rajagopal J, et al. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell 2007; 13: 103–114. [DOI] [PubMed] [Google Scholar]
- 9. Mosevitsky MI, Novitskaya VA, Iogannsen MG, et al. Tissue specificity of nucleo-cytoplasmic distribution of HMG1 and HMG2 proteins and their probable functions. Eur J Biochem 1989; 185: 303–310. [DOI] [PubMed] [Google Scholar]
- 10. Müller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med 2004; 255: 332–343. [DOI] [PubMed] [Google Scholar]
- 11. Nemeth MJ, Curtis DJ, Kirby MR, et al. Hmgb3: an HMG-box family member expressed in primitive hematopoietic cells that inhibits myeloid and B-cell differentiation. Blood 2003; 102: 1298–1306. [DOI] [PubMed] [Google Scholar]
- 12. Rouhiainen A, Zhao X, Vanttola P, et al. HMGB4 is expressed by neuronal cells and affects the expression of genes involved in neural differentiation. Sci Rep 2016; 6: 32960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bianchi ME, Agresti A. HMG proteins: dynamic players in gene regulation and differentiation. Curr Opin Genet Dev 2005; 15: 496–506. [DOI] [PubMed] [Google Scholar]
- 14. Pil PM, Lippard SJ. Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science 1992; 256: 234–237. [DOI] [PubMed] [Google Scholar]
- 15. Agresti A, Bianchi ME. HMGB proteins and gene expression. Curr Opin Genet Dev 2003; 13: 170–178. [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999; 285: 248–251. [DOI] [PubMed] [Google Scholar]
- 17. Bianchi ME, Crippa MP, Manfredi AA, et al. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev 2017; 280: 74–82. [DOI] [PubMed] [Google Scholar]
- 18. Cheng KJ, Alshawsh MA, Mejia Mohamed EH, et al. HMGB1: an overview of its versatile roles in the pathogenesis of colorectal cancer. Cell Oncol (Dordr). Epub ahead of print 1 November 2019. DOI: 10.1007/s13402-019-00477-5. [DOI] [PubMed] [Google Scholar]
- 19. Wang L-K, Xie X-N, Song X-H, et al. Upregulation of miR-200b inhibits hepatocellular carcinoma cell proliferation and migration by targeting HMGB3 protein. Technol Cancer Res Treat 2018; 17: 1533033818806475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu L, Yang L. The function and mechanism of HMGB1 in lung cancer and its potential therapeutic implications. Oncol Lett 2018; 15: 6799–6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fu D, Li J, Wei J, et al. HMGB2 is associated with malignancy and regulates Warburg effect by targeting LDHB and FBP1 in breast cancer. Cell Commun Signal 2018; 16: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou G-H, Lu Y-Y, Xie J-L, et al. Overexpression of miR-758 inhibited proliferation, migration, invasion, and promoted apoptosis of non-small cell lung cancer cells by negatively regulating HMGB. Biosci Rep 2019; 39: BSR20180855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu T, Jiang L, Wang Z. The progression of HMGB1-induced autophagy in cancer biology. Onco Targets Ther 2018; 12: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ueda T, Yoshida M. HMGB proteins and transcriptional regulation. Biochim Biophys Acta 2010; 1799: 114–118. [DOI] [PubMed] [Google Scholar]
- 25. Bianchi ME, Falciola L, Ferrari S, et al. The DNA binding site of HMG1 protein is composed of two similar segments (HMG boxes), both of which have counterparts in other eukaryotic regulatory proteins. EMBO J 1992; 11: 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee KB, Thomas JO. The effect of the acidic tail on the DNA-binding properties of the HMG1,2 class of proteins: insights from tail switching and tail removal. J Mol Biol 2000; 304: 135–149. [DOI] [PubMed] [Google Scholar]
- 27. Gong W, Li Y, Chao F, et al. Amino acid residues 201–205 in C-terminal acidic tail region plays a crucial role in antibacterial activity of HMGB1. J Biomed Sci 2009; 16: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 2003; 22: 5551–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J, Kokkola R, Tabibzadeh S, et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med 2003; 9: 37–45. [PMC free article] [PubMed] [Google Scholar]
- 30. Ohndorf UM, Rould MA, He Q, et al. Basis for recognition of cisplatin-modified DNA by high-mobility-group proteins. Nature 1999; 399: 708–712. [DOI] [PubMed] [Google Scholar]
- 31. Webb M, Thomas JO. Structure-specific binding of the two tandem HMG boxes of HMG1 to four-way junction DNA is mediated by the A domain. J Mol Biol 1999; 294: 373–387. [DOI] [PubMed] [Google Scholar]
- 32. Ugrinova I, Pasheva E. HMGB1 protein: a therapeutic target inside and outside the cell. Adv Protein Chem Struct Biol 2017; 107: 37–76. [DOI] [PubMed] [Google Scholar]
- 33. Marekov LN, Beltchev BG, Pivec L. High mobility group proteins HMG1 and HMG2 do not decrease the melting temperature of DNA. Biochem Biophys Res Commun 1984; 120: 782–788. [DOI] [PubMed] [Google Scholar]
- 34. Riuzzi F, Sorci G, Donato R. RAGE expression in rhabdomyosarcoma cells results in myogenic differentiation and reduced proliferation, migration, invasiveness, and tumor growth. Am J Pathol 2007; 171: 947–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stemmer C, Leeming DJ, Franssen L, et al. Phosphorylation of maize and arabidopsis HMGB proteins by protein kinase CK2α. Biochemistry 2003; 42: 3503–3508. [DOI] [PubMed] [Google Scholar]
- 36. Pasheva E, Sarov M, Bidjekov K, et al. In vitro acetylation of HMGB-1 and -2 proteins by CBP: the role of the acidic tail. Biochemistry 2004; 43: 2935–2940. [DOI] [PubMed] [Google Scholar]
- 37. Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017; 45: W98–W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nemeth MJ, Cline AP, Anderson SM, et al. Hmgb3 deficiency deregulates proliferation and differentiation of common lymphoid and myeloid progenitors. Blood 2005; 105: 627–634. [DOI] [PubMed] [Google Scholar]
- 39. Yu Y, Tang D, Kang R. Oxidative stress-mediated HMGB1 biology. Front Physiol 2015; 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kang R, Livesey KM, Zeh HJ, et al. HMGB1: a novel Beclin 1-binding protein active in autophagy. Autophagy 2010; 6: 1209–1211. [DOI] [PubMed] [Google Scholar]
- 41. Venereau E, De Leo F, Mezzapelle R, et al. HMGB1 as biomarker and drug target. Pharmacol Res 2016; 111: 534–544. [DOI] [PubMed] [Google Scholar]
- 42. Lu B, Antoine DJ, Kwan K, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A 2014; 111: 3068–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Wheeler D, Tang Y, et al. Calcium/calmodulin-dependent protein kinase (CaMK) IV mediates nucleocytoplasmic shuttling and release of HMGB1 during lipopolysaccharide stimulation of macrophages. J Immunol 2008; 181: 5015–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goh WW, Fan M, Low HS, et al. Enhancing the utility of Proteomics Signature Profiling (PSP) with Pathway Derived Subnets (PDSs), performance analysis and specialised ontologies. BMC Genomics 2013; 14: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Musumeci D, Roviello GN, Montesarchio D. An overview on HMGB1 inhibitors as potential therapeutic agents in HMGB1-related pathologies. Pharmacol Ther 2014; 141: 347–357. [DOI] [PubMed] [Google Scholar]
- 46. Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 2002; 3: 995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang R, Zhang Q, Zeh HJ, III, et al. HMGB1 in cancer: good, bad, or both? Clin Cancer Res 2013; 19: 4046–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ueno H, Matsuda T, Hashimoto S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med 2004; 170: 1310–1316. [DOI] [PubMed] [Google Scholar]
- 49. Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med 2014; 40: 1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bonne-Andrea C, Harper F, Sobczak J, et al. The role of HMG1 protein in nucleosome assembly and in chromatin replication. Adv Exp Med Biol 1984; 179: 479–488. [DOI] [PubMed] [Google Scholar]
- 51. Kang R, Tang D, Schapiro NE, et al. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene 2014; 33: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Javaherian K, Sadeghi M, Liu LF. Nonhistone proteins HMG1 and HMG2 unwind DNA double helix. Nucleic Acids Res 1979; 6: 3569–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh J, Dixon GH. High mobility group proteins 1 and 2 function as general class II transcription factors. Biochemistry 1990; 29: 6295–6302. [DOI] [PubMed] [Google Scholar]
- 54. Lee H, Shin N, Song M, et al. Analysis of nuclear high mobility group box 1 (HMGB1)-binding proteins in colon cancer cells: clustering with proteins involved in secretion and extranuclear function. J Proteome Res 2010; 9: 4661–4670. [DOI] [PubMed] [Google Scholar]
- 55. Daston MM, Ratner N. Expression of P30, a protein with adhesive properties, in Schwann cells and neurons of the developing and regenerating peripheral nerve. J Cell Biol 1991; 112: 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Fuentes E, Rojas A, Palomo I. Role of multiligand/RAGE axis in platelet activation. Thromb Res 2014; 133: 308–314. [DOI] [PubMed] [Google Scholar]
- 57. Passalacqua M, Zicca A, Sparatore B, et al. Secretion and binding of HMG1 protein to the external surface of the membrane are required for murine erythroleukemia cell differentiation. FEBS Lett 1997; 400: 275–279. [DOI] [PubMed] [Google Scholar]
- 58. Santambrogio L, Rammensee H-G. Contribution of the plasma and lymph degradome and peptidome to the MHC ligandome. Immunogenetics 2019; 71: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 2007; 220: 35–46. [DOI] [PubMed] [Google Scholar]
- 60. Seyedin SM, Pehrson JR, Cole RD. Loss of chromosomal high mobility group proteins HMG1 and HMG2 when mouse neuroblastoma and friend erythroleukemia cells become committed to differentiation. Proc Natl Acad Sci U S A 1981; 78: 5988–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pistoia V, Raffaghello L. Damage-associated molecular patterns (DAMPs) and mesenchymal stem cells: a matter of attraction and excitement. Eur J Immunol 2011; 41: 1828–1831. [DOI] [PubMed] [Google Scholar]
- 62. Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci 2009; 122: 3203–3208. [DOI] [PubMed] [Google Scholar]
- 63. Balani P, Boulaire J, Zhao Y, et al. High mobility group box2 promoter-controlled suicide gene expression enables targeted glioblastoma treatment. Mol Ther 2009; 17: 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wu ZB, Cai L, Lin SJ, et al. High-mobility group box 2 is associated with prognosis of glioblastoma by promoting cell viability, invasion, and chemotherapeutic resistance. Neuro Oncol 2013; 15: 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van Beijnum JR, Nowak-Sliwinska P, van den Boezem E, et al. Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene 2013; 32: 363–374. [DOI] [PubMed] [Google Scholar]
- 66. van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of Toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008; 11: 91–99. [DOI] [PubMed] [Google Scholar]
- 67. Zhu L, Li X, Chen Y, et al. High-mobility group box 1: a novel inducer of the epithelial-mesenchymal transition in colorectal carcinoma. Cancer Lett 2015; 357: 527–534. [DOI] [PubMed] [Google Scholar]
- 68. Kuniyasu H, Chihara Y, Kondo H. Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int J Cancer 2003; 104: 722–727. [DOI] [PubMed] [Google Scholar]
- 69. Sharma S, Evans A, Hemers E. Mesenchymal-epithelial signalling in tumour microenvironment: role of high-mobility group box 1. Cell Tissue Res 2016; 365: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011; 278: 16–27. [DOI] [PubMed] [Google Scholar]
- 71. Zhang C, Ge S, Hu C, et al. MiRNA-218, a new regulator of HMGB1, suppresses cell migration and invasion in non-small cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2013; 45: 1055–1061. [DOI] [PubMed] [Google Scholar]
- 72. Yao S, Zhao T, Jin H. Expression of microRNA-325-3p and its potential functions by targeting HMGB1 in non-small cell lung cancer. Biomed Pharmacother 2015; 70: 72–79. [DOI] [PubMed] [Google Scholar]
- 73. Xiao P, Liu W-L. MiR-142-3p functions as a potential tumor suppressor directly targeting HMGB1 in non-small-cell lung carcinoma. Int J Clin Exp Pathol 2015; 8: 10800–10807. [PMC free article] [PubMed] [Google Scholar]
- 74. Han Q, Xu L, Lin W, et al. Long noncoding RNA CRCMSL suppresses tumor invasive and metastasis in colorectal carcinoma through nucleocytoplasmic shuttling of HMGB2. Oncogene 2019; 38: 3019–3032. [DOI] [PubMed] [Google Scholar]
- 75. Li W, Wang Q, Feng Q, et al. Oncogenic KSHV-encoded interferon regulatory factor upregulates HMGB2 and CMPK1 expression to promote cell invasion by disrupting a complex lncRNA-OIP5-AS1/miR-218-5p network. PLoS Pathog 2019; 15: e1007578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mo Y, Fang R-H, Wu J, et al. MicroRNA-329 upregulation impairs the HMGB2/β-catenin pathway and regulates cell biological behaviors in melanoma. J Cell Physiol 2019; 234: 23518–23527. [DOI] [PubMed] [Google Scholar]
- 77. Wang J, Sheng Z, Cai Y. Effects of microRNA-513b on cell proliferation, apoptosis, invasion, and migration by targeting HMGB3 through regulation of mTOR signaling pathway in non-small-cell lung cancer. J Cell Physiol 2019; 234: 10934–10941. [DOI] [PubMed] [Google Scholar]
- 78. Yamada Y, Nishikawa R, Kato M, et al. Regulation of HMGB3 by antitumor miR-205-5p inhibits cancer cell aggressiveness and is involved in prostate cancer pathogenesis. J Hum Genet 2018; 63: 195–205. [DOI] [PubMed] [Google Scholar]
- 79. Xie X, Pan J, Han X, et al. Downregulation of microRNA-532-5p promotes the proliferation and invasion of bladder cancer cells through promotion of HMGB3/Wnt/β-catenin signaling. Chem Biol Interact 2019; 300: 73–81. [DOI] [PubMed] [Google Scholar]
- 80. Zhang Z, Chang Y, Zhang J, et al. HMGB3 promotes growth and migration in colorectal cancer by regulating WNT/β-catenin pathway. PLoS One 2017; 12: e0179741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Barreiro-Alonso A, Cámara-Quílez M, Salamini-Montemurri M, et al. Characterization of HMGB1/2 interactome in prostate cancer by yeast two hybrid approach: potential pathobiological implications. Cancers (Basel) 2019; 11: 1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kwon J-H, Kim J, Park JY, et al. Overexpression of high-mobility group box 2 is associated with tumor aggressiveness and prognosis of hepatocellular carcinoma. Clin Cancer Res 2010; 16: 5511–5521. [DOI] [PubMed] [Google Scholar]
- 83. Zhang P, Lu Y, Gao S. High-mobility group box 2 promoted proliferation of cervical cancer cells by activating AKT signaling pathway. J Cell Biochem 2019; 120: 17345–17353. [DOI] [PubMed] [Google Scholar]
- 84. Zhao B, Liu P, Fukumoto T, et al. Topoisomerase 1 cleavage complex enables pattern recognition and inflammation during senescence. Nat Commun 2020; 11: 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bianco C, Mohr I. Ribosome biogenesis restricts innate immune responses to virus infection and DNA. Elife 2019; 8: e49551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Völp K, Brezniceanu M-L, Bösser S, et al. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut 2006; 55: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. van Beijnum JR, Eijgelaar WJ, Griffioen AW. Towards high-throughput functional target discovery in angiogenesis research. Trends Mol Med 2006; 12: 44–52. [DOI] [PubMed] [Google Scholar]
- 88. Lin Q, Yang XP, Fang D, et al. High-mobility group box-1 mediates toll-like receptor 4-dependent angiogenesis. Arterioscler Thromb Vasc Biol 2011; 31: 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abraham AB, Bronstein R, Chen EI, et al. Members of the high mobility group B protein family are dynamically expressed in embryonic neural stem cells. Proteome Sci 2013; 11: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Li S, Huang Y, Huang Y, et al. The long non-coding RNA TP73-AS1 modulates HCC cell proliferation through miR-200a-dependent HMGB1/RAGE regulation. J Exp Clin Cancer Res 2017; 36: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20: 197–216. [DOI] [PubMed] [Google Scholar]
- 92. Matzinger P. The danger model: a renewed sense of self. Science 2002; 296: 301–305. [DOI] [PubMed] [Google Scholar]
- 93. Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol 2011; 30: 16–34. [DOI] [PubMed] [Google Scholar]
- 94. Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci 2011; 36: 625–632. [DOI] [PubMed] [Google Scholar]
- 95. Huttunen HJ, Fages C, Kuja-Panula J, et al. Receptor for advanced glycation end products-binding COOH-terminal motif of amphoterin inhibits invasive migration and metastasis. Cancer Res 2002; 62: 4805–4811. [PubMed] [Google Scholar]
- 96. Xu D, Young J, Song D, et al. Heparan sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE). J Biol Chem 2011; 286: 41736–41744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ibrahim ZA, Armour CL, Phipps S, et al. RAGE and TLRs: relatives, friends or neighbours? Mol Immunol 2013; 56: 739–744. [DOI] [PubMed] [Google Scholar]
- 98. Hreggvidsdóttir HS, Lundberg AM, Aveberger A-C, et al. High mobility group box protein 1 (HMGB1)-partner molecule complexes enhance cytokine production by signaling through the partner molecule receptor. Mol Med 2012; 18: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ivanov S, Dragoi A-M, Wang X, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood 2007; 110: 1970–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yang H, Hreggvidsdottir HS, Palmblad K, et al. A critical cysteine is required for HMGB1 binding to toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 2010; 107: 11942–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yang H, Lundbäck P, Ottosson L, et al. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med 2012; 18: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102. Hreggvidsdottir HS, Ostberg T, Wähämaa H, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol 2009; 86: 655–662. [DOI] [PubMed] [Google Scholar]
- 103. Tsung A, Klune JR, Zhang X, et al. HMGB1 release induced by liver ischemia involves toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 2007; 204: 2913–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hoppe G, Talcott KE, Bhattacharya SK, et al. Molecular basis for the redox control of nuclear transport of the structural chromatin protein Hmgb1. Exp Cell Res 2006; 312: 3526–3538. [DOI] [PubMed] [Google Scholar]
- 105. Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 2012; 209: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schiraldi M, Raucci A, Munoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012; 209: 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Collins PJ, McCully ML, Martinez-Munoz L, et al. Epithelial chemokine CXCL14 synergizes with CXCL12 via allosteric modulation of CXCR4. FASEB J 2017; 31: 3084–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Pawig L, Klasen C, Weber C, et al. Diversity and inter-connections in the CXCR4 chemokine receptor/ligand family: molecular perspectives. Front Immunol 2015; 6: 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 110. Carbone M, Yang H. Mesothelioma: recent highlights. Ann Transl Med 2017; 5: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yang H, Rivera Z, Jube S, et al. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci U S A 2010; 107: 12611–12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Napolitano A, Antoine DJ, Pellegrini L, et al. HMGB1 and its hyperacetylated isoform are sensitive and specific serum biomarkers to detect asbestos exposure and to identify mesothelioma patients. Clin Cancer Res 2016; 22: 3087–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cornelissen R, Lievense LA, Maat AP, et al. Ratio of intratumoral macrophage phenotypes is a prognostic factor in epithelioid malignant pleural mesothelioma. PLoS One 2014; 9: e106742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cottone L, Capobianco A, Gualteroni C, et al. Leukocytes recruited by tumor-derived HMGB1 sustain peritoneal carcinomatosis. Oncoimmunology 2016; 5: e1122860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mittal D, Saccheri F, Vénéreau E, et al. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J 2010; 29: 2242–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cottone L, Capobianco A, Gualteroni C, et al. 5-Fluorouracil causes leukocytes attraction in the peritoneal cavity by activating autophagy and HMGB1 release in colon carcinoma cells. Int J Cancer 2015; 136: 1381–1389. [DOI] [PubMed] [Google Scholar]
- 117. Bald T, Quast T, Landsberg J, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014; 507: 109–113. [DOI] [PubMed] [Google Scholar]
- 118. Galluzzi L, Buque A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017; 17: 97–111. [DOI] [PubMed] [Google Scholar]
- 119. Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58: 862–870. [DOI] [PubMed] [Google Scholar]
- 120. Krysko DV, Garg AD, Kaczmarek A, et al. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12: 860–875. [DOI] [PubMed] [Google Scholar]
- 121. Kazama H, Ricci JE, Herndon JM, et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 2008; 29: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Campana L, Bosurgi L, Rovere-Querini P. HMGB1: a two-headed signal regulating tumor progression and immunity. Curr Opin Immunol 2008; 20: 518–523. [DOI] [PubMed] [Google Scholar]
- 123. White E. The role for autophagy in cancer. J Clin Invest 2015; 125: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sun X, Tang D. HMGB1-dependent and -independent autophagy. Autophagy 2014; 10: 1873–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tang D, Kang R, Livesey KM, et al. High-mobility group box 1 is essential for mitochondrial quality control. Cell Metab 2011; 13: 701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol 2011; 12: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Scarffe LA, Stevens DA, Dawson VL, et al. Parkin and PINK1: much more than mitophagy. Trends Neurosci 2014; 37: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol 1998; 72: 8586–8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hoyer-Hansen M, Jäättelä M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 2007; 14: 1576–1582. [DOI] [PubMed] [Google Scholar]
- 130. Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell 2005; 122: 927–939. [DOI] [PubMed] [Google Scholar]
- 131. Luo Y, Yoneda J, Ohmori H, et al. Cancer usurps skeletal muscle as an energy repository. Cancer Res 2014; 74: 330–340. [DOI] [PubMed] [Google Scholar]
- 132. Su Z, Wang T, Zhu H, et al. HMGB1 modulates Lewis cell autophagy and promotes cell survival via RAGE-HMGB1-Erk1/2 positive feedback during nutrient depletion. Immunobiology 2015; 220: 539–544. [DOI] [PubMed] [Google Scholar]
- 133. Wang Z, Wang N, Liu P, et al. AMPK and cancer. Exp Suppl 2016; 107: 203–226. [DOI] [PubMed] [Google Scholar]
- 134. Verbrugge I, Johnstone RW, Smyth MJ. SnapShot: Extrinsic apoptosis pathways. Cell 2010; 143: 1192, 1192.e1191–1192. [DOI] [PubMed] [Google Scholar]
- 135. Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 2008; 9: 231–241. [DOI] [PubMed] [Google Scholar]
- 136. Wang Z, Wang X, Li J, et al. HMGB1 knockdown effectively inhibits the progression of rectal cancer by suppressing HMGB1 expression and promoting apoptosis of rectal cancer cells. Mol Med Rep 2016; 14: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 137. Zhang CC, Gdynia G, Ehemann V, et al. The HMGB1 protein sensitizes colon carcinoma cells to cell death triggered by pro-apoptotic agents. Int J Oncol 2015; 46: 667–676. [DOI] [PubMed] [Google Scholar]
- 138. Tang D, Kang R, Cheh CW, et al. HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 2010; 29: 5299–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 2000; 52: 237–268. [PubMed] [Google Scholar]
- 140. van Beijnum JR, Dings RP, van der Linden E, et al. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood 2006; 108: 2339–2348. [DOI] [PubMed] [Google Scholar]
- 141. Sipos F, Galamb O. Epithelial-to-mesenchymal and mesenchymal-to-epithelial transitions in the colon. World J Gastroenterol 2012; 18: 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Li H, Zhang H, Wang Y. Centromere protein U facilitates metastasis of ovarian cancer cells by targeting high mobility group box 2 expression. Am J Cancer Res 2018; 8: 835–851. [PMC free article] [PubMed] [Google Scholar]
- 143. Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest 2018; 128: 1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Sun L, Wu J, Du F, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013; 339: 786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017; 550: 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kim H-K, Kang MA, Kim M-S, et al. Transcriptional repression of high-mobility group box 2 by p21 in radiation-induced senescence. Mol Cells 2018; 41: 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Stordal B, Davey M. Understanding cisplatin resistance using cellular models. IUBMB Life 2007; 59: 696–699. [DOI] [PubMed] [Google Scholar]
- 148. Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007; 7: 573–584. [DOI] [PubMed] [Google Scholar]
- 149. Chao JC, Wan XS, Engelsberg BN, et al. Intracellular distribution of HMG1, HMG2 and UBF change following treatment with cisplatin. Biochim Biophys Acta 1996; 1307: 213–219. [DOI] [PubMed] [Google Scholar]
- 150. Redmond AM, Byrne C, Bane FT, et al. Genomic interaction between ER and HMGB2 identifies DDX18 as a novel driver of endocrine resistance in breast cancer cells. Oncogene 2015; 34: 3871–3880. [DOI] [PubMed] [Google Scholar]
- 151. An Y, Zhang Z, Shang Y, et al. miR-23b-3p regulates the chemoresistance of gastric cancer cells by targeting ATG12 and HMGB2. Cell Death Dis 2015; 6: e1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Guo S, Wang Y, Gao Y, et al. Knockdown of high mobility group-box 3 (HMGB3) expression inhibits proliferation, reduces migration, and affects chemosensitivity in gastric cancer cells. Med Sci Monit 2016; 22: 3951–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mukherjee A, Huynh V, Gaines K, et al. Targeting the high-mobility group box 3 protein sensitizes chemoresistant ovarian cancer cells to cisplatin. Cancer Res 2019; 79: 3185–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chen X, Zeng L. Ginkgo biloba extract 761 enhances 5-fluorouracil chemosensitivity in colorectal cancer cells through regulation of high mobility group-box 3 expression. Am J Transl Res 2018; 10: 1773–1783. [PMC free article] [PubMed] [Google Scholar]
- 155. Shi J, Wang H, Feng W, et al. Long non-coding RNA HOTTIP promotes hypoxia-induced glycolysis through targeting miR-615-3p/HMGB3 axis in non-small cell lung cancer cells. Eur J Pharmacol 2019; 862: 172615. [DOI] [PubMed] [Google Scholar]
- 156. Zhang C, Samanta D, Lu H, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m6A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A 2016; 113: E2047–E2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Gu J, Xu T, Huang Q-H, et al. HMGB3 silence inhibits breast cancer cell proliferation and tumor growth by interacting with hypoxia-inducible factor 1α. Cancer Manag Res 2019; 11: 5075–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zheng W, Yang J, Dong Z, et al. High mobility group box 3 as an emerging biomarker in diagnosis and prognosis of hepatocellular carcinoma. Cancer Manag Res 2018; 10: 5979–5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Masuda K, Ono A, Aikata H, et al. Serum HMGB1 concentrations at 4 weeks is a useful predictor of extreme poor prognosis for advanced hepatocellular carcinoma treated with sorafenib and hepatic arterial infusion chemotherapy. J Gastroenterol 2018; 53: 107–118. [DOI] [PubMed] [Google Scholar]
- 160. Tang HR, Luo XQ, Xu G, et al. High mobility group-box 3 overexpression is associated with poor prognosis of resected gastric adenocarcinoma. World J Gastroenterol 2012; 18: 7319–7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ladoire S, Penault-Llorca F, Senovilla L, et al. Combined evaluation of LC3B puncta and HMGB1 expression predicts residual risk of relapse after adjuvant chemotherapy in breast cancer. Autophagy 2015; 11: 1878–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Wu D, Ding Y, Wang S, et al. Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. J Pathol 2008; 216: 167–175. [DOI] [PubMed] [Google Scholar]
- 163. Nagy Á, Lánczky A, Menyhárt O, et al. Author correction: validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep 2018; 8: 11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Liu Y, Xie C, Zhang X, et al. Elevated expression of HMGB1 in squamous-cell carcinoma of the head and neck and its clinical significance. Eur J Cancer 2010; 46: 3007–3015. [DOI] [PubMed] [Google Scholar]
- 165. Cui G, Cai F, Ding Z, et al. HMGB2 promotes the malignancy of human gastric cancer and indicates poor survival outcome. Hum Pathol 2019; 84: 133–141. [DOI] [PubMed] [Google Scholar]
- 166. Amann R, Peskar BA. Anti-inflammatory effects of aspirin and sodium salicylate. Eur J Pharmacol 2002; 447: 1–9. [DOI] [PubMed] [Google Scholar]
- 167. Ulloa L, Ochani M, Yang H, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci U S A 2002; 99: 12351–12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Han Y, Englert JA, Yang R, et al. Ethyl pyruvate inhibits nuclear factor-κB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther 2005; 312: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 169. Nishibori M, Mori S, Takahashi HK. Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J Pharmacol Sci 2019; 140: 94–101. [DOI] [PubMed] [Google Scholar]
- 170. Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature 2000; 405: 354–360. [DOI] [PubMed] [Google Scholar]
- 171. Lippitz BE, Harris RA. A translational concept of immuno-radiobiology. Radiother Oncol 2019; 140: 116–124. [DOI] [PubMed] [Google Scholar]
- 172. Dong Xda E, Ito N, Lotze MT, et al. High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother 2007; 30: 596–606. [DOI] [PubMed] [Google Scholar]
- 173. Tesniere A, Schlemmer F, Boige V, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29: 482–491. [DOI] [PubMed] [Google Scholar]
- 174. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13: 54–61. [DOI] [PubMed] [Google Scholar]
- 175. Lin T-J, Lin H-T, Chang W-T, et al. Shikonin-enhanced cell immunogenicity of tumor vaccine is mediated by the differential effects of DAMP components. Mol Cancer 2015; 14: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Chang C-L, Hsu Y-T, Wu C-C, et al. Immune mechanism of the antitumor effects generated by bortezomib. J Immunol 2012; 189: 3209–3220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplements for Biological functions and theranostic potential of HMGB family members in human cancers by Liaoran Niu, Wanli Yang, Lili Duan, Xiaoqian Wang, Yiding Li, Chengchao Xu, Chao Liu, Yujie Zhang, Wei Zhou, Jinqiang Liu, Qingchuan Zhao, Yu Han, Liu Hong and Daiming Fan in Therapeutic Advances in Medical Oncology