Abstract
The object of the present study was to identify markers for predicting urinary bladder cancer progression by comparative proteome analysis of bladder cancers and paired normal mucosas. We found that DDX39 was overexpressed in four of six bladder cancers examined compared with respective control tissues. Immunohistochemical analysis using 303 bladder cancer specimens revealed that DDX39 was inversely correlated to pT stage and histological grade progression. The incidence of DDX39high tumors (positive cells ≥50%) was 68.6%, 43.5%, 20.0%, and 5.3% in pTa, pT1, pTis, and ≥pT2 tumors, respectively, and 65.2%, 60.7%, and 19.6% in G1, G2, and G3 tumors, respectively. The incidence of DDX39high tumors was significantly lower in pT1 and ≥pT2 compared to pTa tumors, and also significantly lower in G3 compared to G1 and G2 tumors. Follow‐up analysis (n = 105) revealed that DDX39low tumors (positive cells <50%) were associated with disease progression (hazard ratio 7.485; P = 0.0083). Furthermore, DDX39‐knockdown bladder cancer cells increased their invasion ability compared to negative control cells. These results suggest that DDX39 is a suppressor of invasion and loss of its function predicts disease progression in bladder cancers. (Cancer Sci 2012; 103: 1363–1369)
Urinary bladder cancers account for approximately 54% of cancers of the urinary system (kidney, renal pelvis, ureters, bladder, and urethra).1 Approximately 90% of all bladder cancers are urothelial carcinomas.2 At initial presentation, up to 70% of tumors are non‐muscle‐invasive, whereas the remainder present with muscle‐invasive disease.3 The treatment for bladder cancer completely differs depending on stage. Generally, non‐muscle‐invasive bladder cancer (NMIBC) requires transurethral resection of the bladder tumor (TUR‐Bt), whereas most muscle‐invasive bladder cancer (MIBC) requires radical cystectomy with or without systemic chemotherapy. However, the prognosis for advanced bladder cancer is poor despite recent therapeutic advances.4 To date, pathological data, including grade, stage, and associated carcinoma in situ (CIS) at initial presentation, have provided some insight into predicting the likelihood of progression of bladder cancer.5, 6 Nevertheless, the ability to predict progression remains a challenge as bladder tumors with the same stage and grade have a heterogeneous clinical outcome. This might be due to differences in molecular expression profile. Furthermore, understanding the molecular biology of bladder cancer may provide new therapeutic strategies.
Various molecules have been reported to be associated with the progression of bladder cancer. Tumor suppressor genes, such as p53, have been widely studied in bladder cancer, however, its predictive value in assessing the risk of disease progression remains controversial.7, 8, 9 Ki‐67 has some prognostic value for predicting recurrence, however, further studies are necessary and the marker is not yet clinically applicable.10, 11 Recently, proteome analysis has been widely used in the study of urine from bladder cancer patients to identify biomarkers.12 Various urinary markers for the early detection of bladder cancer have been reported, but reliable urinary markers capable of predicting cancer progression have not been established. This is partly due to the fact that some of them are not expressed in bladder tissues.13 Therefore, investigation of protein expression profiling in bladder cancer tissues will facilitate not only understanding the behavior of cancer cells but also identification of markers of progression of bladder cancer.
The purpose of the present study is to identify markers of bladder cancer progression by comparative proteome analyses of human bladder cancer and paired normal tissues using QSTAR Elite liquid chromatography with tandem mass spectrometry and iTRAQ technology.
Materials and Methods
Patients
Six pairs of snap‐frozen bladder urothelial carcinomas and normal mucosa from cystectomy specimens were used for proteome analyses. The clinicopathological characteristics of the bladder carcinomas was as follows: case 1 was pT1, G3; case 2 was pTa, G3; case 3 was pT1, G3; case 4 was pT1, G2; case 5 was pT2a, G3; and case 6 was pT3, G3. Four cases (1–4) were NMIBCs and the remaining cases (5 and 6) were MIBCs. Immunohistochemical analysis was carried out on samples from 303 patients who were treated for bladder cancer by TUR‐Bt or cystectomy at Osaka City University Hospital (Osaka, Japan) between 2000 and 2009. There were 248 men (81.8%) and 55 women (18.2%), and the median age was 68 years (range, 33–90 years). Among these patients, those with CIS and those who were incompletely resected and lost to follow‐up were excluded from the study. The patients who were treated by total cystectomy were also excluded, because almost all of these patients had already progressed to muscle‐invasive disease. One hundred and five TUR‐Bt cases (between 2004 and 2007), for which full clinical data were available, were used for follow‐up analysis. Pathologic staging was carried out according to the 2002 TNM classification system,14 and grading was done according to the 1973 World Health Organization criteria for continuity of the study, as many samples were obtained before the 2004 criteria were published. The Institutional Review Board at Osaka City University Graduate School of Medicine approved the use of the specimens and clinical data in accordance with the Declaration of Helsinki and guidelines of Osaka City University Graduate School of Medicine.
Proteome analyses
Pathologic diagnoses of the six urothelial cancers and paired control tissues were confirmed before proteome analyses. Reagents, except for those specifically noted, were obtained from AB Sciex (Foster City, CA, USA). The specimens were homogenized and dissolved in 300 μL T‐PER Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL, USA) with protease inhibitor. After brief ultrasonication, insoluble material was removed by centrifugation at 13 000g for 15 min at 4°C. Protein concentration of the supernatant was measured by the BCA Protein Assay Kit (Pierce, Rockford, IL, USA). Protein reduction, alkylation, and subsequent peptide labeling were carried out using iTRAQ Reagent Multiplex Kit (AB Sciex, Foster City, CA, USA). Samples (100 μg of each) were resuspended in 20 μL of dissolution buffer (0.5 M triethylammonium bicarbonate at pH 8.5). One microliter of denaturant (2% SDS) and 2 μL reducing reagent (50 nM tris‐(2‐carboxyethyl) phosphine) were added and incubated at 60°C for 60 min. Free sulfhydryl groups of cysteines were blocked with 1 μL cysteine blocking reagent (20 mM methyl methanethiosulfonate) and incubated at room temperature for 10 min. Trypsin solution (10 μg) was added to each sample and incubated at 37°C overnight. Tryptic peptides of each sample were labeled with iTRAQ tags by incubation at room temperature for 1 h. Each of the samples was then mixed in one tube and fractionated by six concentrations of KCl solutions (10, 50, 70, 100, 200, and 350 mM) using ICAT cation exchange cartridge. Supernatant was evaporated in a vacuum centrifuge. Peptides of each fraction were resuspended into 2 mL of 2% acetonitrile and desalted using Sep‐Pak Light C18 cartridge (Waters, Milford, MA, USA). The supernatant was dissolved in 20 μL of 0.1% formic acid.
Proteome analysis was carried out with a DiNa‐AI nano system (KYA Technologies, Tokyo, Japan) coupled to a QSTAR EliteHybrid mass spectrometer through a NanoSpray ion source. Protein identification was done with ProteinPilot 2.0 software (AB Sciex).
Immunohistochemical analysis of DDX39
Formalin‐fixed, paraffin‐embedded tissues of 303 patients with bladder cancer were analyzed by immunohistochemical staining. Sections (3 μm‐thick) were cut and deparaffinized in xylene and rehydrated in alcohols and distilled water. Endogenous peroxidase was blocked with 3% hydrogen peroxide in distilled water for 5 min, followed by washing in PBS three times. Sections were then incubated with 1.5% goat serum in PBS for 15 min to bind non‐specific antigens and then with rabbit polyclonal antibody to DDX39 (ab96621, 1:500; Abcam, Cambridge, MA, USA) at 4°C overnight. This was followed by incubation with biotinylated goat anti‐rabbit IgG for 30 min and avidin–biotin peroxidase complex for 30 min at room temperature. Antigen was detected with 3,3‐diaminobenzidine and counterstaining with hematoxylin.
Immunohistochemical analysis was carried out by two pathologists who were blinded to the clinical data (S.Y. and H.W.). Immunoreactivity of DDX39 was observed in nuclei of bladder tissues but not in the normal urothelium. Under a microscope at ×200 magnification on six random fields per sample, tissues with ≥50% cancer cells immunoreactive for DDX39 were defined as DDX39high, and those with <50% cells immunoreactive for DDX39 were defined as DDX39low.
Cell lines
Human bladder cancer cell lines T24, TCCSUP, and UMUC3 were purchased from ATCC (Rockville, MD, USA). All cells were maintained as monolayer cultures at 37°C and 5% CO2. T24 was grown in McCoy's medium and TCCSUP and UMUC were grown in MEM. All media were supplemented with 10% FBS.
Western blot analyses
Whole cell lysates were collected using a cell scraper and resupended in CelLytic MT (Sigma, St Louis, MO, USA) with protease inhibitor. The amount of total protein was determined using a BCA protein assay kit (Pierce). Protein (15 μg of each) was loaded on 10% SDS–polyacrylamide gels. Proteins were transferred to a PVDF membrane and blocked with 5% skimmed milk in TBS buffer containing 0.1% Tween‐20. The membrane was probed with primary antibody for DDX39 (ab50697, 1:100; Abcam) or β‐actin (ab49900, 1:100 000; Abcam) for 1 h at room temparature. After washing, the membrane was incubated for 1 h at room temperature linked with HRP‐conjugated secondary antibody (#sc‐2004, 1:10 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunoreactive bands were detected using the ECL Plus Western blotting system (GE Healthcare, Piscataway, NY, USA) and LAS‐3000 image analysis system (Fujifilm, Tokyo, Japan).
Real‐time PCR
Total RNA was extracted from cell lines using the RNeasy Mini kit (Qiagen, Tokyo, Japan) according to the manufacturer's instructions. RNA concentration was determined by Nanodrop (Thermo Scientific). RNA (1 μg) was used for cDNA synthesis using Advantage RT‐for‐PCR kit (Takara Bio, Tokyo, Japan). The real time RT‐PCR assay was carried out with the Applied Biosystems 7500 Fast real‐time PCR machine (Applied Biosystems, Foster City, CA, USA). Real‐time RT‐PCR reactions consisted of 10 μL of 2× TaqMan FAST Universal Master Mix (Applied Biosystems), 1 μL of 20× TaqMan Gene Expression Assay (Applied Biosystems), and 1 μg cDNA solution. The assay IDs used for real‐time RT‐PCR were as follows: DDX39, Hs00271794_m1; and GAPDH, Hs00266705_g1. The thermal cycle program was: 20 s at 95°C followed by 40 cycles of 3 s at 95°C and 30 s at 60°C. The data were then quantified using the comparative C t method for relative gene expression compared with GAPDH as internal control.
Knockdown of DDX39
DDX39 expression was transiently knocked down using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. DDX39‐specific siRNAs (Silencer Select SiRNA; Cat.# s19917 and s19918) were obtained from Life Technologies (Grand Island, NY, USA). Non‐targeting control siRNA (Pre‐miR miRNA Precursor Starter Kit, Cat.# AM1540) was obtained from Life Technologies. T24 cells (3 × 105) were transiently transfected with 10 nM s19917, s19918, or control SiRNA in a six‐well plate. After 24 h, cells were trypsinized and used in cell proliferation and cell invasion assays.
Cell proliferation assay
T24 cells (1 × 104/well) were seeded in a 96‐well plate and transfected with 10 nM DDX39 siRNAs and control siRNA. After 24 h, cell proliferation was measured using a Cell Counting Kit‐8 (Dojindo Laboratories, Tokyo, Japan) according to the manufacturer's instructions. The number of cells was measured with a microplate reader (Bio‐Rad, Tokyo, Japan) at 450 nm.
Cell invasion assay
Invasion was assessed in a QCM cell invasion assay (Millipore, Billerica, MA, USA), according to the manufacturer's protocol. Briefly, transfectants (2 × 105 cells) were seeded in the upper chamber, whereas the lower chamber was loaded with medium containing 10% FBS. After a 24‐h incubation at 37°C, the cells that invaded the reverse side of the insert were dislodged by incubating the insert in 225 μL cell detachment buffer for 30 min at room temparature. Lysis buffer and CyQuant GR Dye mixture (75 μL each) were added in detachment buffer and the plate was incubated for 15 min in the dark. Then 200 μL of the mixture was transferred to a 96‐well plate and measured with a fluorescence plate reader at 480/520 nm.
Statistical analysis
Statistical analyses were carried out with SPSS version 19 (IBM, Armonk, NY, USA). Fisher's exact test was used to evaluate the differences in incidence of DDX39 expression patterns among clinical and pathological parameters. The progression‐free survival was defined as the time between the date of surgery and the last date of follow‐up or date of progression in pT status. The curves were done using the Kaplan–Meier method with the log–rank test to assess statistical significance. Cox proportional hazards analysis was used to determine the relative contribution of various factors to the risk of progression. P < 0.05 was considered statistically significant.
Results
Proteome analysis
We identified 493 proteins by proteomic analysis of six sample pairs. Overexpressed proteins were selected according to the criteria that the fold difference had to be >1.2.15 Fifteen proteins were overexpressed in cancer tissues compared to adjacent normal tissues in four or more of six sample pairs (Table 1). To validate the results of the proteome analysis, immunohistochemical staining of the above proteins in 303 bladder specimens was carried out, except for those that have already been evaluated in bladder cancers (Ezrin, nucleophosmin, prothymosin alpha, S100 calcium binding protein A11, and S100 calcium binding protein P).16, 17, 18, 19 Actin‐related protein 3 homolog B was not evaluated as no commercial antibody was available.
Table 1.
Upregulated proteins in cancer tissues using liquid chromatography with tandem mass spectrometry
| Symbol | Case | Full name | Location | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| ACTR3B | 4.200 | 2.359 | ND | 1.718 | 1.818 | 16.933 | Actin‐related protein 3 homolog B (yeast) | Unknown |
| BCAP31 | 2.797 | 2.186 | ND | 1.38 | 2.704 | ND | B‐cell receptor‐associated protein 31 | Cytoplasm |
| CCT4 | 4.008 | 1.561 | 1.650 | 1.296 | ND | 7.610 | Chaperonin containing TCP1, subunit 4 (delta) | Cytoplasm |
| DDX39 | 4.685 | 1.858 | 2.116 | ND | 1.900 | ND | DEAD (Asp‐Glu‐Ala‐Asp) box polypeptide 39 | Nucleus |
| EZR | 4.961 | 2.315 | 1.536 | 1.776 | 3.113 | 4.704 | Ezrin | Plasma membrane |
| FKBP4 | 19.763 | 3.820 | 2.308 | ND | 3.500 | ND | FK506 binding protein 4 | Nucleus |
| IDH1 | 4.618 | 3.137 | 1.278 | 1.580 | 2.662 | ND | Isocitrate dehydrogenase 1 (NADP+), soluble | Cytoplasm |
| KRT19 | 3.101 | 4.917 | ND | 1.437 | 5.312 | 4.614 | Keratin 19 | Cytoplasm |
| MYH9 | 2.918 | 2.888 | 1.413 | 1.473 | 2.438 | 6.401 | Myosin, heavy chain 9, non‐muscle | Cytoplasm |
| NPM1 | 1.529 | 1.240 | ND | ND | 1.684 | 11.318 | Nucleophosmin (nucleolar phosphoprotein B23, numatrin) | Nucleus |
| P4HB | 3.690 | 1.431 | 1.378 | ND | 2.286 | 10.049 | Prolyl 4‐hydroxylase, beta polypeptide | Cytoplasm |
| PTMA | 3.477 | 1.165 | 1.860 | ND | 2.102 | 3.062 | Prothymosin, alpha | Nucleus |
| S100A11 | 8.414 | 1.991 | 1.311 | 1.512 | 5.261 | 9.114 | S100 calcium binding protein A11 | Cytoplasm |
| S100P | 6.842 | 5.731 | ND | 2.128 | 8.834 | 3.450 | S100 calcium binding protein P | Cytoplasm |
| YBX1 | 2.978 | 1.711 | 1.644 | ND | 1.912 | ND | Y box binding protein 1 | Nucleus |
The numbers listed under cases 1–6 indicate the fold change of the protein expression level of cancer tissue compared to normal tissue in each case. ND, not detectable.
Results of immunohistochemistry analyses of DDX39, B‐cell receptor‐associated protein 31, chaperonin containing TCP1, FK506 binding protein 4, isocitrate dehydrogenase 1, keratin 19, myosin heavy chain 9 non‐muscle, prolyl 4‐hydroxylase beta polypeptide, and Y box binding protein 1 revealed that DDX39, but not the other proteins, was expressed in a different manner according to cancer stage and grade as described below, although all of them showed high expression levels in cancer compared to control tissues.
Expression of DDX39 in bladder cancers
The clinicopathological parameters of the patients whose tissues were used for immunohistochemical analysis are summarized in Table 2. As shown in Figure 1, nuclear staining of DDX39 was not observed in normal urothelium (Fig. 1D). Unexpectedly, expression levels of DDX39 were apparently lower in MIBCs when compared with NMIBCs. As summarized in Table 2, the incidence of DDX39low cancers was 31%, 56%, 80%, and 95% in pTa, pT1, pTis, and ≥pT2 tumors, respectively. The incidence of DDX39low cancers was significantly higher in ≥pT2 compared to pT1 and pTa cancers, and also significantly higher in pT1 when compared to pTa. Furthermore, the incidence of DDX39low cancers was 35%, 39%, and 80% in G1, G2, and G3 tumors, respectively, and significantly higher in G3 when compared to G1 and G2 tumors.
Table 2.
Clinicopathological characteristics of patients (n = 303), stages and grades of bladder cancer, and DDX39 expression
| Parameters | DDX39 high expression tumors (%) | P‐value |
|---|---|---|
| Age (68 ± 10.5 years) | ||
| <65 years (n = 116) | 52 (44.8) | NS |
| ≥65 years (n = 187) | 103 (55.1) | |
| Gender | ||
| Male (n = 248, 81.8%) | 127 (51.2) | NS |
| Female (n = 55, 18.2%) | 28 (50.1) | |
| Stage | ||
| pTa (n = 169, 55.7%) | 116 (68.6) | |
| pT1 (n = 62, 20.5%) | 27 (43.5) | 0.0005* |
| ≥pT2 (n = 57, 18.8%) | 3 (5.3) |
<0.0001* <0.0001† |
| pTis (n = 15, 5.0%) | 3 (20.0) | 0.0003* |
| Grade | ||
| G1 (n = 66, 21.8%) | 43 (65.2) | |
| G2 (n = 145, 47.9%) | 88 (60.7) | |
| G3 (n = 92, 30.4%) | 18 (19.6) | <0.0001**,†† |
*Statistically significant from pTa. **Statistically significant from G1. †Statistically significant from pT1. ††Statistically significant from G2. NS, not significant.
Figure 1.
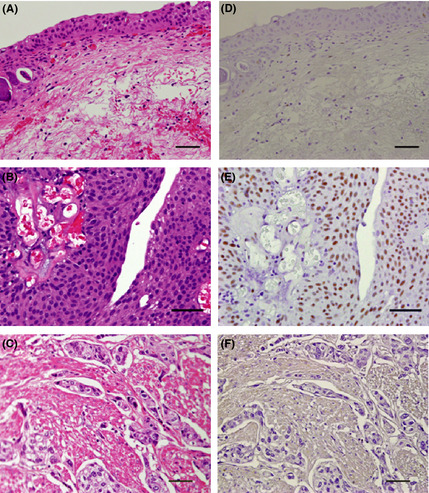
Hematoxylin–eosin staining (A–C) and immunohistochemical staining (D–F) for DDX39 of normal urothelium and bladder cancers. (A,D) Normal bladder urothelium; (B,E) DDX39high (positive cells ≥50%) pTa cancer; (C,F) DDX39low (positive cells <50%) pT2 cancer. Bar = 50 μm.
Follow‐up of patient outcomes and survival analysis
Correlation analysis of DDX39 expression level and recurrence/progression‐free survival in 105 bladder cancer patients who were treated by TUR‐Bt revealed that DDX39low cancers showed rapid disease progression (P = 0.0083; Fig. 2A). Moreover, rapid disease progression was also evident in DDX39low cancers when the 78 pTa cancers were analyzed from the above cases (P = 0.0027; Fig. 2B). No association was found with recurrence‐free survival in either of the analyses (data not shown).
Figure 2.
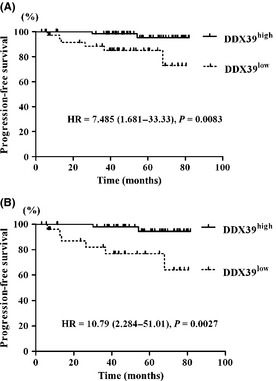
DDX39low cancers (positive cells <50%) showed rapid disease progression in pTa and pT1 cancers (A) (n = 105) and pTa cancers (B) (n = 78). HR, hazard ratio.
Univariate and multivariate analyses
Univariate and multivariate analyses of clinicopathological parameters and progression‐free survival revealed that low expression of DDX39 was an independent risk factor for progression (Tables 3, 4).
Table 3.
Univariate analyses of various clinicopathological parameters in relation to progression‐free survival of patients with bladder cancer
| Patients | Progression‐free survival | ||
|---|---|---|---|
| Parameters | No. cases (%) | No. events | P‐valuea |
| Age (years) | |||
| <65 | 46 (44) | 2 | 0.2971 |
| ≥65 | 59 (56) | 6 | |
| Gender | |||
| Male | 84 (80) | 6 | 0.6708 |
| Female | 21 (20) | 2 | |
| Stage | |||
| pTa | 78 (74) | 8 | 0.1050 |
| pT1 | 27 (26) | 0 | |
| Grade | |||
| G1 + G2 | 90 (86) | 6 | 0.3695 |
| G3 | 15 (14) | 2 | |
| Concominant CIS | |||
| No | 98 (93) | 7 | 0.4638 |
| Yes | 7 (7) | 1 | |
| No. tumors | |||
| Single | 76 (72) | 4 | 0.1624 |
| Multiple (2–7) | 29 (28) | 4 | |
| Tumor size | |||
| <3 cm | 101 (96) | 8 | 0.6123 |
| ≥3 cm | 4 (4) | 0 | |
| Tumor status | |||
| Primary | 76 (72) | 4 | 0.1830 |
| Recurrent | 29 (28) | 4 | |
| DDX39 expression | |||
| Low (positive cells <50%) | 39 (37) | 6 | 0.0083 |
| High (positive cells ≥50%) | 66 (63) | 2 | |
Log–rank test. CIS, carcinoma in situ.
Table 4.
Multivariate analyses for progression free survival
| Variables | Progression‐free survival | |
|---|---|---|
| OR (95% CI) | P‐value | |
| Grade | ||
| G1 + G2 | 1 | – |
| G3 | 1.084 (0.140–8.420) | 0.938 |
| Carcinoma in situ | ||
| − | 1 | – |
| + | 2.369 (0.178–31.484) | 0.514 |
| No. tumors | ||
| Single | 1 | – |
| Multiple | 2.532 (0.570–11.250) | 0.222 |
| Prior recurrence rate | ||
| Primary | 1 | – |
| Recurrence | 1.035 (0.337–3.176) | 0.952 |
| DDX39 expression | ||
| High | 1 | – |
| Low | 7.171 (1.284–40.063) | 0.025 |
–, normalized.
Expression levels of DDX39 and invasion ability of bladder cancer cells
The mRNA expression levels of DDX39 were analyzed by real‐time PCR in T24, TCCSUP, and UMUC3 cells. T24 cells showed the highest expression level of DDX39 (Fig. 3A) but the lowest invasion ability among the three bladder cancer cell lines (Fig. 3B). As T24 showed an inverse relationship between the expression level of DDX39 and its invasion ability, similar to that observed in the bladder cancer specimens analyzed above, we used T24 cells to investigate the effects of DDX39 knockdown on invasion ability.
Figure 3.
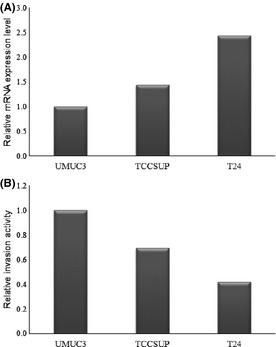
mRNA expression of DDX39 (A) and cell invasion ability (B) of UMUC3, TCCSUP, and T24 bladder cancer cells. The DDX39 expression level was highest, but the invasion ability was lowest, in T24 cells compared to the other two cell lines.
Effect of DDX39 knockdown on cell proliferation and invasion ability of T24 cells
Western blot analysis showed a remarkable reduction in protein level of DDX39 in T24 cells transfected with si‐DDX39 A (s19917) and B (s19918) compared with the negative control (Fig. 4A). Furthermore, real‐time PCR analysis showed that si‐DDX39 A and B reduced DDX39 mRNA expression levels by 71% and 74%, respectively, compared with the negative control (Fig. 4B). Although knockdown of DDX39 by si‐DDX39 A and B had no effect on cell proliferation (Fig. 5A), T24 cells transfected with si‐DDX39 A and si‐DDX39 B showed 2.36‐ and 2.65‐fold higher invasion activity, respectively, compared to the negative control (Fig. 5B).
Figure 4.
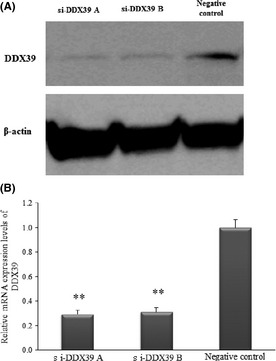
Two siRNAs for DDX39 (si‐DDX39 A and si‐DDX39 B) downregulated the protein (A) and mRNA expression of DDX39 (B). **P < 0.01.
Figure 5.
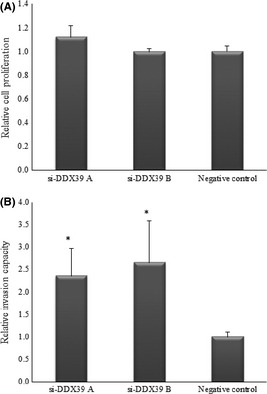
siRNA knockdown effects on cell proliferation and invasion ability using T24 bladder cancer cells. (A) No significant difference in cell proliferation was found between si‐DDX39 transfected cells and control cells. (B) Cells transfected with si‐DDX39 A and si‐DDX39 B gained significantly higher invasion ability compared to control cells. *P < 0.05.
Discussion
The results of the present study indicated that the expression level of DDX39 is significantly lower in MIBCs compared to NMIBCs. We also found that the DDX39 expression level was significantly correlated with pT stage and grade, and DDX39low cancers showed rapid disease progression. Furthermore, knockdown of DDX39 increased the invasion ability of bladder cancer cells. These findings indicated that DDX39 is a suppressor of invasion and loss of its function predicts disease progression in bladder cancers. To the best of our knowledge, the present study showed for the first time the relationship between DDX39 expression and cancer cell invasion.
A member of the RNA helicases, DDX39 is involved in pre‐mRNA splicing.20 RNA helicase is thought to be required for the export of mRNA out of the nucleus, transcription, splicing, and transport of mRNA.21 Although several other RNA helicases were reported to be dysregulated in cancer, and loss of normal function of RNA helicase could result in abnormal RNA processing,22 little is known about the exact roles of RNA helicases in carcinogenesis. Sugiura et al.23 reported that DDX39 was upregulated in lung squamous cell cancer and promoted cancer cell growth. However, we found that DDX39 inhibits the invasion, but had no effect on proliferation, of bladder cancer cells. Although the exact role of DDX39 in bladder carcinogenesis is not known, the fact that the expression level of DDX39 is significantly higher in NMIBCs compared to MIBCs, and that DDX39 has no effect on cell proliferation and is not expressed in normal bladder mucosa, suggested that DDX39 in NMIBCs may exert a protective role against bladder cancer invasion. Furthermore, based on the finding that DDX39low pTa cancers showed rapid disease progression, DDX39 might serve as a marker for NMIBCs that are likely to progress; those showing low levels of DDX39 may require more intensive therapy and closer follow‐up. Further study is needed to evaluate the underlying mechanisms by which DDX39 inhibits the invasion of bladder cancer.
Matrix metalloproteinase 2 and 9 and E‐cadherin were reported to be associated with bladder cancer invasion.24, 25 In the present study, the mRNA expression level of MMP2, MMP9, and E‐cadherin did not change significantly by DDX39 knockdown (data not shown). These results suggested that DDX39 inhibited invasion by mechanisms independent of these proteins.
Recurrence and progression are the main problems for NMIBCs, but few reports are available identifying molecules that could predict progression. The European Association of Urology (EAU) guideline on non‐muscle‐invasive urothelial carcinoma of the bladder has already proven to be useful to predict recurrence and progression,26 but its classification system is quite complicated to apply clinically. In addition, stratification based on the EAU guidelines for recurrence is not fully applicable to Japanese patients with bladder cancers.27 In the present study, cancer stage, histological grade, concomitant CIS, number of tumors, size of tumors, and prior recurrence rate could not predict cancer progression. This might be partially due to the sample size of our patients' dataset. But the results of the present study showed that only DDX39 had prognostic value for predicting progression of NMIBCs.
In conclusion, our results suggest that DDX39 is a suppressor of invasion and could be a useful molecular marker for predicting progression of urothelial carcinoma and a novel target for clinical therapy.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We are grateful to Rie Onodera, Kaori Nakakubo, Azusa Inagaki, Keiko Sakata, and Yuko Hisabayashi (Osaka City University, Osaka, Japan) for their technical assistance, to Yukiko Iura (Osaka City University) for her assistance in the preparation of this manuscript, and to Dr. Samuel M. Cohen (University of Nebraska Medical Center, Omaha, NE, USA) for reviewing this manuscript. This work was supported in part by a grant from the Ministry of Health, Labor and Welfare of Japan.
References
- 1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol 1998; 22: 1435–48. [DOI] [PubMed] [Google Scholar]
- 3. Rubben H, Lutzeyer W, Fischer N, Deutz F, Lagrange W, Giani G. Natural history and treatment of low and high risk superficial bladder tumors. J Urol 1988; 139: 283–5. [DOI] [PubMed] [Google Scholar]
- 4. Thurman SA, DeWeese TL. Multimodality therapy for the treatment of muscle‐invasive bladder cancer. Semin Urol Oncol 2000; 18: 313–22. [PubMed] [Google Scholar]
- 5. Althausen AF, Prout GR Jr, Daly JJ. Non‐invasive papillary carcinoma of the bladder associated with carcinoma in situ. J Urol 1976; 116: 575–80. [DOI] [PubMed] [Google Scholar]
- 6. Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15‐year outcome. J Urol 2000; 163: 60–1; discussion 1–2. [PubMed] [Google Scholar]
- 7. Habuchi T, Marberger M, Droller MJ et al Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology 2005; 66: 64–74. [DOI] [PubMed] [Google Scholar]
- 8. Esrig D, Spruck CH III, Nichols PW et al p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol 1993; 143: 1389–97. [PMC free article] [PubMed] [Google Scholar]
- 9. Goebell PJ, Groshen SG, Schmitz‐Drager BJ. p53 immunohistochemistry in bladder cancer–a new approach to an old question. Urol Oncol 2010; 28: 377–88. [DOI] [PubMed] [Google Scholar]
- 10. Kamai T, Takagi K, Asami H, Ito Y, Oshima H, Yoshida KI. Decreasing of p27(Kip1) and cyclin E protein levels is associated with progression from superficial into invasive bladder cancer. Br J Cancer 2001; 84: 1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Korkolopoulou P, Christodoulou P, Konstantinidou AE, Thomas‐Tsagli E, Kapralos P, Davaris P. Cell cycle regulators in bladder cancer: a multivariate survival study with emphasis on p27Kip1. Hum Pathol 2000; 31: 751–60. [DOI] [PubMed] [Google Scholar]
- 12. Letarte S, Brusniak MY, Campbell D et al Differential plasma glycoproteome of p19 skin cancer mouse model using the corra label‐free LC‐MS proteomics platform. Clin Proteomics 2008; 4: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsui KH, Tang P, Lin CY, Chang PL, Chang CH, Yung BY. Bikunin loss in urine as useful marker for bladder carcinoma. J Urol 2010; 183: 339–44. [DOI] [PubMed] [Google Scholar]
- 14. Kirkali Z, Chan T, Manoharan M et al Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology 2005; 66: 4–34. [DOI] [PubMed] [Google Scholar]
- 15. Sui J, Zhang J, Tan TL, Ching CB, Chen WN. Comparative proteomics analysis of vascular smooth muscle cells incubated with S‐ and R‐enantiomers of atenolol using iTRAQ‐coupled two‐dimensional LC‐MS/MS. Mol Cell Proteomics 2008; 7: 1007–18. [DOI] [PubMed] [Google Scholar]
- 16. Palou J, Algaba F, Vera I, Rodriguez O, Villavicencio H, Sanchez‐Carbayo M. Protein expression patterns of ezrin are predictors of progression in T1G3 bladder tumours treated with nonmaintenance bacillus Calmette–Guerin. Eur Urol 2009; 56: 829–36. [DOI] [PubMed] [Google Scholar]
- 17. Tsui KH, Juang HH, Lee TH, Chang PL, Chen CL, Yung BY. Association of nucleophosmin/B23 with bladder cancer recurrence based on immunohistochemical assessment in clinical samples. Acta Pharmacol Sin 2008; 29: 364–70. [DOI] [PubMed] [Google Scholar]
- 18. Tzai TS, Tsai YS, Shiau AL, Wu CL, Shieh GS, Tsai HT. Urine prothymosin‐alpha as novel tumor marker for detection and follow‐up of bladder cancer. Urology 2006; 67: 294–9. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Kaygusuz G, Wang L et al Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol 2007; 31: 673–80. [DOI] [PubMed] [Google Scholar]
- 20. Pryor A, Tung L, Yang Z, Kapadia F, Chang TH, Johnson LF. Growth‐regulated expression and G0‐specific turnover of the mRNA that encodes URH49, a mammalian DExH/D box protein that is highly related to the mRNA export protein UAP56. Nucleic Acids Res 2004; 32: 1857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sugiura T, Sakurai K, Nagano Y. Intracellular characterization of DDX39, a novel growth‐associated RNA helicase. Exp Cell Res 2007; 313: 782–90. [DOI] [PubMed] [Google Scholar]
- 22. Abdelhaleem M. Over‐expression of RNA helicases in cancer. Anticancer Res 2004; 24: 3951–3. [PubMed] [Google Scholar]
- 23. Sugiura T, Nagano Y, Noguchi Y. DDX39, upregulated in lung squamous cell cancer, displays RNA helicase activities and promotes cancer cell growth. Cancer Biol Ther 2007; 6: 957–64. [DOI] [PubMed] [Google Scholar]
- 24. Lipponen PK, Eskelinen MJ. Reduced expression of E‐cadherin is related to invasive disease and frequent recurrence in bladder cancer. J Cancer Res Clin Oncol 1995; 121: 303–8. [DOI] [PubMed] [Google Scholar]
- 25. Kumar B, Koul S, Petersen J et al p38 mitogen‐activated protein kinase‐driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP‐2 and MMP‐9 activity. Cancer Res 2010; 70: 832–41. [DOI] [PubMed] [Google Scholar]
- 26. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou‐Redorta J. EAU guidelines on non‐muscle‐invasive urothelial carcinoma of the bladder. Eur Urol 2008; 54: 303–14. [DOI] [PubMed] [Google Scholar]
- 27. Sakano S, Matsuyama H, Takai K et al Risk group stratification to predict recurrence after transurethral resection in Japanese patients with stage Ta and T1 bladder tumours: validation study on the European Association of Urology guidelines. BJU Int 2011; 107: 1598–604. [DOI] [PubMed] [Google Scholar]


