Abstract
Bone is one of the most frequent targets of small cell lung cancer (SCLC) metastasis and is closely associated with a poor prognosis, but the specific cellular gene alterations responsible for SCLC with bone metastasis are unclear. Zinc finger E‐box binding homeobox 1 (ZEB1) as an E‐box transcriptional repressor has been suggested that an important inducer of the epithelial‐mesenchymal transition (EMT) and a promoter of tumor metastasis in colon, breast and lung cancers. However, the relationship between ZEB1 and SCLC with bone metastasis is unclear. In this study, ZEB1 was found to be highly expressed in bone‐metastatic SCLC tissues and cell lines as compared with those that were non‐metastatic (P < 0.05). Using a lentivirus RNA interference technique to knockdown ZEB1 expression in bone‐metastatic SCLC cells (SBC‐5 cell line), we found that ZEB1 siRNA could inhibit the invasive and migratory ability and decrease parathyroid hormone‐related protein expression, as determined by invasion assays and enzyme‐linked immunosorbent assays. Besides, ZEB1 siRNA significantly inhibited the bone metastasis of SBC‐5 cells in vivo. Furthermore, overexpression of ZEB1 in SBC‐3 cells, which demonstrate promoted bone‐metastatic potential, dramatically promoted their invasive and migratory ability and parathyroid hormone‐related proteinexpression as well as increased the number and sites of bone metastases in vivo compare to the control group. We also found that SBC‐3 cells underwent EMT, as indicated by decreased epithelial markers and increased mesenchymal marker expression. Taken together, these results indicate that ZEB1 promoted the invasive ability and bone metastasis of SCLC cells, and that this was partially mediated via the EMT pathway. (Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02347.x, 2012)
Small cell lung cancer (SCLC) is the lung neoplasia with the poorest prognosis, due to its high ability of metastasis. SCLC in the advanced stage frequently metastasizes to multiple organs, including the liver, lungs, lymph nodes and bone.1 Bone is one of the most frequent targets of SCLC metastasis (as well as the brain and liver) and the bone metastasis is associated with high morbidity and poor prognosis2, 3 due to the consequences of skeletal‐related events, including bone pain, pathologic and radiologic fractures and hypercalcemia.4, 5, 6 However, the mechanisms underlying this rapid metastatic capacity of SCLC are unknown.
Previous study shows that SBC‐3 and SBC‐5 are derived a similar genetic background from human SCLC, which have the same metastatic ablility of visceral organs except for bone. SBC‐5 has a higher capability of bone metastasis than SBC‐3.7 We previously explored the preliminary mechanism of bone metastasis using SBC‐5 and SBC‐3.8, 9, 10
Zinc finger E‐box binding homeobox 1 (ZEB1) is an E‐box transcriptional inducer that has emerged as a key player in cancer progression. The epithelial‐mesenchymal transition (EMT) plays an important role in tumor invasion and is closely related with development and progression of tumors.11, 12 ZEB1 has been described as an inducer of EMT in cancer of epithelial origin, like breast cancer,13, 14 pancreatic cancer15, 16 and lung cancer.17, 18, 19, 20 However, whether or not ZEB1 is involved in SCLC with bone metastasis has not been reported.
In the present study, we found that ZEB1 was highly expressed in bone‐metastatic SCLC tissues and SBC‐5 cells. We used a lentivirus‐delivered siRNA technique and an overexpression vector to observe the effects of knockdown and upregulation of ZEB1 on cell invasion and migration and parathyroid hormone‐related protein (PTHrP) expression in vitro as well as the bone metastatic ability of SCLS cells in vivo. The results demonstrate that ZEB1 contributes to an EMT‐like transformation, invasion and metastasis in bone‐metastatic SCLC. Thus, we present the first evidence that ZEB1 promotes the occurrence of bone metastases in SCLC via the EMT pathway.
Materials and Methods
Cell lines and culture
The human SCLC cell lines SBC‐3 and SBC‐5 were gifted from Dr Saburo Sone and Seiji Yano (University of Tokushima School of Medicine, Japan).7 The SBC‐3, SBC‐5 and alveolar macrophage‐derived NR8383 (American Type Culture Collection, Manassas, VA, USA) cell lines were maintained in RPMI‐1640 supplemented with 10% heat‐inactivated PBS and 1% gentamicin. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Tissue collection and immunohistochemistry
Primary‐site tissues from 32 non‐metastatic SCLC and 31 bone‐metastatic SCLC were obtained from patients who underwent surgery at the Department of Pathology in Tangdu Hospital, Xi'an, China. Patients having surgical tissues dissected for this study all signed an informed consent. All cases of SCLC were clinically and pathologically proven. The protocols used in the study were approved by the hospital's Protection of Human Subjects Committee. Sections (5 μm) of formalin‐fixed paraffin‐embedded specimens were cut, and slides were dewaxed, rehydrated and incubated in 10% normal goat serum and 0.3% Triton X‐100 in PBS for 1 h. The slides were then incubated with a monoclonal anti‐ZEB1 antibody (1:100; Sigma‐Aldrich, St. Louis, MO, USA) and then washed in PBS three times for 5 min each. The tissues were incubated in biotin‐labeled rabbit anti‐mouse serum (1:200) for 30 min, rinsed with PBS, and incubated with avidin–biotin peroxidase complex for 1 h. The signal was detected using 3,3‐diaminobenzidine as the chromogen. Negative control slides omitting the primary antibody were included in all assays. All sections were examined independently by two investigators. Results were evaluated using a formula described previously:21 staining score = the intensity of immunoreactivity (IR) × the proportion of positive staining cells. The intensity of IR was stratified into four categories: 0, none; 1, weak; 2, moderate; and 3, strong. The proportion of positive cells was classified into four groups: 0, 0–5% tumor cells exhibiting IR; 0.33, 5–33% of the tumor cells exhibiting IR; 0.67, 33–67% of the tumor cells exhibiting IR; and 1, 67–100% of the tumor cells exhibiting IR.
Zinc finger E‐box binding homeobox 1 RNAi lentivirus generation
Three pairs of specific oligonucleo tides were designed (ZEB1‐si1:5′‐GCTGTTGTTCTGCCAACAGTT, 5′‐TTCAAGAGAACTTGGCAGAACAACAGC;ZEB1‐si2:5′‐CCTCTCTGAAAGAACACATTA, 5′‐TTCAAGAGATAATGTGTTCTTTCAGAGAGG; and ZEB1‐si3: 5′CGGCGCAATAACGTTACAAAT, 5′‐TTCAAAGAATTTGTAACGTTATTGCGCCG). After testing for knockdown efficiencies, the stem‐loop oligonucleotides were synthesized and cloned into the lenti‐virus‐based vector PsicoR (Addgene, Boston, MA, USA). A nontargeting (scrambled) stem‐loop DNA PsicoR vector was also generated as a negative control. Lentivirus particles were prepared as described previously.22 SCLC cells were infected with a ZEB1‐siRNA lentivirus or the negative control virus at 7 days and examined at 10 days.
Lentivirus infection
Cells were incubated with lentivirus in a small volume of serum‐free DMEM at 37°C for 4 h. Then, 10% DMEM was added, and the cells were placed in an incubator for an additional period of time (as indicated) for the following experiments. GFP indicated that the infection efficiency of SBC‐5 cells was approximately 90% at a multiplicity of infection (MOI) of 20, at which concentration no virus toxicity was observed. Subsequently, the following experiments were performed using viruses at an MOI of 20, unless indicated otherwise. SBC‐5 cells were transfected with ZEB1‐siRNA, scramble RNA designated lenti‐siRNA/ZEB1 and src‐siRNA, respectively.
Zinc finger E‐box binding homeobox 1 cDNA vector
The complementary DNA sequence of ZEB1 was designed from the full‐length ZEB1 sequence by Shanghai GeneChem Company (Shanghai, China). The recombinant sense expression vector for ZEB1 were constructed as described previously (5′‐AAGAATTCACAGTGGAGAGAAGCCA‐3′, as 5′‐CGTTTCTTGCAGTTTGGGCATT‐3′). In brief, recombinant sense expression vector pcDNA3.1‐ZEB was constructed by subcloning the cDNA fragment of ZEB1 that contained the complete coding sequence, and was introduced into the SBC‐3 cells. Stably transfected cell clones were obtained by limiting dilution culture under the pressure of G418. To avoid the effects of clonal variety, three random clones of each group were used in all the experiments.
Real‐time RT‐PCR
Primary SCLC cancer tissues were collected. RNA was extracted with RNAzol (Biogenesis) according to the manufacturer's instructions. cDNA synthesis was performed using a Moloney murine leukemia virus cDNA synthesis kit (Gibco BRL). Taqman PCR primers were designed based on mRNA sequences using Primer Express software (Perkin‐Elmer) supplied by Sigma Genosys (Haverhill, UK). The PCR primers used were as follows: ZEB1 forward 5′‐CAGGCAGATGAAGCAGGATG and reverse 5′‐CAGCAGTGTCTTGTTGTTGTAG and β‐actin forward 5′‐GGCGGCACCACCATGTACCCT and reverse 5′‐AGGGGCCGGACTCGTCATACT.
Western blot analysis
After protein quantitation using a Coomassie brilliant blue assay, 50 μg of protein was boiled in loading buffer, resolved on 10% SDS‐polyacrylamide gels, electrotransferred to nitrocellulose membranes, and incubated overnight with mouse monoclonal antibodies against ZEB1 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), E‐cadherin (1:2500; Abcam, Cambridge, UK), keratin (1:1000; Abcam), fibronectin (1:1000; Abcam), vimentin (1:1000; Abcam) and β‐actin (1:5000; Sigma‐Aldrich, St. Louis, MO, USA). The secondary antibodies (1:1000; anti‐mouse or anti‐rabbit peroxidase conjugated IgG) were applied, and the relative content of the target proteins was detected by chemiluminescence.
In vitro invasion and migration assays
In vitro cell invasion assays were performed as described by others.23 using Transwell chambers (8‐μM pore size; Corning Costar Corp., Acton, MA, USA). Cells were allowed to grow to subconfluency (75–80%) and were serum‐starved for 24 h. After detachment with trypsin, cells were washed with PBS, resuspended in serum‐free medium and 250 μL of cell suspension (2 × 105 cells/mL) was added to the upper chamber. Complete medium was added to the bottom wells of the chamber. The cells that had not migrated were removed from the upper face of the filters using cotton swabs, and the cells that had migrated to the lower face of the filters were fixed with 5% glutaraldehyde solution and stained with 0.5% solution of toluidine blue in 2% sodium carbonate. Images of three random × 10 fields were captured from each membrane and the number of migratory cells was counted. The mean of triplicate assays for each experimental condition was used. Similar inserts coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) were used to determine invasive potential in the invasion assay.
ELISA
SBC‐5 and SBC‐3 cells at 80% confluence were harvested, plated into 6‐well tissue culture plates (1 × 105 cells/2 mL per well), and incubated for 24 h at 37°C in 5% CO2. After a 72‐h incubation at 37°C, culture supernatants were collected and the concentrations of PTHrP and vascular endothelial growth factor (VEGF) were determined using radioimmunoassays (Kitasato Otsuka Virus Assay Laboratories, Tokushima, Japan) and ELISA (R&D Systems, Minneapolis, MN, USA), respectively.
Tail‐vein bone metastatic assay
Mice were handled using best humane practices and cared for in accordance with National Institutes of Health Animal Care and Use Committee guidelines. Cells were harvested from tissue culture flasks using trypsin and washed three times with PBS. Mice were injected through the tail vein with 1 × 106 cells in 0.1 mL PBS and monitored for overall health and total body weight. Four weeks after injection, the mice were killed. The number of sites of bone metastasis on the body surface was counted. Sites of bone metastasis were serially sectioned for X‐ray analysis and observed under a light microscope. Each experimental group contained 5–10 mice.
Immunofluorescence
For immunofluorescent staining, cells were grown for 2 days in 10‐well glass microscope slides (VWR International, Fontenay‐sous‐Bois, France). Cells were washed and fixed in acetone/methanol (1:1) solution for 3 min on ice. After rinsing, the slides were incubated with primary antibodies for 1 h at room temperature, rinsed and incubated with Alexa 488‐conjugated rabbit anti‐mouse IgG (Pierce, Rockford, IL, USA) for 1 h. The anti‐E–cadherin and anti‐fibronectin antibodies were from BD Biosciences, Transduction Laboratories (Franklin Lakes, NJ, USA). Cells were examined and photographed using a confocal inverted microscope (Axiovert 200M; Zeiss, Oberkochen, Germany). To monitor BrdU incorporation, cells were pulse labeled with BrdU for 40 min and stained with DAPI according to the protocols supplied with the Detection Kit I (Roche Applied Science, Basel, Switzerland).
Statistical analysis
Statistical analysis was performed using the Kruskal–Wallis rank test, and the Mann–Whitney U‐test was used to calculate P‐values and to compare the differences between groups for immunohistochemistry. Assays for characterizing cell phenotypes were analyzed using Student's t‐test. The statistical SPSS software package (SPSS, Chicago, IL, USA) was used to analyze the data. Differences were considered statistically different at P < 0.05.
Results
Zinc finger E‐box binding homeobox 1 is overexpressed in bone‐metastatic small cell lung cancer tissues and cell lines
Zinc finger E‐box binding homeobox 1 was significantly overexpressed in SCLC tissues with bone metastasis (n = 31) mainly located in the nucleus as compared to control non‐metastatic tissues (n = 32; Fig. 1A). The average staining score in the SCLC tissues with bone metastasis group was significantly higher than that in the control group (Fig. 1B) (2.21 ± 0.34 vs 1.49 ± 0.23; P < 0.01). As shown in Table 1, a positive rate of ZEB1 expression in non‐metastatic tissues was 56.3% (18/32), lower than the 90.3% (28/31) in SCLC tissues with bone metastasis. To further confirm these observations, western blot analysis was performed using four human SCLC specimens with metastasis (M) and four human SCLC specimens without metastasis (N), the specimens were from different patients (Fig. 1C). The results showed that the tumor non‐metastatic tissue specimens had drastically decreased ZEB1 expression compared with the metastatic tissues, which was consistent with the level of ZEB1 protein expression determined by immunohistochemical staining. Real‐time PCR confirmed the same result in mRNA level (Fig. 1D). Western blot analysis of nuclear proteins from SCLC cells indicated that ZEB1 was highly expressed in the SBC‐5 cell line and had lower expression in the SBC‐3 and the alveolar macrophage‐derived NR8383 cell line (Fig. 1E). Similarly, differential expression of the ZEB1 mRNA was confirmed by RT‐PCR (Fig. 1F). These results indicated that ZEB1 expression closely correlated with SCLC with bone metastasis.
Figure 1.
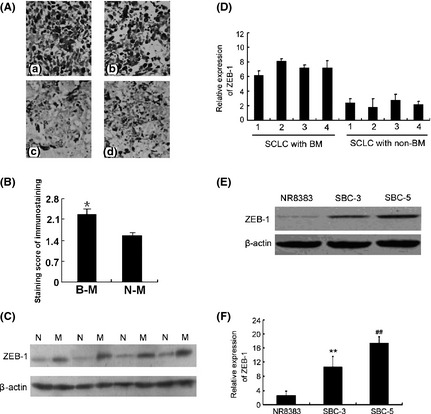
Zinc finger E‐box binding homeobox 1 (ZEB1) is overexpressed in small cell lung cancer (SCLC) with bone metastasis. Monoclonal mouse anti‐ZEB1 antibodies were used to stain paraffin sections. (A) ZEB1 protein expression in SCLC tissues with bone metastasis and SCLC with no metastasis (400×). (a,b) small cell lung cancer with bone metastasis. (c,d) small cell lung cancer with no metastasis. (B) The results of immunohistochemical staining were evaluated by staining scores as described in the Materials and Methods. B‐M, bone metastatic; N‐M, non‐metastatic. (C) Expression of ZEB1 in SCLC with metastasis (M) and non‐metastatic tissues (N). SCLC tissues with metastasis had higher ZEB1 protein levels than SCLC tissues with no metastasis (P < 0.01). (D) The effect of ZEB1 mRNA expression in non‐metastatic (NM) compared with metastatic (BM) tissues. (E) Expression of ZEB1 protein and (F) mRNA in SBC‐5 cells were significantly higher than those in SBC‐3 or NR8383 cells (P < 0.01 versus SBC‐3 or NR8383 cells).
Table 1.
Intensity of immunoreactivity of zinc finger E‐box binding homeobox 1 in bone metastatic and non‐metastatic small cell lung cancer
| Cases | − | + | ++ | +++ | |
|---|---|---|---|---|---|
| Bone metastatic SCLC | 31 | 3 | 15 | 8 | 5 |
| Non‐metastatic SCLC | 32 | 14 | 9 | 6 | 3 |
SCLC, small cell lung cancer.
Lenti‐siRNA/zinc finger E‐box binding homeobox 1 inhibits cell invasion, migration, and the expression of parathyroid hormone‐related protein in vitro and inhibits the number of bone metastases in vivo
To further this study, we constructed four lentivirus‐delivered vectors: three ZEB1‐specific siRNA vectors (ZEB1‐si1, ZEB1‐si2 and ZEB1‐si3) and a scrambled control siRNA vector (src‐siRNA). As shown in Figure 2(A), after infection with any of the ZEB1‐targeting lentivirus vectors, ZEB1 expression decreased. However, ZEB1‐si2 and ZEB1‐si3 effectively downregulated ZEB1 in SBC‐5 cells, whereas the effect of ZEB1‐si1 was minimal (Fig. 2B,C). Therefore, ZEB1‐si2 and ZEB1‐si3 were chosen for further assays.
Figure 2.
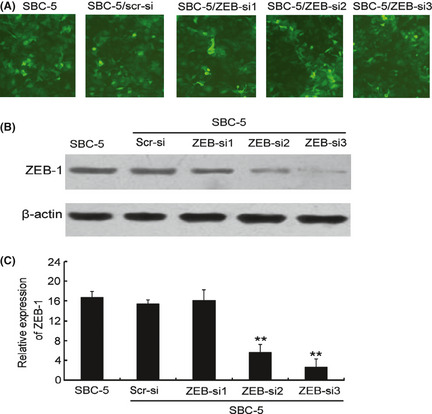
The effect of lentivirus‐mediated knockdown of zinc finger E‐box binding homeobox 1 (ZEB1) expression in SBC‐5 cells. Lentivirus vectors for the ZEB1 siRNA were constructed and shown to be specific and potent for silencing ZEB1 expression in the SBC‐5 cell line. (A) To observe the infection efficiency of ZEB1 siRNA (ZEB1‐si) and scramble siRNA (Src‐si) by fluorescence microscope. (B) Western blot analysis of ZEB1 protein expression in SBC‐5 cells infected with the lenti‐siRNA/ZEB1 and src‐siRNA. (C) RT‐PCR analysis indicating ZEB1 mRNA levels in SBC‐5 cells transfected with the lenti‐siRNA/ZEB1 vectors or the src‐siRNA vector. The src‐siRNA‐transfected cells were used as a blank control.
Cell migrated and invasion in vitro assays showed that downregulation of ZEB1 in SBC‐5 cells significantly decreased their invasive ability (Fig. 3A). ZEB1‐si2/si3‐transfected cells demonstrated decreased migration compared with control‐transfected cells (P < 0.01; Fig. 3B). ZEB1‐si2/si3‐transfected cells demonstrated a decreased invasion compared with control‐transfected cells (P < 0.001; Fig. 3C,D). To investigate the effect of ZEB1 on SCLC with bone metastasis, we examined the bone metastatic specificity molecule PHTrP by ELISA.24, 25 Our results indicated that ZEB1‐si2 and ZEB1‐si3 could significantly inhibit the expression of PHTrP (Fig. 3E,F).
Figure 3.
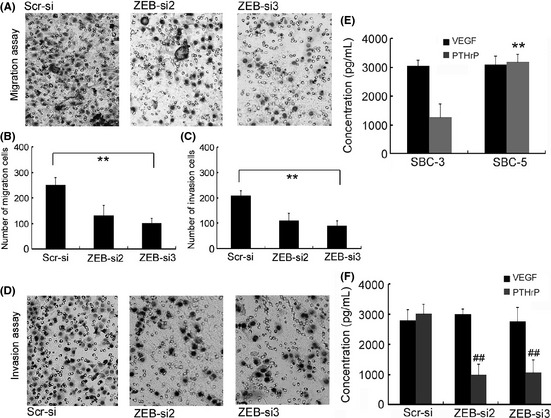
Zinc finger E‐box binding homeobox 1 (ZEB1)‐siRNA inhibits cell invasion, migration and expression of PTHrP in vitro. (A) Cell migration was evaluated by counting cells invading through a transwell membrane with an 8‐μm pore size. (B) Results of migration analysis were evaluated as described in the Materials and Methods. *P < 0.01 versus scr‐siRNA treatment. (C) Results of invasion analysis were evaluated as described in the Materials and Methods. *P < 0.01 versus scr‐siRNA treatment. (D) Cell invasion was evaluated by counting cells invading through a Matrigel‐coated transwell membrane with an 8‐μm pore size. Cells attached to the Matrigel were counted under a light microscope. (E) parathyroid hormone‐related protein (PTHrP) was expressed at higher levels in SBC‐5 cells compared to that in SBC‐3 cells, as determined by ELISA. However, there was no obvious change in vascular endothelial growth factor (VEGF) expression. (F) Knockdown of ZEB1 expression resulted in a significant decrease in expression of PTHrP, but there was no obvious change in VEGF expression.
Furthermore, in vivo bone metastasis assays for SCLC were adopted to examine the proliferative ability of SBC‐5 cells. NK‐cell depleted SCID mice injected with SBC‐5 cells developed bone metastatic lesions in the limbs, the vertebral bones, the pelvis and the scapulae.7 Compared with control cells transfected with an empty vector, the injection of ZEB1‐si2 and ZEB1‐si3‐transfected SBC‐5 cells led to a significant decrease in bone metastatic lesions (Fig. 4A,B). Both in vitro and in vivo assays suggested that ZEB1 siRNA had the potential to inhibit the ability of bone metastatic lesions of SCLC.
Figure 4.
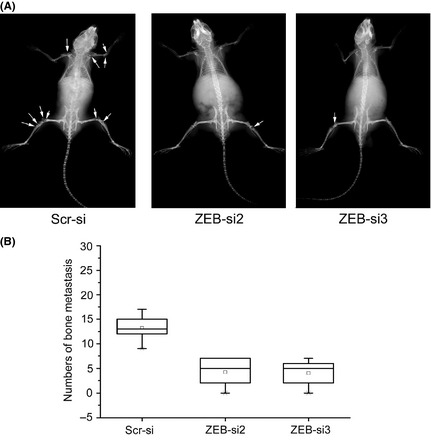
Zinc finger E‐box binding homeobox 1 (ZEB1)‐siRNA inhibits the number of bone metastases in vivo. Inhibition of bone metastasis by SBC‐5 cells transfected with the lenti‐siRNA/ZEB1 vectors or scr‐siRNA vector in NK‐cell depleted SCID mice. SBC‐5 cells (1 × 106) were intravenously inoculated into NK‐cell depleted SCID mice. After 35 days, evidence of bone metastasis was evaluated using X‐ray photographs. (A) Analysis of SBC‐5 cell lines transfected with ZEB1 knockdown or control vectors. Arrows indicate bone metastasis. (B) Results evaluated the number of bone metastases as described in the Materials and Methods. *P < 0.01 versus scr‐siRNA treatment.
Upregulation of zinc finger E‐box binding homeobox 1 expression promotes invasion, migration and parathyroid hormone‐related protein expression in SBC‐3 cells and increases the number of bone metastases in vivo
To further explore the exact mechanism of ZEB1 in metastasis of SCLC, a ZEB1 sense vectors was constructed and then transfected into SBC‐3 cells that is a non bone‐metastasis SCLC and normally have low expression of ZEB1. As shown in Figure 5(A,B), SBC‐3 cells transfected with ZEB1 sense vector significantly upregulated ZEB1 expression. Cell invasion in vitro assays showed that upregulation of ZEB1 in SBC‐3 cells significantly increased their migration and invasive ability compared with control cells (Fig. 5C,D). We also examined the expression of PTHrP in ZEB1‐cDNA‐transfected cells via ELISA. The results showed that force overexpression of ZEB1 significantly increased the expression of PHTrP (Fig. 5E). To investigate the role of ZEB1 overexpression in ability of bone metastasis, we injected ZEB1‐cDNA‐transfected cells into NK‐cell depleted SCID mice and assayed for the development of bone metastatic lesions (Fig. 5F). Compared with the control group, the injection of ZEB1‐cDNA‐transfected cells led to a significant increase in bone metastatic lesions (Fig. 5G). Taken together, these results suggested that ZEB1 overexpression had the potential to promote the invasion, migration and metastatic ability of SCLC bone metastatic cells both in vitro and in vivo.
Figure 5.
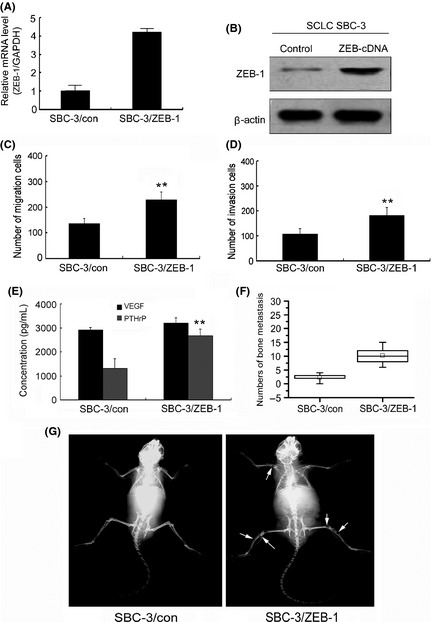
Upregulation of zinc finger E‐box binding homeobox 1 (ZEB1) expression promotes invasion, migration and parathyroid hormone‐related protein (PTHrP) expression in SBC‐3 cells and increases the number of bone metastases in vivo. (A) RT‐PCR of the ZEB1 mRNA from SBC‐3 cells transfected with the ZEB1‐cDNA or control vector. (B) Western blot analysis of ZEB1 protein expression in SBC‐3 cells transfected with the ZEB1‐cDNA or control vector. (C) Results of migration analysis were evaluated as described in the Materials and Methods. *P < 0.01 versus control treatment. (D) Results of invasion analysis were evaluated as described in the Materials and Methods. *P < 0.01 versus control treatment. (E) Upregulation of ZEB1 expression significantly increased the expression of PTHrP, but there was no obvious change in vascular endothelial growth factor (VEGF) expression. (F) Results of animal experiments were evaluated as described in the Materials and Methods. *P < 0.01 versus control treatment. (G) Animals experiments utilizing SBC‐3 cell lines transfected with ZEB1 overexpression or control vectors. Arrows indicate bone metastases.
Zinc finger E‐box binding homeobox 1 promotes bone metastasis in small cell lung cancer via the epithelial‐mesenchymal transition pathway
After transfection with the ZEB1 sense vector, we noticed that the morphology of the SBC‐3/ZEB cells changed from round to fusiform (Fig. 6A). To gain insight into the mechanism of ZEB1‐induced EMT, we next examined the expression of well‐characterized EMT markers. Immunofluorescence staining showed that the epithelial marker E‐cadherin had nearly disappeared and the mesenchymal marker fibronectin was dramatically increased in the SBC‐3/ZEB cells compared to the staining observed in the control cells. This change represents one of the hallmarks of EMT (Fig. 6B). To determine whether the molecular alteration consistent with EMT occurred in these cells, we examined the expression of several proteins, such as E‐cadherin, keratin, fibronectin and vimentin. Western bolt analyses revealed high expression of mesenchymal markers fibronectin, vimentin, and nearly a complete loss of epithelial markers E‐cadherin and keratin expression (Fig. 6C). Therefore, both the morphological and molecular changes observed in the ZEB1‐cDNA‐transfected SBC‐3 cells indicated that these cells had undergone EMT.
Figure 6.
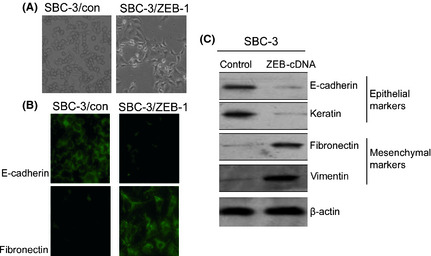
Zinc finger E‐box binding homeobox 1 (ZEB1) is a transcriptional repressor of E‐cadherin expression in SBC‐3 cells. (A) Morphological changes of SBC‐3 cells transfected with the ZEB1‐cDNA vector or without plasmid were observed using a phase‐contrast microscope, and representative photographs are shown; ×200 original magnification. (B) Immunohistochemistry for E‐cadherin and fibronectin expression in SBC‐3 cells transfected with the ZEB1‐cDNA vector or control. (C) Western blot analysis of epithelial markers (i.e. E‐cadherin and keratin) and mesenchymal markers (i.e. fibronectin and vimentin) in SBC‐3 cells transfected with the ZEB1‐cDNA vector or control.
Discussion
Bone is one of the most frequent targets of SCLC metastasis. Compared with breast cancer26, 27 and prostate cancer,28 the research on lung cancer bone metastasis is only in its infancy. ZEB1 is a crucial factor of the EMT pathway and has been found to be expressed in many kinds of human cancers,29, 30 including breast cancer,11, 12 colon cancer,31, 32 gastric cancer33 and hepatocellular carcinoma.34 Recently, some studies have indicated that ZEB1 may play an import role in NSCLC progression,17, 18 but its exact role in SCLC with bone metastasis is not fully elucidated.
In this study, we present the first evidence of the critical role of ZEB1 in SCLC with bone metastasis. We identified that ZEB1 was significantly overexpressed in bone‐metastatic SCLC tissues and cell lines, and that its average expression level in bone‐metastatic SCLC was significantly higher compared to non‐metastatic SCLC, as indicated by immunohistochemistry, western blot and RT‐PCR analyses. In addition, ZEB1 expression was found to be upregulated in SBC‐5 cells compared to SBC‐3 cells, which have a similar genetic background but demonstrate different capabilities for bone metastasis; SBC‐5 cells have a higher capability for promoting bone metastasis than SBC‐3 cells. This observation suggests that ZEB1 plays an important role in SCLC with bone metastasis.
Lentivirus is one kind of reversal viruses, it could provide with the basic feature and structure of reversal. Lentiviruses can integrate into the host genome and demonstrate long‐term expression of integrated genes. As such, these viruses can serve as efficient promoters for transgenic animals.35 Lentiviruses have higher titers than retroviral viruses and not only infect dividing phase cells, but also infect nondividing cells. Using lentivirus vectors, three pairs of siRNA targeting ZEB1 were transfected into SBC‐5 cells. Cell invasion and migration experiments confirmed that knockdown of ZEB1 expression by siRNA could significantly inhibit these processes, indicating that ZEB1 functioned to promote invasion and metastasis in SCLC with bone metastasis.
It is known that bone lesions caused by bone metastases are mediated by various factors,36, 37 including receptor activator of nuclear factor κB (RANK) ligand and PTHrP, which have been implicated in the enhancement of osteoclast formation and bone destruction in malignant diseases.36 PTHrP is reported to indirectly activate osteoclasts by induction or stimulation of RANK ligand expression and, hence, augment bone resorption. In the present study, we demonstrated that PTHrP was overexpressed in SBC‐5 cells and that knockdown of ZEB1 expression could significantly decrease the expression of PTHrP without an obvious change in VEGF expression, but, to our knowledge, there are no studies considering the relationship between ZEB1 and PTHrP.
In the previous report,7 Sone S et al. established an SCLC cell line that demonstrated high bone metastasis (SBC‐5) in NK3 cell‐depleted SCID mice. In this model, SBC‐5 cells metastasized to multiple organs, such as the lung, liver and kidneys, and especially to bone, similar to that observed in patients with SCLC. We found that knockdown of ZEB1 expression inhibited the number and location of bone metastasis in this mouse model.
We then employed a ZEB1 overexpression vector to infected SBC‐3, a low bone metastasis SCLC cell lines. examined the influence of ZEB1 overexpression on the invasion and migration ability of tumor cells in vitro and bone metastasis ability in vivo. It was observed that ZEB1 overexpression promoted the invasive and migration ability and upregulated the expression of PTHrP in SBC‐3 cells. ZEB1 overexpression also significantly increased the number of bone lesions in vivo as compared with the control group. Taken together, these data confirmed that ZEB1 functioned to promote SCLC invasion and bone metastasis.
Recent research shows that a novel view related metastasis about heterogeneity of SCLC. The researcher using mouse model for SCLC previously found that tumors are often composed of phenotypically different cells with either a neuroendocrine markers(such as Syp1,Ash1,NCAM) or mesenchymal markers profile(such nestin, Vimentin). And these crosstalk between mesenchymal and neuroendocrine cells strongly influenced their behavior. When engrafted as a mixed population, the mesechmal cell endowed the neuroendocrine cells with metastatic capacity. These data give us a good evidence that poorest prognosis of SCLC cells is due to cells with mesenchymal character.38
The EMT has been proposed as a key step during carcinoma progression and metastasis development. The hallmark of EMT is the loss of epithelial marker (e.g. E‐cadherin, catenin) expression and induced expression of mesenchymal markers (e.g. N‐cadherin, vimentin, SMS‐actin).39, 40, 41 ZEB1 is a transcriptional repressor that functions as an inducer of EMT.42 In this study we examined the role of EMT in ZEB1‐promoted bone metastasis of SCLC cells. We used the ZEB1 overexpression vector to transfect SBC‐3 cells. After transfection, the cell morphology changed dramatically from round to fusiform, the expression of epithelial markers was decreased, and the expression of mesenchymal markers was increased. Thus, we considered that ZEB1 likely promoted SCLC invasion and bone metastasis via the EMT pathway, but the precise role of EMT and its heterogeneity character in SCLC with bone metastasis still requires further study.
In conclusion, we demonstrated that ZEB1 promoted bone metastasis in SCLC via the EMT pathway. Knockdown of ZEB1 expression significantly inhibited the progress of bone metastasis in SCLC both in vitro and in vivo. Upregulation of ZEB1 expression promoted invasion and bone metastasis in SCLC both in vitro and in vivo, and both morphological and molecular changes were observed. These results indicated that ZEB1 is a promising therapeutic target for SCLC with bone metastasis, however the mechanisms underling ZEB1's role in these processes still requires further study.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30873028). The cell lines SBC‐3 and SBC‐5 was gifted by Dr Saburo sone and Seiji Yano, Department of Internal Medicine and Molecular Therapeutics,University of Tokushima School of Medicine, Tokushima, Japan
References
- 1. Yano S, Zhang H, Hanibuchi M et al Combined therapy with a new bisphosphonate, minodronate (YM529), and chemotherapy for multiple organ metastases of small cell lung cancer cells in severe combined immunodeficient mice. Clin Cancer Res 2003; 14: 5380–5. [PubMed] [Google Scholar]
- 2. Al Husaini H, Wheatley‐Price P, Clemons M, Shepherd FA. Prevention and management of bone metastases in lung cancer: a review. J Thorac Oncol 2009; 4: 251–9. [DOI] [PubMed] [Google Scholar]
- 3. Hirsh V, Major PP, Lipton A et al Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclast activity. J Thorac Oncol 2008; 3: 228–36. [DOI] [PubMed] [Google Scholar]
- 4. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–93. [DOI] [PubMed] [Google Scholar]
- 5. Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther 2007; 6: 2609–17. [DOI] [PubMed] [Google Scholar]
- 6. Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia 2005; 10: 169–80. [DOI] [PubMed] [Google Scholar]
- 7. Miki T, Yano S, Hanibuchi M, Sone S. Bone metastasis model with multiorgan dissemination of human small‐cell lung cancer (SBC‐5) cells in natural killer cell‐depleted SCID mice. Oncol Res 2000; 12: 209–17. [DOI] [PubMed] [Google Scholar]
- 8. Zhang H, Yano S, Miki T et al A novel bisphosphonate minodronate (YM529) specifically inhibits osteolytic bone metastasis produced by human small‐cell lung cancer cells in NK‐cell depleted SCID mice. Clin Exp Metastasis 2003; 20: 153–9. [DOI] [PubMed] [Google Scholar]
- 9. Ma N, Liu L, Min J et al The effect of down regulation of calcineurin Aα by lentiviral vector‐mediated RNAi on the biological behavior of small‐cell lung cancer and its bone metastasis. Clin Exp Metastasis 2011; 28: 765–78. [DOI] [PubMed] [Google Scholar]
- 10. Liu Y, Zhang Y, Min J et al Calcineurin promotes proliferation, migration, and invasion of small cell lung cancer. Tumour Biol 2010; 31: 199–207. [DOI] [PubMed] [Google Scholar]
- 11. Spaderna S, Schmalhofer O, Wahlbuhl M et al The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res 2008; 68: 537–44. [DOI] [PubMed] [Google Scholar]
- 12. Mejlvang J, Kriajevska M, Vandewalle C et al Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell 2007; 18: 4615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial‐mesenchymal transition in human breast cancer‐observations in vitro and in vivo. Cells Tissues Organs 2007; 185: 191–203. [DOI] [PubMed] [Google Scholar]
- 14. Yang S, Du J, Wang Z et al BMP‐6 promotes E‐cadherin expression through repressing delta EF1 in breast cancer cells. BMC Cancer 2007; 7: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arumugam T, Ramachandran V, Fournier KF et al Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res 2009; 69: 5820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, VandenBoom TG, 2nd, Kong D et al Up‐regulation of miR‐200 and let‐7 by natural agents leads to the reversal of epithelial‐to‐mesenchymal transition in gemcitabine‐resistant pancreatic cancer cells. Cancer Res 2009; 69: 6704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takeyama Y, Sato M, Horio M et al Knockdown of ZEB1, a master epithelial‐to‐mesenchymal transition (EMT) gene, suppresses anchorage‐independent cell growth of lung cancer cells. Cancer Lett 2010; 296: 216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gemmill RM, Roche J, Potiron VA et al ZEB1‐responsive genes in non‐small cell lung cancer. Cancer Lett 2011; 300: 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clarhaut J, Gemmill RM, Potiron VA et al ZEB1, a repressor of the semaphorin 3F tumor suppressor gene in lung cancer cells. Neoplasia 2009; 11: 157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dohadwala M, Yang SC, Luo J et al Cyclooxygenase‐2–depende nt regulation of E‐cadherin: prostaglandin E(2) induces transcriptional repressors ZEB1 and snail in non‐small cell lung cancer. Cancer Res 2006; 66: 5338–45. [DOI] [PubMed] [Google Scholar]
- 21. Liu N, Bi F, Pan Y et al Reversal of the malignant phenotype of gastric cancer cells by inhibition of RhoA expression and activity. Clin Cancer Res 2004; 10: 6239–47. [DOI] [PubMed] [Google Scholar]
- 22. Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue‐specific expression of transgenes delivered by lentiviral vectors. Science 2002; 295: 868–72. [DOI] [PubMed] [Google Scholar]
- 23. Wang F, Reierstad S, Fishman D. Over‐expression in MCF‐7 cells enhances cellular invasiveness and progelatinase activation. Cancer Lett 2005; 236: 292–301. [DOI] [PubMed] [Google Scholar]
- 24. Muguruma H, Yano S, Kakiuchi S et al Reveromycin A inhibits osteolytic bone metastasis of small‐cell lung cancer cells, SBC‐5, through an antiosteoclastic activity. Clin Cancer Res 2005; 11: 8822–8. [DOI] [PubMed] [Google Scholar]
- 25. Miki T, Yano S, Hanibuchi M, Kanematsu T, Muguruma H, Sone S. Parathyroid hormone‐related protein (PTHrP) is responsible for production of bone metastasis, but not visceral metastasis, by human small cell lung cancer SBC‐5 cells in natural killer cell‐depleted SCID mice. Int J Cancer 2004; 108: 511–5. [DOI] [PubMed] [Google Scholar]
- 26. Zhang XH, Wang Q, Gerald W et al Latent bone metastasis in breast cancer tied to Src‐dependent survival signals. Cancer Cell 2009; 16: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pathi SP, Kowalczewski C, Tadipatri R, Fischbach C. A novel 3‐D mineralized tumor model to study breast cancer bone metastasis. PLoS One 2010; 5: e8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Msaouel P, Pissimissis N, Halapas A, Koutsilieris M. Mechanisms of bone metastasis in prostate cancer: clinical implications. Best Pract Res Clin Endocrinol Metab 2008; 22: 341–55. [DOI] [PubMed] [Google Scholar]
- 29. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415–28. [DOI] [PubMed] [Google Scholar]
- 30. Tse JC, Kalluri R. Mechanisms of metastasis: epithelial–to–mesen chymal transition and contribution of tumor microenvironment. J Cell Biochem 2007; 101: 816–29. [DOI] [PubMed] [Google Scholar]
- 31. Brabletz T, Hlubek F, Spaderna S et al Invasion and metastasis in colorectal cancer: epithelial‐mesenchymal transition, mesenchymal ‐epithelial transition, stem cells and beta‐catenin. Cells Tissues Organs 2005; 179: 56–65. [DOI] [PubMed] [Google Scholar]
- 32. Spaderna S, Schmalhofer O, Hlubek F et al A transient, EMT‐linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 2006; 131: 830–40. [DOI] [PubMed] [Google Scholar]
- 33. Yan‐Qi Z, Xue‐Yan G, Shuang H et al Expression and significance of TWIST basic helix‐loop‐helix protein over‐expression in gastric cancer. Pathology 2007; 39: 470–5. [DOI] [PubMed] [Google Scholar]
- 34. Miyoshi A, Kitajima Y, Kido S et al Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer 2005; 92: 252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lever AM, Strappe PM, Zhao J. Lentiviral vectors. J Biomed Sci 2004; 11: 439–49. [DOI] [PubMed] [Google Scholar]
- 36. Roodman GD. Biology of osteoclast activation in cancer. J Clin Oncol 2001; 19: 3562–71. [DOI] [PubMed] [Google Scholar]
- 37. Orr FW, Lee J, Duivenvoorden WC, Singh G. Pathophysiologic interactions in skeletal metastasis. Cancer 2000; 88: 2912–8. [DOI] [PubMed] [Google Scholar]
- 38. Calbo J, Montfort E, Proost N et al A fuctional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011; 19: 244–56. [DOI] [PubMed] [Google Scholar]
- 39. Prudkin L, Liu DD, Ozburn NC et al Epithelial‐to‐mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol 2009; 22: 668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cowin P, Rowlands TM, Hatsell SJ. Cadherins and catenins in breast cancer. Curr Opin Cell Biol 2005; 17: 499–508. [DOI] [PubMed] [Google Scholar]
- 41. Junghans D, Haas IG, Kemler R. Mammalian cadherins and protocadherins: about cell death, synapses and processing. Curr Opin Cell Biol 2005; 17: 446–52. [DOI] [PubMed] [Google Scholar]
- 42. Adachi Y, Takeuchi T, Nagayama T, Ohtsuki Y, Furihata M. Zeb1‐mediated T‐cadherin repression increases the invasive potential of gallbladder cancer. FEBS Lett 2009; 583: 430–6. [DOI] [PubMed] [Google Scholar]


