Abstract
Background
To achieve malaria elimination, it is important to determine the role of human mobility in parasite transmission maintenance. The Alto Juruá basin (Brazil) exhibits one of the largest vivax and falciparum malaria prevalence in the Amazon. The goal of this study was to estimate the contribution of human commutes to malaria persistence in this region, using data from an origin-destination survey.
Methods
Data from an origin-destination survey were used to describe the intensity and motivation for commutations between rural and urban areas in two Alto Juruá basin (Brazil) municipalities, Mâncio Lima and Rodrigues Alves. The relative time-person spent in each locality per household was estimated. A logistic model was developed to estimate the effect of commuting on the probability of contracting malaria for a certain residence zone inhabitant commuting to another zone.
Results
The main results suggest that the assessed population is not very mobile. A total of households reported spending over of their annual person-hour in areas within the same residence zone. Study and work were the most prevalent commuting motivations, calculated at and respectively. Spending person-hours in urban Rodrigues Alves conferred relative protection to urban Mâncio Lima residents. The opposite effect was observed for those spending time in rural areas of both municipalities.
Conclusion
Residence area is a stronger determinant for contracting malaria than commuting zones in the Alto Juruá region. As these municipalities are a hotspot for Plasmodium transmission, understanding the main local human fluxes is essential for planning control strategies, since the probability of contracting malaria is dependent on the transmission intensity of both the origin and the displacement area. The natural conditions for the circulation of certain pathogens, such as Plasmodium spp., combined with the Amazon human mobility pattern indicate the need for disease control perspective changes. Therefore, intersectoral public policies should become the basis for health mitigation actions.
Keywords: Malaria, Household survey, Mobility, Commutation, Logistic model
Background
In 2015, Brazil launched The Malaria Elimination Plan, in alignment with the 2030 Sustainable Development Agenda [1]. Achieving this goal will require a better understanding of local malaria dynamics in remaining transmission hotspots. In this regard, malaria burden in Brazil is concentrated in the Amazon Basin [2]. Transmission is spatially heterogeneous within the Amazon Basin [3, 4] with high malaria transmission pockets associated to fish farming in both rural and urban areas [5], the arrival of susceptible individuals in new rural settlements located in forest fringes and illegal activities, such as mining and logging [6, 7].
Malaria risk factors display different determinant levels, from individual to household levels [8–12]. At the individual level, immunity, genetic background, nutrition, work activities, adherence to preventive practices and travel history, are important exposure and disease determinants, whereas type of household construction, distance to mosquito breeding sites, household income source, preventive habits and customs are important household level determinants [6, 9]. At the eco-social (community) level, type of landscape, economic activities and human occupation and mobility are noted as important determinants [8, 13, 14].
The focus of this study is human mobility and its contribution to malaria persistence. Human movements, seasonal, circular, or linear, within and across borders, are considered strong drivers for malaria (re)introduction [13, 15]. In the Amazon region, mobility drivers include seasonal economic activities, the search for urban services and illegal commodity transportation, among others [4, 7, 16]. Commuting requires long hours in small boats or 4 × 4 vehicles to cross rivers or poorly maintained dirty roads. The cost of travelling often implies in staying away from home for days. Carrasco-Escobar et al. [8] reported that human mobility in Peru is an important malaria persistence determinant in riverine communities, while Wesolowski et al. [13] indicated that human mobility influences the risk for malaria importation into low transmission areas in Kenya. The same scenario is noted in the Amazon Basin, i.e. in the state of Tocantins, which is a low endemicity area maintained by case imports [2, 14].
An important malaria transmission site in the Brazilian Amazon is the Alto Juruá region, in the state of Acre [16]. Plasmodium vivax is the main local pathogen, accounting for of all reported cases, followed by Plasmodium falciparum [2]. In 2018, the annual parasite index (API) was of 121.7 positive exams per 1000 inhabitants for vivax malaria and an API of 30 for P. falciparum. Vivax malaria is considered a neglected tropical disease and its elimination is one of the 2030 Agenda goals [1].
Reis et al. [5] reported a strong correlation between a malaria incidence time series in six Northwest Acre municipalities (Cruzeiro do Sul, Mâncio Lima, Rodrigues Alves, Marechal Thaumaturgo, Porto Walter, and Tarauacá). All but Tarauacá are located in the Alto Juruá region. The first three are connected by a single paved road, while the others are only accessible by waterways. Reis et al. [5, 17] postulated that shared environmental and social drivers could explain the observed malaria synchronicity in this region, contributing to high disease receptivity and vulnerability.
A household survey conducted in 2015 in 40 localities throughout this region detected a malaria trend along urban-rural gradient [9]. Although malaria was not clustered in any specific region, the odds of a household having malaria-infected inhabitants increased significantly along the assessed urban-rural axis, from in urban households to ca. in riverine households. Lana et al. [9] also reported increased odds of contracting malaria in rural households accessible by roads in comparison to those accessible by river only. These estimations did not take into account the time spent by individuals away from their residential areas.
The goal of this study is to revisit this household survey to estimate the contribution of human commuting to malaria persistence in the Alto Juruá region. In this article, it is postulated that commutation between urban and rural areas is important to the maintenance of high regional malaria indices. Understanding malaria dynamics along urban-rural gradient is useful to increase intervention strategy precision, by directing efforts towards main risk groups and areas.
To achieve this, a probabilistic model to estimate mobility contribution to the probability of contracting malaria at the household level was proposed, by using data from an origin-destination questionnaire that was part of the aforementioned household survey. First, estimates concerning the relative time spent in each locality by each individual during one year were postulated, followed by estimations concerning commuting contribution to the probability of contracting malaria.
Methods
Study area
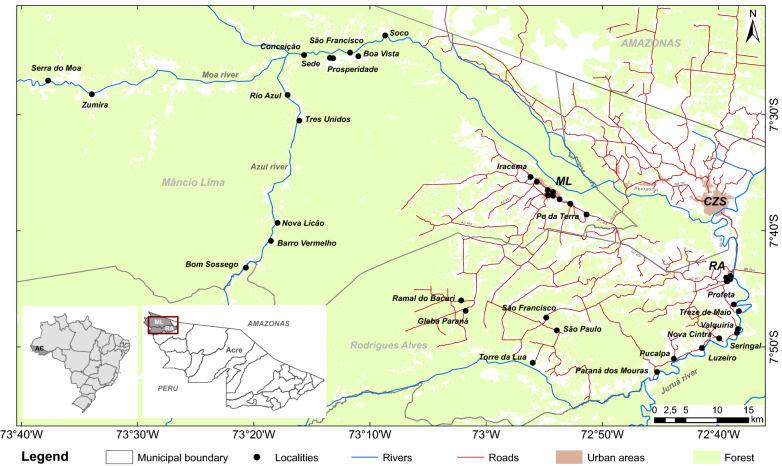
The study area comprises a set of 40 urban and rural localities in two municipalities located in the Alto Juruá river basin, Acre, Brazil, Mâncio Lima (ML) and Rodrigues Alves (RA). These are predominantly rural and forested municipalities, inhabited by indigenous populations, traditional forest product extractivists, rural settlers and small-scale agriculture and fish farming businesses. The main administrative centre of the region, Cruzeiro do Sul, is located 12 km away from RA and 43 km from ML. Cruzeiro do Sul (CZS) is 700 km distant from Rio Branco, the state capital (Fig. 1).
Fig. 1.
Map of the study area in the Alto Juruá region, state of Acre, Brazil. The black dots represent the surveyed communities. These communities are connected by dirt roads (red lines) and rivers (blue lines). Source: IBGE [18], PRODES [40], TerraClass [41], ANA [42] and the household survey described in Lana et al. [9]
ML ( S, W) is the westernmost municipality of Brazil. Population density is low (2.79 inhabitants/km2), with of the 15,206 inhabitants living in the town and the remaining living in rural settlements scattered along dirt roads and along the Moa river and its effluents [18]. Plasmodium vivax is the most prevalent malaria parasite, with every year since at least 2003. Malaria transmission in this municipality has being partially attributed to fish farming, which contributes to mosquito infestations, increasing risks for families practicing this activity [17]. The largest malaria activity peak was registered in 2005/2006, during a large malaria epidemic (), followed by a downwards trend after this date. In 2018, an increase in the number of malaria cases in Brazil was noted, attributed, in part, to the deterioration of malaria control programme [19]. During this period, ML experienced a surge in the number of vivax malaria cases, both in urban and rural settings ().
RA ( S, W) is predominantly rural (4.68 inhabitants/km2). Only of the 14,389 inhabitants live in the town, which is located by the Juruá river. RA is small compared to ML, with less public services. Residents often commute to CZS, 12 km distant, in search of urban services. RA is located in a drier area, away from the flooded forest, and no fish farms are located within the town perimeter. Most of the population () lives in rural settlements scattered along a network of over 1000 km of dirt roads. Several riverine localities are accessible only by the Juruá river and its effluents during the rainy season [18]. As in ML, vivax malaria is the most prevalent, with an API always above 150 since 2004, with a peak noted in 2006 () [2]. Urban malaria is less frequent in RA compared to ML, ranging from to most of the time, except during the 2006 epidemic, when API was 290. In contrast, malaria was more frequent in rural RA than in rural ML up to 2018 (), peaking at 1135 in 2006, decreasing to 227 only after 2018.
In the present study, data were compiled for four residence zones: MLu = urban Mâncio Lima, MLr = rural Mâncio Lima, RAu = urban Rodrigues Alves, RAr = rural Rodrigues Alves. Each residence locality belongs to only one of the four zones.
Data
The Lana et al. 2015 survey assessed demographic and behaviour traits associated with self-reported malaria at the household level. Briefly, a total of 520 households were surveyed, distributed throughout 40 localities, as follows: nine in MLu (), five in RAu (), 13 in MLr () and 13 in RAr (). The questionnaire was applied to a member of the household, who answered questions regarding him/herself and other members of the household. Information for a total of 2274 residents was obtained. Information on malaria was obtained with the question: “Have you had at least one episode of malaria in the last 12 months?”. A total of 233 () households reported at least one episode of malaria in the last 12 months, with in MLu, in MLr, in RAu, and in RAr [9].
One of the components of the survey was an origin-destination questionnaire. The member of the household responded how often he/she travelled to other localities to study, work, and other reasons, in the last 12 months. The time spent at each destination per year was recorded. After removing one household due to inaccurate information, the final data set for the analysis comprised 519 households.
Mobility indicators
From the origin-destination data, the fraction of time spent at each destination was computed for each individual. To harmonize differences in time units that emerged between responses, whenever possible, the length of stay per trip was converted to hours and compiled for a period of 12 months, providing a measure of the total amount of hours per pair of origin-destination, per motive. In the case of pendular displacements for work and study, i.e. same-day round trips, standard work- and school -hours were applied, as described in more detail in the following paragraphs. Concerning a small set of responses (3/2274), the member of the household reported an area instead of a specific destination. A similarly small number of responses (6/2274) reported the locality but not the corresponding municipality. In these cases, the information based on the most likely destinations observed among neighbours was used.
Displacement for school attendance
The time spent at a destination for schooling was estimated assuming standard school hours, hours per day [20, 21] and day-classes in a year [22], not accounting for extra time spent waiting for transportation or attending extra-curricular activities, among other activities.
Formally, the total amount of time spent at a given locality l by individual i, , is given by: where is a matrix where if the individual i attends classes at locality l, and 0 otherwise. If a household has cohabitants, with students, the total household person-time spent at each destination for studying will be .
Displacement for work
Information on the average time spent and how frequently a household member travelled to a certain destination for work during the last 12 months was available for only a fraction of the responses. In these cases, the amount of time spent at the destination received the reported value. When the destination was mentioned but the time spent was absent, this information was imputed using the maximum working hours in Brazilian labour law, hours per week. A total of 61/208 responses with only partial information regarding time spent were obtained, reporting total number of worked days but not how many hours per day, and another 45/208 reporting regular workdays. For the former, it was assumed 8 hours for each reported day. For the latter, it was assumed 5 days over 52 weeks, with 8 hours of work per day. The total person-time spent at each locality l by the workers in a household is , where where is a matrix where if the individual i works at locality l, and 0 otherwise.
Total displacement
The total time spent away from home to work or study was calculated on two spatial scales: First, the time spent in every locality l was counted, where locality was considered as any settlement or neighbourhood mentioned during the survey. Then, these localities were aggregated by residence zone (urban ML, rural ML, urban RA, rural RA, urban CZS, rural CZS and other). With this, local and regional mobility patterns can be assessed. Only commutations for work and study were considered in this calculation, since these are the main reasons for regular commuting.
The total person-time spent on each locality l by the cohabitants of household h is simply the sum of the time spent away for studying and for working, . Complementary, it was assumed that the remaining time was spent at the residence locality:
| 1 |
Since households have different sizes, a normalized measure was computed by dividing by the total person-time for all household members (number of dwellers × 24 hs × 365 days):
| 2 |
The computation of residence zone level mobility was performed by replacing the person-hours spent at each locality l by the sum throughout localities belonging to the same zone z, .
Statistical model
A logistic model was fitted to the origin-destination data to estimate the effect of commuting on the probability of contracting malaria for a household member living in a residence zone z and commuting to a zone . is the number of people in a certain household h with cohabitants mentioning at least one episode of malaria in the past 12 months. If is the probability of a malaria case in the last 12 months at household h, be modelled as a binomial process, dependent on the mobility pattern of its household members:
| 3 |
| 4 |
with as given in Eq. (2) calculated per zone. The model has no intercept and each model coefficient can be interpreted as the probability of contracting malaria in the last 12 months when all people in a certain household spent their time at zone z.
Inferences for the model coefficients were performed under the Bayesian approach based on the approximation implemented in the R function bayesglm from the package arm [23]. In order to avoid numerical instability, relatively vague prior distributions were used for the model coefficients. These priors assume that the probability of contracting malaria in the absence of commutation is of approximately () ranging from to . This is in accordance with the average annual parasite index observed in the area in 2015. Mathematically,
| 5 |
For any location l, the probability of a malaria case in the last 12 months is estimated as
| 6 |
where is a normalized average of the ratio of person-time spent at zone by individuals from location l. That is
| 7 |
where is the number of respondents in location l.
Scenarios
The fitted model was used to calculate the expected probability of contracting malaria under different commuting scenarios. The baseline is zero mobility, that is, a scenario with nobody leaving their home. This baseline was compared with scenarios where individuals spent up to of their time in ML and RA rural or urban areas.
The calculated effect is obtained from the fitted model as the ratio of person-hours spent at zone for a resident of z resident, with the difference in malaria case probability calculated as:
| 8 |
The first term is the estimated malaria case probability given the mobility pattern scenario, while the second term is the probability without leaving the residence zone (baseline). Therefore, it can be interpreted as the destination’s malaria probability contribution at origin. To take into account statistical uncertainties, 1000 samples were generated from the posterior distribution of each parameter obtained from the logistic model.
Along with R package arm [23] used for the aforementioned statistical model, the tidyverse [24] package was used for data processing, ggplot2 [25], and Inkscape [26] for plot and graph styling, the seriation [27] package for heatmap plot ordering, and cytoscape [28] for network representation.
Results
Mobility to areas outside the study region
As a whole, the participants reported 19 destinations outside the study area visited in the last 12 months, totaling 17 in Brazil, one in Peru and one in Bolivia. In Brazil, the following destinations were cited: nine in Acre, one in Rondônia, one in São Paulo, one in Rio de Janeiro, one in Rio Grande do Norte and four in Amazonas (see Additional file 1). Of the 519 households, only 92 reported at least one travel to localities outside the study area. Of those, only 10 households reported destinations outside the state of Acre. The main reasons for travelling to places outside the study area were for medical assistance and leisure activities.
Mobility within the study region
In general, respondents exhibited a low rate of displacement to destinations outside their residence zone, with of all households reporting over of their annual person-hour spent at localities within the same zone (Fig. 2). In fact, of the households reported at least of person-hours spent in the same residence locality. For reference, a typical Brazilian student will spend at least of his/her annual person-hours at school, while a 40 h per week job results in at least about of annual person-hours at work. No household members travelled outside their residence zone for either work or study in and urban ML and RA households, respectively. Percentages were higher among rural residents, reaching and for ML and RA, respectively.
Fig. 2.
Heatmap of person-hours annual ratio spent in each zone by locality of residence. Color gradient based on the log-scale of the ratio, ranging from white () to dark blue (1). Locality (rows) and zone (columns) names are coloured by their type: urban (blue), rural (brown), or nonspecific (gray). Localities’ names have a prefix of M.L or R.A. to indicate the corresponding municipality, ML, or RA. The suffixes u and r in each destination zone indicate urban and rural zones in the corresponding municipality, respectively
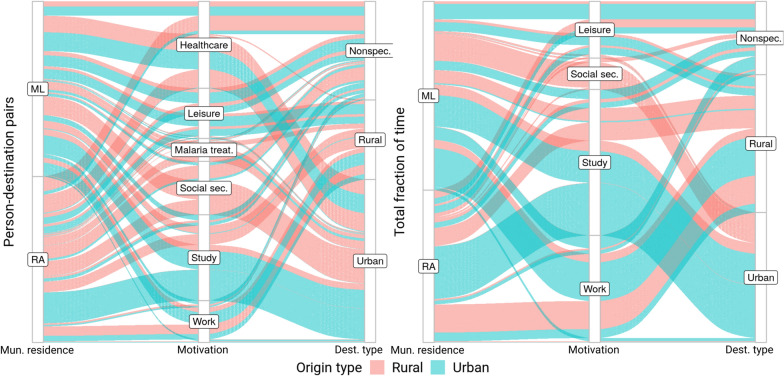
Mobility motivation
Figure 3 displays the time distribution spent away from a residence per motivation. The panels in Fig. 4 present the amount of person-destination pairs reported (left panel) and the total fraction of time spent in each destination (right panel). The former provides information on the diversity of destinations, while the latter is essential to assess actual impact in terms of exposure over the 12 month-period. Unfortunately, the time spent on trips motivated by seeking health services was not possible to ascertain.
Fig. 3.
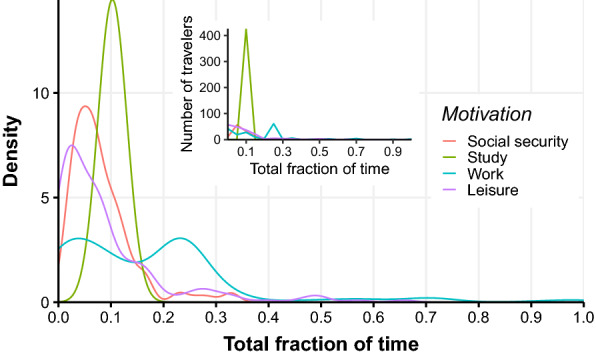
Fraction of time spent in the last 12 months per destination, by respondent. Each curve corresponds to a specific travel motivation: social security (red), study (green), work (blue) and leisure (purple). The main plot represents the kernel density distribution, while the inset shows the histogram with the number of respondents per bin of size 0.05
Fig. 4.
Alluvial diagram of reported displacements. The left panel presents the number of reported destinations per respondent, stratified by municipality and type of origin (red: rural, blue: urban), motivation, and destination type. The right panel presents the total fraction of hours spent in destination per respondent, stratified by municipality and type of origin (red: rural, blue: urban), motivation, and destination type
In terms of person-destination pairs, it is clear that healthcare and study are the main displacement drivers, followed by work, leisure and banking. Participants listed rural localities as their main leisure destinations while referring to urban localities when the motivation was to seek healthcare, study or receive social security benefits. In the case of study-related displacement, the diagram indicates that urban residents are able to remain in urban areas. Concerning rural residents that reported study displacements, the number of residents from ML was similar concerning travellers to urban and rural areas, while those from RA remain in rural areas. On the other hand, individuals that leave their residence for work almost always exhibit rural destinations, regardless of their residence locality.
When taking into account the actual amount of time spent outside their original locality, the main motivations comprise work and study. Although almost no flow from urban to rural areas for study reasons is noted, the opposite is considerable, particularly for ML residents. Conversely, in terms of work displacement, an almost non-observable flow to urban localities is noted, while rural destinations attract residents of both urban and rural areas from both municipalities, with considerable total time spent at the destination. A detailed description of the mobility motivation among respondents is presented in Additional file 1.
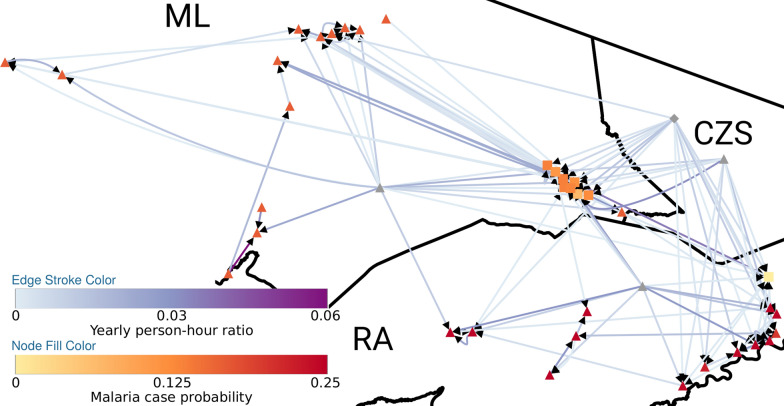
Mobility and malaria
Figure 5 summarizes the effect of travelling on malaria contraction probability at each locality. It is a weighted directed graph (Fig. 5), where nodes represent the localities and directed edges represent the contribution of time spent at the destination on the malaria probability at the origin (arrow). The darker the line color, the more important is the commuting effect on malaria probability at the origin. Nodes are coloured based on the estimated malaria probability using (Eq. 6). It is clear that all localities are well-connected in this network.
Fig. 5.
Mobility and malaria probability network. Each node represents a locality surveyed or reported as a place of work/study. Localities outside of the surveyed area were aggregated by municipality (ML, RA, or CZS) and zone type (rural, urban, or nonspecific) and are gray shaded. Nodes’ shape represents rural (triangles), urban (squares), and unspecified (diamonds) zones. Nodes from the surveyed area are displayed according to their geolocation. Nodes representing aggregated localities are displayed in the corresponding municipality. Nodes are coloured according to the estimated malaria case probability based on typical mobility pattern, with a gradient from light yellow (0) to dark red (0.25). Edge’s direction and color reflect the annual person-hours ratio contribution of the locality of edge’s origin to the probability of observing a malaria case at the locality of edge’s destination, with a gradient from light blue (0) to dark purple (0.06). Please refer to the online article for high resolution image
A strong connectivity between residence zones is observed. A detailed description of the estimated probability of contracting malaria considering the typical median time spent at each destination is provided in the Additional file 2, since the values reported in the network comprise the medians of the subsequent distribution. This calculation does not differentiate between residing or visiting a locality. The probability is highest in rural RA, followed by rural ML. Urban ML shows a slightly lower risk compared to its urban counterpart. Only urban RA presented a very low malaria contraction probability. Spending time in rural CZS also resulted in a protective effect, albeit not statistically significant (not shown).
Scenarios
The results of the logistic model are presented in Table 1. The coefficients correspond to the marginal effect of spending of the person-time of a certain household in a rural or urban zone at ML, RA or CZS. These data can be used to estimate the probability of malaria cases in each zone with no displacement. This is computed from the inverse of the logit function (Eq. 6) with , and for .
Table 1.
Logistic model results
| Zone | Coefficients | Std. error | p value |
|---|---|---|---|
| MLr | 0.10405 | < 0.001 | |
| MLu | 0.10605 | < 0.001 | |
| RAr | 0.09384 | < 0.001 | |
| RAu | 0.18589 | < 0.001 | |
| CZS | 3.95460 | 0.4309 | |
| CZSr | 7.72548 | 0.0356 |
Figure 6 indicates how the probability of contracting malaria for urban resident changes as they spend more time in the other residence zones. Spending person-hours in RAu conferred relative protection to MLu residents. On the other hand, spending time in RAr increased the probability of contracting malaria, although not significant for MLr. For RAu residents, spending time in MLu, MLr and RAr increased the probability of contracting malaria, more so if travelling to the latter.
Fig. 6.
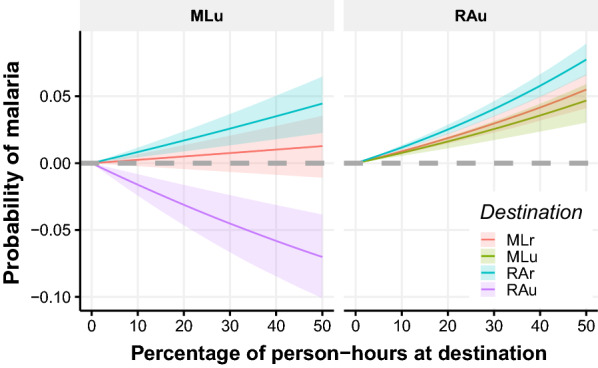
The expected probability of contracting malaria under different commuting scenarios. Contribution in urban areas from ML and RA based on the percentage of person-hours spent at different destination zones. Results obtained from 1000 samples of the posterior distribution of exposure effect of each zone and simulating single-destination mobility profiles
Discussion
Mâncio Lima and Rodrigues Alves are important targets for malaria prevention. In 2015, the official system reported a falciparum API of 48 and 64 and vivax API of 294 and 220, respectively, for ML and RA [2]. It was a relatively good year considering the historical trend of these municipalities, but a bad year considering the Amazon Basin as a whole. Risk factors for malaria transmission exist both in urban and rural areas, such as the presence of fish farming, proximity to flooded areas, living at the forest fringe and working in logging activities [9], as well as the few available health teams that must travel to distant places in search of cases. Understanding the mobility of this population can result in new approaches towards malaria control in this region.
The analysis suggests that the Mâncio Lima and Rodrigues Alves populations are not very mobile, with most residents rarely leaving their home area. When they do, most destinations are located within their municipalities. The major commutation hub is CZS, the regional administrative center. Low mobility can be attributed to fuel costs, the scarcity of routes between most rural localities and the difficulty of using the existing routes year round. For example, dirt roads often close during the rainy season while small rivers become too shallow to navigate by larger boats during the dry season [9]. However, it is important to note that some types of commutes, such as those motivated by illegal activities (hunting, drug tracking) may have been omitted by the respondents. Moreover, it is possible that a household survey may have failed to capture truly mobile populations, that is, those without a fixed residence, or those that were away from home during the survey. To capture hidden populations, other study designs are required, including snowball sampling or respondent-driven-sampling.
Malaria cases imports by resident commutation is likely to be low. On the other hand, malaria case exports to other municipalities by ML and RA residents should be monitored. Exports depend on travelling rates and the probability of being infected. Although travelling is infrequent, malaria prevalence is high. Guajará (in the state of Amazonas), and Rio Branco (in the state of Acre) (see Additional file 1) are at risk for malaria imports due to their connectivity with the studied population [2, 5]. Low mobility rates should not be disregarded in a malaria control programme. In such a situation, active traveller testing should be considered to avoid their reinfection, while testing in other Amazon areas is also required. Some studies characterize circulating Plasmodium strains, and have demonstrated the importance of long journeys on infection imports [29, 30]. Saita et al. [31] reported a positive association between malaria prevalence during the dry season in the Thai-Myanmar border and road quality, a mobility rate proxy.
The probability of contracting malaria in the Alto Juruá is, overall, more associated with residence area than displacement area, due to low mobility rates. However, some types of commutes result in higher risks than others. It was demonstrated herein that an urban RA resident exhibits an increase of only in the probability of contracting malaria by spending of their time in rural RA, but as high as if spending the same time in ML areas (Fig. 6). The effectiveness of the malaria control programme can be improved by placing testing sites along these routes.
Differences in malaria prevalence along rural-urban gradients can be explained by ecological, social, and behavioural factors [7–9, 31]. In general, urban areas present certain conditions, such as housing, drainage network, pavement and easier access to health care, that contribute to decreased malaria prevalence compared to rural areas [32]. According to Braz et al. [33], a spatial dependency is noted for malaria in the Amazon Basin, with differing characteristics between municipalities, states and international borders, reinforcing the importance of monitoring malaria clusters and integrative control actions. The Alto Juruá, as a whole, is highly receptivity to malaria, since most of the population lives at the forest fringes. This forest is seasonally flooded, creating favourable breeding conditions for the malaria vector [34]. Fish farming also contributes to a high abundance of vectors in both urban and peri-urban areas. Fish-ponds began to be built around 2000 as an important local economic activity. A large fraction of these ponds was completed by 2005 [35], with over 80 fish-ponds built each year [5]. Fish farming contributed to maintaining urban ML as a significant malaria transmission area, despite efforts to remove natural breeding sites. The same was not observed in urban RA, as fish farming conditions are not so suitable.
Studies carried out in Colombia, Peru and Namibia using molecular markers to measure the gene flow between different study areas have suggested the presence of malaria corridors that allow the slow and progressive movement of parasite populations throughout endemic areas, with few evidence of direct human displacement [8, 29, 30, 36]. This is an important issue to consider for the Alto Juruá region when observing prevalence differences throughout urban and rural zones and neighbouring municipalities. The studied localities in rural ML are traditional communities living along the Moa and Azul rivers. Their way of life is mainly based on traditional forest product extractivism and subsistence agriculture. Their mobility is very low and occurs strictly by boat [9]. Conversely, rural RA contains several newly implemented settlements, which means that the new populations were not probably exposed to malaria. Recently arriving in a rural settlement is a known risk for symptomatic malaria [6]. This region comprises more commuting individuals, mainly for work, which follows a season-dependent pattern mostly related to agricultural populations, as reported by Saita et al. [31].
This study exhibits certain limitations. It is a cross-sectional survey, which provides only one measure in time. The interviews were performed as a self-reported questionnaire, with potential memory bias, since events were reported up to 12 months prior to the day of the interview. Another limitation is the fact that the time of day spent in each locality was not accounted for, which can affect the probability for malaria infection, due to vector activity preferences. Seasonality attributed to malaria transmission dynamics may display an important influence in this study, as reported in the literature [3, 31, 32, 34, 37].
Conclusion
The natural conditions for pathogen circulation, such as Plasmodium spp., combined with the Amazon human mobility pattern clearly indicate the need for changes in disease control perspectives. It is paramount that intersectoral public policies be the basis for health mitigation actions. Areas with fish farms must follow adequate policies to support this economic activity, and this policy must comprise the entire process, from the fish-pond concession through maintenance training, as well as the final product destination. This will avoid fish-pond abandonment by owners who are unaware of the malaria-related risks and who are often unable to afford maintenance costs, which is common in the region [38] (personal communication). This is especially true concerning areas close to the forest fringe, as recent settlements have been established and higher active case monitoring is required to improve the opportunity concerning diagnoses and treatment responses, which can, in turn, reduce malaria transmission in these areas. The state of Acre is a reference concerning these protocols and won the Malaria Champion of the Americas award for three consecutive years (2011–2013) [39]. The current issue comprises changes to these policies and investments in Malaria Control Programme. Another important action to consider is to include malaria as a transverse activity in local school curricula, as well as in other Amazon Basin regions. In workshops held with teachers in the region, it was identified that schools focus more on other diseases such as dengue (manuscript in preparation), which results in a higher regional disease burden (submitted manuscript).
Supplementary information
Additional file 1. The table with information about origin-destination pairs by a total of hours, motivation, and type of zone destination and the pdf file with the quantitative description of mobility motivation.
Additional file 2. The estimated malaria case probability based on typical person-hour-origin-destination matrix and the estimated effect of each locality to the probability of observing a malaria case. Points represent the median and vertical lines the 95% CI over 1000 samples of the posterior distribution.
Additional file 3. The dataset used to conduct the analysis.
Acknowledgements
Acknowledgements are due to the Mâncio Lima and Rodrigues Alves City Halls, Endemias de Mâncio Lima and Rodrigues Alves, to Mâncio Lima and Rodrigues Alves residents, SESACRE, and the household survey team. We would also like to thank Ana Paula Dal’Asta for Figure 1 and the reviewers for their important comments.
Authors’ contributions
RML and CTC developed the questionnaire, designed the study, conducted the household survey and consolidated the database. MFCG and RML organized the database for analysis. MFCG and LSB analyzed the data. RML, MFCG and CTC wrote the manuscript. All authors revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by Grant E-26/102.287/2013 from the Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro-FAPERJ (http://www.faperj.br), Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (http://www.cnpq.br) 305553/2014-3, 454665/2014-8. The household survey was conducted as part of RML’s PhD. RML was supported by a PhD fellowship from the Brasil Sem Miséria Programme, funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (http://www.capes.gov.br). RML is supported by a PDJ INOVA Fiocruz fellowship. Funding agencies had no role in the design, collection, analysis or interpretation of the data.
Availability of data and materials
The dataset supporting the conclusions of this article is available in Additional file 3.
Ethics approval and consent to participate
Ethical considerations. The study protocol was approved by the Ethical Review Board of the National Public Health School, Brazil (Number 861.871), and written informed consent was obtained from each adult participant.
Consent for publication
All participants signed an informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marcelo F. C. Gomes, Email: marcelo.gomes@fiocruz.br
Cláudia T Codeço, Email: claudia.codeco@fiocruz.br.
Leonardo S. Bastos, Email: leonardo.bastos@fiocruz.br
Raquel M. Lana, Email: raquelmlana@gmail.com
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12936-020-03474-4.
References
- 1.UN/SDG. The United Nations Sustainable Development Goals 2030. United Nations; 2015. https://nacoesunidas.org/pos2015/agenda2030/.
- 2.SIVEP. Sistema de Informação de Vigilância Epidemiológica. Brasília, Brasil: Ministério da Saúde; 2019. http://200.214.130.44/sivep_malaria/.
- 3.Carlos BC, Rona LDP, Christophides GK, Souza-Neto JA. A comprehensive analysis of malaria transmission in Brazil. Pathog Glob Health. 2019;113:1–13. doi: 10.1080/20477724.2019.1581463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Souza PF, Xavier DR, Mutis MCS, Mota JCd, Peiter PC, Matos VPd, et al. Spatial spread of malaria and economic frontier expansion in the Brazilian Amazon. PLoS ONE. 2019;14:e0217615. doi: 10.1371/journal.pone.0217615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis IC, Honório NA, de Barros FSM, Barcellos C, Kitron U, Camara DCP, et al. Epidemic and endemic malaria transmission related to fish farming ponds in the Amazon Frontier. PLoS ONE. 2015 doi: 10.1371/journal.pone.0137521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva-Nunes M, Codeço CT, Malafronte RS, Da Silva NS, Juncansen C, Muniz PT, et al. Malaria on the Amazonian frontier: transmission dynamics, risk factors, spatial distribution, and prospects for control. Am J Trop Med Hyg. 2008;79:624–635. doi: 10.4269/ajtmh.2008.79.624. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira MU, Castro MC. Challenges for malaria elimination in Brazil. Malar J. 2016;15:284. doi: 10.1186/s12936-016-1335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrasco-Escobar G, Gamboa D, Castro MC, Bangdiwala SI, Rodriguez H, Contreras-Mancilla J, et al. Micro-epidemiology and spatial heterogeneity of P. vivax parasitaemia in riverine communities of the Peruvian Amazon: a multilevel analysis. Sci Rep. 2017;7:8082. https://www.nature.com/scientificreports/. [DOI] [PMC free article] [PubMed]
- 9.Lana RM, Riback TIS, Lima TFM, Da Silva-Nunes M, Cruz OG, Oliveira FGS, et al. Socioeconomic and demographic characterization of an endemic malaria region in Brazil by multiple correspondence analysis. Malar J. 2017;16:397. doi: 10.1186/s12936-017-2045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosha JF, Sturrock HJ, Brown JM, Hashim R, Kibiki G, Chandramohan D, et al. The independent effect of living in malaria hotspots on future malaria infection: an observational study from Misungwi, Tanzania. Malar J. 2014;13:445. http://www.malariajournal.com/content/13/1/445. [DOI] [PMC free article] [PubMed]
- 11.Rosas-Aguirre A, Ponce OJ, Carrasco-Escobar G, Speybroeck N, Contreras-Mancilla J, Gamboa D, et al. Plasmodium vivax malaria at households: spatial clustering and risk factors in a low endemicity urban area of the northwestern Peruvian coast. Malar J. 2015;14:176. doi: 10.1186/s12936-015-0670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woyessa A, Deressa W, Ali A, Lindtjørn B. Malaria risk factors in Butajira area, south-central Ethiopia: a multilevel analysis. Malar J. 2013;12:1. http://www.malariajournal.com/content/12/1/273. [DOI] [PMC free article] [PubMed]
- 13.Wesolowski A, Eagle N, Tatem AJ, Smith DL, Noor AM, Snow RW, et al. Quantifying the impact of human mobility on malaria. Science. 2012;338:267–270. http://www.sciencemag.org/content/338/6104/267.full.pdf. [DOI] [PMC free article] [PubMed]
- 14.Parise ÉV, de Araújo GC, Pinheiro RT. Análise espacial e determinação de áreas prioritárias para o controle da malária, no Estado no Tocantins, 2003-2008. Rev Soc Bras Med Trop. 2011;44:63–69. http://www.scielo.br/pdf/rsbmt/v44n1/15.pdf. [DOI] [PubMed]
- 15.Smith C, Whittaker M. Beyond mobile populations: A critical review of the literature on malaria and population mobility and suggestions for future directions. Malar J. 2014;13:307. http://www.malariajournal.com/content/13/1/307. [DOI] [PMC free article] [PubMed]
- 16.PAHO. Report on the Situation of Malaria in the Americas, 2000–2015. Pan American Health Organization; 2016. https://www.paho.org/hq/dmdocuments/2017/2017-cha-report-situation-malaria-amer-00-15.pdf.
- 17.Reis IC, Codeço CT, Degener CM, Keppeler EC, Muniz MM, Oliveira FGS, et al. Contribution of fish farming ponds to the production of immature Anopheles spp. in a malaria-endemic Amazonian town. Malar J. 2015;14:452. http://www.malariajournal.com/content/14/1/452. [DOI] [PMC free article] [PubMed]
- 18.IBGE. Instituto Brasileiro de Geografia e Estatística | Portal do IBGE | IBGE. IBGE; 2010. https://www.ibge.gov.br/.
- 19.WHO. Global report on insecticide resistance in malaria vectors: 2010–2016. Geneve: World Health Organization; 2018. https://apps.who.int/iris/bitstream/handle/10665/272533/9789241514057-eng.pdf?ua=1.
- 20.Instituto Nacional de Estudos e Pesquisas Educacionais Anísio Teixeira. Censo da Educação Básica 2017. INEP; 2017. http://inep.gov.br/web/guest/censo-escolar.
- 21.Instituto Nacional de Estudos e Pesquisas Educacionais Anísio Teixeira. Sinopse Estatística da Educação Básica 2017. INEP; 2018. http://portal.inep.gov.br/sinopses-estatisticas-da-educacao-basica.
- 22.Brasil. Lei de diretrizes e bases da Educação Nacional. Brasil; 1996. http://www.planalto.gov.br/ccivil_03/LEIS/l9394.htm.
- 23.Gelman A, Su YS, Yajima M, Hill J, Pittau MG, Kerman J, et al. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; 2018. R package version 1.10-1. https://CRAN.R-project.org/package=arm.
- 24.Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the tidyverse. J Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 25.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York; 2016. https://ggplot2.tidyverse.org.
- 26.Inkscape Project. Inkscape. Inkscape; 2018. https://inkscape.org.
- 27.Hahsler M, Hornik K, Buchta C. Getting things in order: An introduction to the R package seriation. J Stat Softw. 2008;25:1–34. http://www.jstatsoft.org/v25/i03/.
- 28.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tessema S, Wesolowski A, Chen A, Murphy M, Wilheim J, Mupiri AR, et al. Using parasite genetic and human mobility data to infer local and cross-border malaria connectivity in Southern Africa. eLife. 2019;8:e43510. doi: 10.7554/eLife.43510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacheco MA, Schneider KA, Céspedes N, Herrera S, Arévalo-Herrera M, Escalante AA. Limited differentiation among Plasmodium vivax populations from the northwest and to the south Pacific Coast of Colombia: A malaria corridor? PLoS Negl Trop Dis. 2019;13:e0007310. doi: 10.1371/journal.pntd.0007310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saita S, Pan-ngum W, Phuanukoonnon S, Sriwichai P, Silawan T, White LJ, et al. Human population movement and behavioural patterns in malaria hotspots on the Thai-Myanmar border: implications for malaria elimination. Malar J. 2019;18:64. doi: 10.1186/s12936-019-2704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amratia P, Psychas P, Abuaku B, Ahorlu C, Millar J, Oppong S, et al. Characterizing local-scale heterogeneity of malaria risk: a case study in Bunkpurugu-Yunyoo district in northern Ghana. Malar J. 2019;18:81. doi: 10.1186/s12936-019-2703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braz RM, Guimarães RF, de Carvalho Júnior OA, Tauil PL. Spatial dependence of malaria epidemics in municipalities of the Brazilian Amazon. Rev Bras Epidemiol. 2014;17:615–628. doi: 10.1590/1809-4503201400030004. [DOI] [PubMed] [Google Scholar]
- 34.Wolfarth-Couto B, Filizola N, Durieux L. Padrão sazonal dos casos de malária e a relação com a variabilidade hidrológica no Estado do Amazonas, Brasil. Rev Bras Epidemiol. 2020;23. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-790X2020000100416&nrm=iso. [DOI] [PubMed]
- 35.Costa KMDM, Almeida WAFD, Magalhães IB, Montoya R, Moura MS, Lacerda MVGD. Malária em Cruzeiro do Sul (Amazônia Ocidental brasileira): análise da série histórica de 1998 a 2008. Rev Panam Salud P. 2010;28:353–360. doi: 10.1590/S1020-49892010001100005. [DOI] [PubMed] [Google Scholar]
- 36.Rosas-Aguirre A, Gamboa D, Manrique P, Conn JE, Moreno M, Lescano AG, et al. Epidemiology of Plasmodium vivax Malaria in Peru. Am J Trop Med Hyg. 2016;95:133–144. doi: 10.4269/ajtmh.16-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss DJ, Mappin B, Dalrymple U, Bhatt S, Cameron E, Hay SI, et al. Re-examining environmental correlates of Plasmodium falciparum malaria endemicity: a data-intensive variable selection approach. Malar J. 2015;14:68. doi: 10.1186/s12936-015-0574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alves MR, Codeço CT, Peiter PC, Souza-Santos R. Malaria and fish farming in the Brazilian Amazon Region: a strengths, weaknesses, opportunities, and threats analysis. Rev Soc Bras Med Trop. 2019;52. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86822019000100350&nrm=iso. [DOI] [PubMed]
- 39.PAHO. Malaria Champion of the Americas - Pan American Health Organization. PAHO; 2015. http://www.paho.org/campeonesmalaria/?lang=es.
- 40.INPE Instituto Nacional de Pesquisas Espaciais. Programa de Monitoramento do Desmatamento da Floresta Amazônica Brasileira por Satélite. INPE; 2010. http://terrabrasilis.dpi.inpe.br/app/map/deforestation?hl=pt-br.
- 41.INPE Instituto Nacional de Pesquisas Espaciais. TerraClass. INPE; 2010. http://www.inpe.br/cra/projetos_pesquisas/dados_terraclass.php.
- 42.ANA Agência Nacional de Águas. Portal de Dados Abertos da Agência Nacional de Águas. Agência Nacional de Águas; Accessed 2020-05-26. Library Catalog: dadosabertos.ana.gov.br. http://dadosabertos.ana.gov.br/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The table with information about origin-destination pairs by a total of hours, motivation, and type of zone destination and the pdf file with the quantitative description of mobility motivation.
Additional file 2. The estimated malaria case probability based on typical person-hour-origin-destination matrix and the estimated effect of each locality to the probability of observing a malaria case. Points represent the median and vertical lines the 95% CI over 1000 samples of the posterior distribution.
Additional file 3. The dataset used to conduct the analysis.
Data Availability Statement
The dataset supporting the conclusions of this article is available in Additional file 3.