Abstract
Tumor progression has been recognized as the product of evolving crosstalk between cancer cells and the surrounding stromal cells. Cancer‐associated orthotopic myofibroblasts may be linked to the progression of gastric carcinomas. To understand the significance of orthotopic myofibroblasts, we examined the effects of cancer‐associated orthotopic myofibroblasts on the malignant phenotype of gastric cancer cells. Three human gastric cancer cell lines (OCUM‐2MD3, OCUM‐12, MKN‐45) and four human gastric fibroblast cell lines (cancer‐associated orthotopic fibroblast [CaF]‐29, CaF‐33, normal orthotopic fibroblast [NF]‐29, NF‐33) were used. The cancer‐associated orthotopic fibroblast cell lines CaF‐29 and CaF‐33 were established from a tumoral gastric wall, and normal orthotopic fibroblast NF‐29 and NF‐33 were established from a non‐tumoral gastric wall. Fibroblasts that were α‐smooth muscle actin‐positive were defined as myofibroblasts. We examined the effects of cancer‐associated orthotopic myofibroblasts on the aggressiveness of gastric cancer cells by wound‐healing assay, invasion assay, and RT‐PCR. The ratios of myofibroblasts in CaF‐29 (33%) and CaF‐33 (46%) were significantly (P < 0.001) greater than those in NF‐29 (11%) or NF‐33 (13%). Although all four orthotopic fibroblast lines increased the motility of gastric cancer cells, including migration and invasion ability, the motility‐stimulating activity of cancer‐associated fibroblasts (CaF‐29 and CaF‐33) was significantly higher than that of normal fibroblasts (NF‐29 and NF‐33). These motility‐stimulating activities of cancer‐associated orthotopic fibroblasts were downregulated by Smad2 siRNA treatment and anti‐transforming growth factor‐β neutralizing antibody. These findings suggest that cancer‐associated orthotopic myofibroblasts may play an important role in the progression of gastric cancers and that transforming growth factor‐β produced by myofibroblasts may be one of the factors associated with the aggressiveness of gastric carcinoma cells. (Cancer Sci 2012; 103: 797–805)
Tumor progression has been recognized as the product of evolving crosstalk between cancer cells and the surrounding tumor stroma.1 The tumor stroma consists of a variety of components, including fibroblasts, macrophages, and extracellular matrix. Of these cells, fibroblasts constitute a major stromal compartment and play a critical role in the regulation of tumor growth. Among the various types of fibroblasts, myofibroblasts, which are distinct from normal fibroblasts in their expression of αSMA, have recently been implicated in important aspects of epithelial solid tumor biology, such as neoplastic progression, tumor growth, and metastasis. Additionally, there may be some differences between the CAF existing in tumoral gastric walls and normal orthotopic fibroblasts in non‐tumoral gastric walls. Although we reported that orthotopic fibroblasts might play an important role in the progression, growth and spread of gastric cancers,2, 3, 4 the differential interactions with cancer cells of CAF and normal orthotopic fibroblasts are still unknown. Also, we reported that TGF‐β produced by orthotopic fibroblasts increases the invasiveness of scirrhous gastric cancer cells.2, 3 Signals from TGF‐β play an important role in the metastatic spread of cancer cells such as migration, invasion and the EMT,5, 6, 7, 8 and TGF‐β activates TGF‐β receptor type I and II, resulting in the activation of transcriptional factors, Smad2/3 and Smad4.6, 9 In this study, to understand the contribution of myofibroblasts to the malignant phenotype of gastric cancer, we examined the effect of cancer‐associated orthotopic myofibroblasts on the motility of gastric cancer cells compared normal orthotopic fibroblasts and analyzed the TGF‐β from myofibroblasts.
Materials and Methods
Cell culture and cell lines
We used three human gastric cancer cell lines, OCUM‐2MD3,10 OCUM‐12,11 and MKN‐45,12 derived from diffuse‐types of gastric carcinomas, and four human gastric fibroblast cell lines, CaF‐29, NF‐29, CaF‐33, and NF‐33, from the gastric wall of gastric carcinoma patients. The CAF, CaF‐29 and CaF‐33, were established from the tumoral gastric wall, and NF‐29 and NF‐33 were from non‐tumoral gastric wall. Both CaF‐29 and NF‐29 were from the same patient, and CaF‐33 and NF‐33 were from the same patient. The fibroblasts were used in the third through twelfth passage in culture, mainly at the fifth passage. The culture medium was composed of DMEM (Nikken, Kyoto, Japan) with the addition of 10% heat‐inactivated FBS (Life Technologies, Grand Island, NY, USA), 100‐IU/mL penicillin, 100‐μg/mL streptomycin, and 0.5‐mm sodium pyruvate. This study was approved by the Osaka City University ethics committee (Osaka, Japan). Informed consent was obtained from the patients prior to the study.
Determination of myofibroblast
To examine incubating myofibroblast content of orthotopic fibroblast, immunohistochemical staining was performed. Fibroblast cells (5 × 104 cells/mL) were seeded into 100‐mm dishes with 10‐mL DMEM containing 10% FCS. The number of fibroblasts in each dish was c. 2.5 × 106 cells after 3 days. Fibroblasts were washed with PBS and fixed with acetone for 5 min. They were then incubated with anti‐αSMA antibody (clone 1A4; 1:200; Dako, Cambridge, UK) for 1 h and with biotinylated rabbit anti‐mouse immunoglobulin G (Nichirei Corporation, Tokyo, Japan), treated with streptavidin‐peroxidase reagent (Nichirei), and counterstained with Mayer's hematoxylin. Cells with αSMA‐positive were determined as myofibroblasts. The percentage of binding cells was calculated as follows: number of myofibroblasts/number of total cells × 100. The percentage of αSMA‐positive myofibroblasts cells was determined in 10 random fields. At least three independent experiments were performed.
Immunofluorescence microscopy
To examine incubating myofibroblast content and TGF‐β expression in fibroblasts, immunofluorescence microscopy was performed with the triple‐immunofluorescence labeling technique. Fibroblasts were fixed with acetone and then blocked with 3% BSA (diluted in PBS). Fibroblasts were further incubated with anti‐human αSMA antibody (1:100; R&D Systems, Minneapolis, MN, USA), vimentin (1:50; Santa Cruz, Santa Cruz, CA, USA), or anti‐TGF‐β1 antibody (1:100; R&D Systems), and DAPI (1:10000; Wako, Osaka, Japan) for 60 min at room temperature. They were then viewed under a fluorescence microscope, Leica Digital Microscopy DMI 6000 (Heidelberg, Germany), with a DAPI filter (365 nm excitation), αSMA fluorescence with a phycoerythrin (PE) filter (546 nm excitation), and vimentin or TGF‐β with an FITC filter (450–490 nm excitation). Cells with αSMA‐positive were determined to be myofibroblasts.
Preparation of CM
We prepared CM from orthotopic fibroblasts and gastric cancer cells by seeding fibroblasts and gastric cancer cells (5.0 × 104 cells/mL) into 100‐mm plastic dishes with 10‐mL DMEM containing 2% FBS and then incubating for 3 days. The number of fibroblasts and gastric cancer cells in each dish was c. 2.5 × 106 cells after 3 days of incubation. To obtain CM, fibroblasts and gastric cancer cells were washed with PBS and then incubated for an additional 3 days in 3‐mL DMEM. Next, CM was collected from each dish. The supernatant was stored as CM at −20°C until use. As a control, DMEM was used instead of CM.
Morphologic findings
Cancer cells were cultured in addition with 50% of CM from orthotopic fibroblasts. Cell morphology was observed microscopically 72 h after the addition.
Wound‐healing assay
Gastric cancer cells were cultured in six‐well plates. After the cells reached semi‐confluence, a wound was created in the cell monolayer with a pipette tip. Cancer cells were cultured with 50% of CM from gastric fibroblasts. Four scratched fields were randomly chosen and the number of cell migrations was counted 48 h after treatment.
Invasion assay
We used the chemotaxicell chambers (Kubota, Osaka, Japan) with a 12‐μm pore membrane filter coated with 50‐μg Matrigel (Collaborative Research Co., Bedford, MA, USA). The chamber (upper component) was placed in a 24‐well culture plate (lower component). Gastric cancer cells were re‐suspended to a final concentration of 4 × 103 cells/mL. Next, 500‐mL cancer cell suspension and 500‐μL DMEM or CM from orthotopic fibroblasts were added to the upper and lower components, respectively (a final concentration: 50% CM of fibroblasts with 2% FBS). After incubation for 72 h, cancer cells on the upper surface of the membrane were removed by wiping and stained with hematoxylin. Cancer cells that invaded through a filter coated with Matrigel into the lower membrane were manually counted under a microscope at ×200 magnification. Six randomly chosen fields were counted for each assay. The mean of six fields was calculated as the sample value. For each group, the culture was done in triplicate.
Quantitative real‐time RT‐PCR
Real‐time RT‐PCR was performed to examine αSMA, vimentin, E‐cadherin, and TGF‐β mRNA expression. Cancer cells and fibroblasts were incubated in 3‐mL DMEM containing 2% FCS with 50% of CM each. After 3 days incubation, the total cellular RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). From 20‐μg RNA using random primers (Invitrogen), cDNA was prepared. To determine fold changes in each gene, RT‐PCR was performed on the ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA), with commercially available gene expression assays for αSMA (Hs00426835), vimentin (Hs00958116), and E‐cadherin (Hs01023894). Then, PCR was performed at 95°C for 15 s and 60°C for 60 s for 40 cycles. Glyceraldehyde‐3‐phosphate dehydrogenase was used as an internal standard to normalize mRNA levels. The threshold cycle (C t) values were used to calculate the relative expression ratios between control and treated cells with the formula described by Pfaffl.13
Effect of TGF‐β and CM from gastric fibroblasts on vimentin and E‐cadherin expression of gastric cancer cells
To examine vimentin and E‐cadherin expression of gastric cancer cells in the presence of CM and TGF‐β (R&D Systems) from fibroblasts, RT‐PCR was performed. Cancer cells were incubated in 3‐mL DMEM containing 2% FCS with 50% of each CM from fibroblasts or 10 ng/mL TGF‐β for 24 h, following which the cells were used for RT‐PCR.
Effect of Smad2 siRNA or anti‐TGF‐β neutralizing antibody on vimentin and E‐cadherin expression of gastric cancer cells
Cancer cells were incubated in 3‐mL DMEM containing 2% FCS with 50% of each CM from fibroblasts, 10‐ng/mL TGF‐β (R&D Systems), and 10‐μm anti‐TGF‐β neutralizing antibody (R&D Systems). After 3 days incubation, vimentin and E‐cadherin expression of each gastric cancer cell line was examined by RT‐PCR. The sequences for Smad2 siRNA are designed as previously reported: 14 Smad2 siRNA sense, 5′‐GUCCCAUGAAAAGACUUAAtt‐3′ and antisense, 5′‐UUAAGUCUUUUCAUGGGACtt‐3′. Control non‐targeting siRNA, Silencer Negative Control siRNA, AM4611, was purchased from Ambion (Austin, TX, USA). The transfection mixture was prepared by incubating 5‐μL siPORT Neo‐Fx (Ambion) and 295‐μL Opti‐MEMI. Next, CAF‐33 cells were prepared at 50–60% confluence in six‐well dishes. The transfection mixture (the final siRNA concentration was 30 nm) was added to the gastric cancer cells in each dish, which contained 2‐mL DMEM with 10% FBS. Then, 24 h after transfection, cancer cells were incubated in CM from CAF‐33 cells. After 3 days incubation, RT‐PCR was performed.
Statistical analysis
Data are expressed as mean ± SD from at least three independent experiments. Significant difference was analyzed using the unpaired Student's t‐test. Values of P < 0.05 were considered statistically significant. Pearson's correlation coefficient (r) was used to analyze the association between αSMA expression and motility or vimentin expression.
Results
Myofibroblast content of each gastric fibroblast
Figure 1(a) shows a representative microscopic photograph of fibroblasts by αSMA immunostain. A larger number of αSMA‐positive fibroblasts (myofibroblasts) were found in CaF‐29 cells than in NF‐29 cells at the fifth passage. Immunofluorescence microscopy also showed that NF‐29 and CaF‐29 fibroblasts contain αSMA‐positive (red) myofibroblast cells (Fig. 1b). The ratios of myofibroblasts in CaF‐29 (33%) and CaF‐33 (46%) were significantly greater (P < 0.001) than in NF‐29 (11%) and NF‐33(13%) at the fifth passage. Also, αSMA mRNA expression of CaF‐29 (4.1‐fold) and CaF‐33 (4.8‐fold) were significantly higher (P < 0.001) than that of NF‐29 (1.0‐fold, the control) and NF‐33 (1.2‐fold). No significant difference in αSMA mRNA expression level was found between the CaF‐29 and CaF‐33 (Fig. 1c). The correlation between the αSMA mRNA level and the αSMA protein level was significant (r = 0.965; Fig. 1d).
Figure 1.
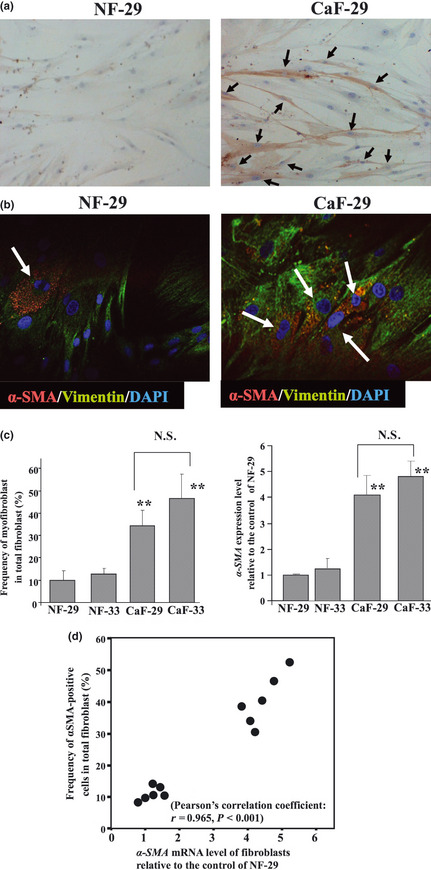
α‐Smooth muscle actin (αSMA) expression in fibroblasts. (a) αSMA immunostaining in CaF‐29 and NF‐29 fibroblasts at the fifth passage. αSMA‐positive myofibroblasts (arrows) were frequently found in CaF‐29, relative to NF‐29. (b) Immunofluorescence of NF‐29 fibroblasts and CaF‐29 fibroblasts. Fibroblasts were stained with αSMA (red) and vimentin (green), and cell nuclei were stained with DAPI (blue). The percentage of αSMA‐positive myofibroblasts (arrows) in CaF‐29 was higher than in NF‐29. (c) The myofibroblast content of CaF‐29 was significantly higher than that of NF‐29. The percentages of αSMA‐positive myofibroblast cells among the total fibroblasts were significantly higher (P < 0.001) in CaF‐29 (33%) and CaF‐33 (46%) than in NF‐29 (11%) and NF‐33(13%). Data are presented as mean ± SD (four samples per group). **P < 0.01. (d) A significant correlation (r = 0.965, P < 0.001) was found between the αSMA mRNA level and myofibroblast frequency among fibroblasts by Pearson's correlation coefficient test. CaF, cancer‐associated orthotopic fibroblast NF, normal orthotopic fibroblast; N.S., not significant.
Effect of CM from gastric fibroblasts on the migration of gastric cancer cells
Figure 2(a) shows phase‐contrast photographs of OCUM‐2MD3 cells. The number of migrating cancer cells from all cell lines significantly increased in the presence CM from each type of orthotopic fibroblast compared to those in the presence of the control. Moreover, the migrating ability of gastric cancer cells was significantly higher with the addition of CM from each CAF, CaF‐29 and CaF‐33, than with that from the normal fibroblasts, NF‐29 and NF‐33 (Fig. 2b). A significant correlation was found between αSMA expression and the migratory ability of gastric cancer cells in all cell lines (Fig. S1). Also, compared with negative siRNA, Smad2 siRNA decreased the migrating ability of OCUM‐2MD3 cells stimulated by CM from CaF‐33 (Fig. 2c).
Figure 2.
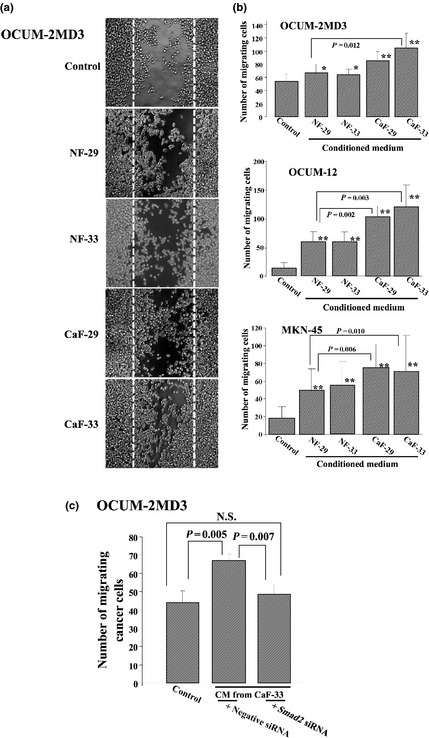
Effect of gastric fibroblasts on the wound‐healing ability of cancer cells. (a) Representative pictures of wound‐healing assay. The number of OCUM‐2MD3 cells able to migrate over the wound line (dotted line) increased in the presence of conditioned medium from fibroblasts but did not do so in the presence of the control. (b) Conditioned medium from fibroblasts significantly stimulated the migration by gastric cancer cells. The migration‐stimulating abilities of the cancer‐associated fibroblasts, CaF‐29 and CaF‐33, were higher than that of the normal fibroblasts, NF‐29 and NF‐33. Data are presented as mean ± SD (four samples per group). *P < 0.05; **P < 0.01. (c) Smad2 siRNA significantly inhibited the migration of OCUM‐2MD3 cells stimulated by conditioned medium from CaF‐33. CaF, cancer‐associated orthotopic fibroblast; CM, conditioned medium; NF, normal orthotopic fibroblast; N.S., not significant.
Effect of CM from gastric fibroblasts on gastric cancer cell invasion
In all gastric cancer cell lines, the number of invading cancer cells was significantly increased in the presence of CM from the CAF, CaF‐29 or CaF‐33, when compared to the control (Fig. 3a). In contrast, the invasion‐stimulating activity of the normal orthotopic fibroblast NF‐29 was only demonstrated for OCUM‐12 cells. A significant correlation was found between αSMA expression and the migratory ability of gastric cancer cells (Fig. S2). Also, Smad2 siRNA significantly inhibited the invasion of OCUM‐2MD3 cells stimulated by CM from CaF‐33 (Fig. 3b).
Figure 3.
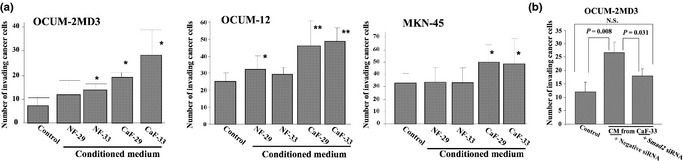
Invasion‐stimulating effect of cancer‐associated fibroblasts on gastric cancer cells. (a) Conditioned medium from cancer‐associated fibroblasts, CaF‐29 or CaF‐33, significantly inhibited invasion by scirrhous gastric cancer cells, but were not affected by the normal fibroblasts, NF‐29 and NF‐33. Data are presented as mean ± SD (four samples per group). *P < 0.05; **P < 0.01. (b) Compared with the negative siRNA control, Smad2 siRNA significantly reduced the invasion of OCUM‐2MD3 cells stimulated by conditioned medium from CaF‐33. CaF, cancer‐associated orthotopic fibroblast; CM, conditioned medium; NF, normal orthotopic fibroblast; N.S., not significant.
Effects of CM from gastric fibroblasts on the morphological characteristics of gastric cancer cells
An increase in the number of cancer cells displaying spindle cells was found after the addition of CM from the CAF, CaF‐29 or CaF‐33. However, these morphological changes were not evident in the presence of CM from orthotopic normal fibroblasts NF‐29 and NF‐33 (Fig. 4).
Figure 4.
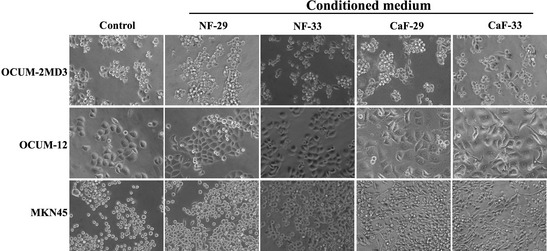
Representative phase‐contrast photographs of gastric cancer cells. The numbers of OCUM‐12, OCUM‐2MD3, and MKN45 cells displaying EMT transition significantly increased in the presence of CM from cancer‐associated fibroblasts, CaF‐29 and CaF‐33, when compared to those in the presence of the control. In contrast, a few gastric cancer cells showed EMT in the presence of CM from NF‐29 and NF‐33. CaF, cancer‐associated orthotopic fibroblast; CM, conditioned medium; EMT, epithelial‐to‐mesenchymal; NF, normal orthotopic fibroblast.
Effect of CM from gastric fibroblasts on vimentin and E‐cadherin expression of gastric cancer cells
Because vimentin and E‐cadherin are surrogate markers for EMT or motility, we examined the effect of CM on the motility of cancer cells in terms of vimentin and E‐cadherin expression. Vimentin expression of all three gastric cancer cells, OCUM‐12, OCUM‐2MD3, and MKN45, was significantly increased with the addition of CM from either cancer‐associated fibroblast, CaF‐29 or CaF‐33, but was not increased by CM from normal fibroblasts, NF‐29 and NF‐33. E‐cadherin expression of all gastric cancer cells, OCUM‐12, OCUM‐2MD3, and MKN45, was significantly decreased with the addition of CM from either cancer‐associated fibroblast, CaF‐29 or CaF‐33, but was not decreased by CM from normal fibroblasts, NF‐29 and NF‐33 (Fig. 5a). Vimentin expression of all gastric cancer cells was significantly correlated with αSMA expression at both the mRNA and protein level (Fig. S3). The expression level of vimentin was significantly increased by the addition of TGF‐β in OCUM‐2MD3, OCUM12, and MKN45 cells compared to that in the control. The neutralizing anti‐TGF‐β antibody downregulated vimentin expression stimulated by TGF‐β, which validated the neutralizing efficiency of anti‐TGF‐β antibody. Vimentin expression was downregulated by Smad2 siRNA and stimulated by TGF‐β in OCUM‐2MD3 cells, which also validated the silencing efficiency of Smad2 siRNA (Fig. 5b). Compared to the control, TGF‐β downregulated E‐cadherin expression of gastric cancer cells. The neutralizing anti‐TGF‐β antibody upregulated E‐cadherin expression, which was downregulated by TGF‐β, validating the neutralizing efficiency of anti‐TGF‐β antibody. In OCUM‐2MD3 cells, Smad2 siRNA upregulated E‐cadherin expression, which was downregulated by TGF‐β, validating the silencing efficiency of Smad2 siRNA (Fig. 5c). Vimentin expression of gastric cancer cells was stimulated by CM from CaF‐33. The anti‐TGF‐β antibody significantly decreased the expression level of vimentin stimulated by CM from CAF‐33. Taken together, Smad2 siRNA also significantly decreased the vimentin expression of OCUM‐2MD3, which was stimulated by CM from CAF‐33. In contrast, anti‐TGF‐β antibody and Smad2 siRNA did not affect the vimentin expression of cancer cells in the presence of CM from NF‐29 (Fig. 5d). E‐cadherin expression of gastric cancer cells was downregulated by CM from CaF‐33. The anti‐TGF‐β antibody significantly increased the expression level of E‐cadherin, which was downregulated by CM from CAF‐33. Taken together, Smad2 siRNA also significantly increased the E‐cadherin expression of OCUM‐2MD3 downregulated by CM from CAF‐33. In contrast, anti‐TGF‐β antibody and Smad2 siRNA did not affect the E‐cadherin expression of cancer cells in the presence of CM from NF‐29 (Fig. 5e).
Figure 5.
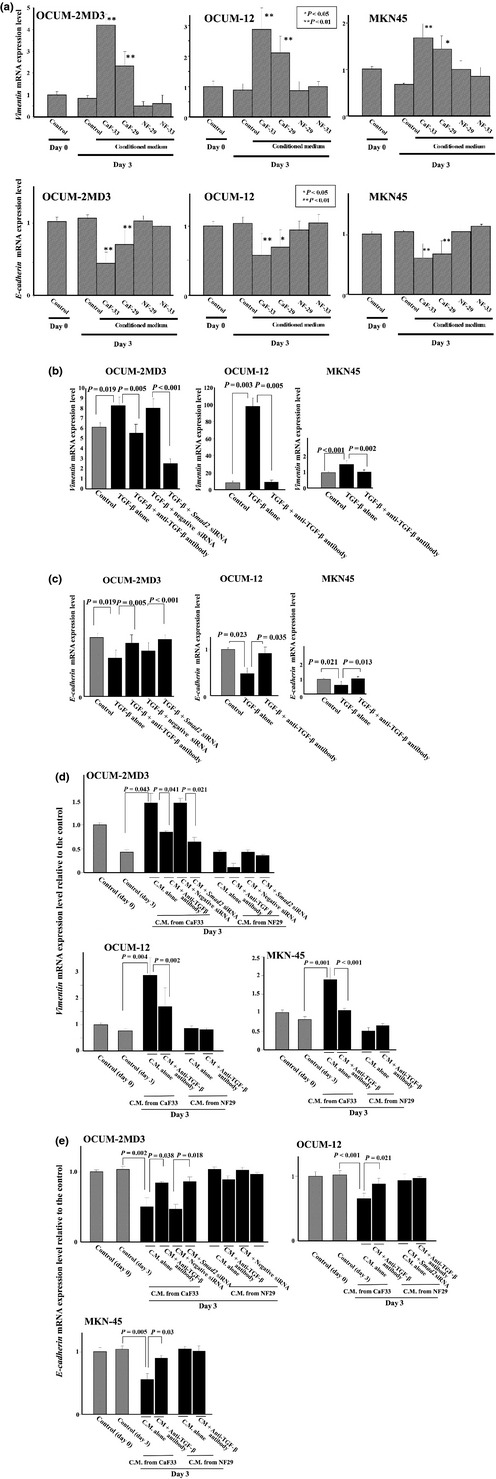
Effect of fibroblasts on the vimentin and E‐cadherin expression of gastric cancer cells. (a) Vimentin expression of all gastric cancer cells was significantly increased by the CM from the cancer‐associated fibroblasts, CaF‐29 and CaF‐33, but not by the CM from NF‐29 and NF‐33. E‐cadherin expression of all gastric cancer cells was significantly decreased by the CM from the cancer‐associated fibroblasts, CaF‐29 or CaF‐33, but not by the CM from NF‐29 and NF‐33. *P < 0.05; **P < 0.01. (b) TGF‐β stimulated the vimentin expression of gastric cancer cells. The neutralizing anti‐TGF‐β antibody downregulated vimentin expression, which was stimulated by TGF‐β. Smad2 siRNA (30 nmol/L) downregulated vimentin expression, which was stimulated by TGF‐β in OCUM‐2MD3 cells. However, the treatment of negative control siRNA (30 nmol/L) had no effect on the expression. The vimentin expression level was relative to the control of MKN45. (c) TGFβ decreased the E‐cadherin expression of gastric cancer cells. The neutralizing anti‐TGFβ antibody up‐regulated E‐cadherin expression decreased by TGFβ. Smad2 siRNA (30 nmol/L) up‐regulated E‐cadherin expression decreased by TGFβ in OCUM‐2MD3 cells, whereas treatment of negative control siRNA (30 nmol/L) had no effect on the expression. The E‐cadherin expression level was relative to the control of MKN45. (d) Smad2 siRNA and anti‐TGF‐β antibody decreased vimentin expression of gastric cancer cells induced by CM from the cancer‐associated fibroblast CaF‐33, but did not affect that of cancer cells in the presence of CM from NF‐29. (e) Smad2 siRNA and anti‐TGFβ antibody increased E‐cadherin expression of gastric cancer cells induced by CM from the cancer‐associated fibroblast CaF‐33, but did not affect that of cancer cells in the presence of CM from NF‐29. CaF, cancer‐associated orthotopic fibroblast; CM, conditioned medium; NF, normal orthotopic fibroblast; TGF‐β, transforming growth factor‐β.
Localization of TGF‐β in gastric fibroblasts
To examine TGF‐β expression in fibroblasts, immunofluorescence microscopy was performed with the triple‐immunofluorescence labeling technique. Frequently, TGF‐β‐positive fibroblasts were found in αSMA‐positive fibroblasts (Fig. 6).
Figure 6.
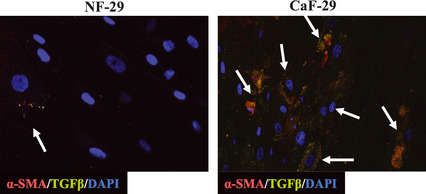
TGF‐β production from αSMA‐positive myofibroblasts. Immunofluorescence microscopic findings showed that TGF‐β expression was found in αSMA‐positive fibroblasts (arrows). αSMA‐positive fibroblasts were frequently found in CaF‐29 relative to in NF‐29. CaF, cancer‐associated orthotopic fibroblast; NF, normal orthotopic fibroblast; SMA, smooth muscle actin; TGF‐β, transforming growth factor‐β.
Discussion
It has been reported that the normal stroma contains few fibroblasts, but there is a dramatic increase in fibroblasts within the tumor stroma.1, 4 In this study, the myofibroblast content in CAF derived from gastric tumors was significantly higher than that of fibroblasts derived from normal gastric tissue. The cancer stroma might contain greater numbers of myofibroblasts than the normal stroma. We previously reported that myofibroblasts in the microenvironment of gastric cancer were significantly associated with an advanced stage, particularly for the macroscopically scirrhous‐type gastric carcinoma.13 These findings suggested that myofibroblasts might contribute to tumor progression.
We have reported that orthotopic fibroblasts might affect the invasiveness of gastric cancer cells.2, 3 We used only normal fibroblasts in the previous study, but the differing effects of orthotopic CAF and orthotopic normal fibroblasts on cancer cell motility remained unclear. Then, we examined the effect of cancer‐associated orthotopic myofibroblasts on the aggressiveness of cancer cells relative to the effect of normal orthotopic fibroblasts. In this study, both CAF and normal fibroblasts increased the motility of gastric cancer cells. The motility‐stimulating activity was higher in CAF than in normal fibroblasts. A significant correlation was found between the αSMA expression of fibroblasts and the motility of gastric cancer cells. These findings suggested that orthotopic CAF, compared to normal orthotopic fibroblasts, might be particularly important for the motility of cancer cells.
It has been reported that there is a multiplicity of molecules involved in the interaction between cancer cells and (myo)fibroblasts.16, 17, 18, 19 Previous studies have reported that TGF‐β signals play an important role in the metastatic spread of cancer cells such as migration, invasion and EMT.5, 6, 7, 8 TGF‐β‐positive fibroblasts were frequently found in αSMA‐positive fibroblasts by immunofluorescence microscopy, which may indicate that αSMA‐positive myofibroblasts frequently produce TGF‐β. When we analyzed the effect of TGF‐β involved with myofibroblasts on the motility of cancer cells, the motility‐stimulating activity of CAF was downregulated by Smad2 siRNA treatment and anti‐TGF‐β antibody. Because vimentin expression contributes to the highly invasive phenotype of cancer cells,20, 21, 22, 23 we used vimentin as a surrogate biomarker to determine the biological aggressiveness of the cancer cells. Both CAF and TGF‐β increased the vimentin expression of gastric cancer cells, and αSMA expression in fibroblasts was significantly correlated with vimentin expression of all gastric cancer cells. The anti‐TGF‐β antibody and Smad2 siRNA significantly decreased the vimentin expression of cancer cells, which was stimulated by TGF‐β or orthotopic CAF in OCUM‐2MD3 cells. These findings suggest that TGF‐β produced from cancer‐associated myofibroblasts may be a factor associated with the aggressive phenotype of gastric carcinoma cells. Recently, we have found that TGF‐β from gastric carcinoma cells also upregulates the number of myofibroblasts in CAF.24 As such, TGF‐β might play an important role in the tumor‐stroma interaction (i.e. paracrine vs autocrine manner) in the microenvironment of gastric carcinoma.
In conclusion, cancer‐associated orthotopic myofibroblasts play an important role in the progression of gastric cancers. TGFβ produced from orthotopic fibroblasts might be one of the factors associated with the aggressive phenotype of gastric carcinoma cells.
Disclosure Statement
The authors have no conflicts of interest.
Abbreviations
- CaF
cancer‐associated orthotopic fibroblast (cell line)
- CAF
cancer‐associated orthotopic fibroblast
- CM
conditioned medium
- EMT
epithelial‐to‐mesenchymal transition
- NF
normal orthotopic fibroblast (cell line)
- SMA
smooth muscle actin
- TGF‐β
transforming growth factor‐β
Supporting information
Fig. S1. Correlation between migratory ability of gastric cancer cells and αSMA expression of fibroblasts.
Fig. S2. Correlation between invasion ability of gastric cancer cells and αSMA expression of fibroblasts.
Fig. S3. Correlation between vimentin expression and αSMA expression in gastric cancer cells.
Acknowledgments
We would like to thank Masako Shinkawa (Osaka City University Graduate School of Medicine) for technical advice on the immunohistochemical staining. This study was supported in part by Grants‐in‐Aid for Scientific Research (KAKENHI) from Japan's Ministry of Education, Science, Sports, Culture and Technology and by the Foundation for Promotion of Cancer Research (Nos. 20591573, 22390262, and 23390329).
References
- 1. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401. [DOI] [PubMed] [Google Scholar]
- 2. Yashiro M, Hirakawa K. Cancer‐stromal interactions in scirrhous gastric carcinoma. Cancer Microenviron 2010; 3: 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inoue T, Chung YS, Yashiro M et al Transforming growth factor‐beta and hepatocyte growth factor produced by gastric fibroblasts stimulate the invasiveness of scirrhous gastric cancer cells. Jpn J Cancer Res 1997; 88: 152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Semba S, Kodama Y, Ohnuma K et al Direct cancer‐stromal interaction increases fibroblast proliferation and enhances invasive properties of scirrhous‐type gastric carcinoma cells. Br J Cancer 2009; 101: 1365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saito H, Tsujitani S, Oka S et al An elevated serum level of transforming growth factor‐beta 1 (TGF‐beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res 2000; 20: 4489–93. [PubMed] [Google Scholar]
- 6. Massagué J. TGFbeta in cancer. Cell 2008; 134: 215–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res 2008; 68: 9107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thiery JP. Epithelial‐mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–54. [DOI] [PubMed] [Google Scholar]
- 9. Heldin CH, Miyazono K, ten Dijke P. TGF‐beta signalling from cell membrane to nucleus through SMAD proteins. Nature 1997; 390: 465–71. [DOI] [PubMed] [Google Scholar]
- 10. Nishimura S, Chung YS, Yashiro M, Inoue T, Sowa M. Role of alpha 2 beta 1‐ and alpha 3 beta 1‐integrin in the peritoneal implantation of scirrhous gastric carcinoma. Br J Cancer 1996; 74: 1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kato Y, Yashiro M, Noda S et al Establishment and characterization of a new hypoxia‐resistant cancer cell line, OCUM‐12/Hypo, derived from a scirrhous gastric carcinoma. Br J Cancer 2010; 102: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Motoyama T, Hojo H, Watanabe H. Comparison of seven cell lines derived from human gastric carcinomas. Acta Pathol Jpn 1986; 36: 65–83. [DOI] [PubMed] [Google Scholar]
- 13. Pfaffl MW. A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 2001; 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawajiri H, Yashiro M, Shinto O et al A novel transforming growth factor beta receptor kinase inhibitor, A‐77, prevents the peritoneal dissemination of scirrhous gastric carcinoma. Clin Cancer Res 2008; 14: 2850–60. [DOI] [PubMed] [Google Scholar]
- 15. Fuyuhiro Y, Yashiro M, Noda S et al Myofibroblasts are associated with the progression of scirrhous gastric carcinoma. Exp Ther Med 2010; 1: 547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9: 239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cat B, Stuhlmann D, Steinbrenner H et al Enhancement of tumor invasion depends on transdifferentiation of skin fibroblasts mediated by reactive oxygen species. J Cell Sci 2006; 119: 2727–38. [DOI] [PubMed] [Google Scholar]
- 18. De WeverO, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 2008; 123: 2229–38. [DOI] [PubMed] [Google Scholar]
- 19. De WeverO, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol 2003; 200: 429–47. [DOI] [PubMed] [Google Scholar]
- 20. Iwatsuki H, Sasaki K, Suda M, Itano C. Vimentin intermediate filament protein as differentiation marker of optic vesicle epithelium in the chick embryo. Acta Histochem 1999; 101: 369–82. [DOI] [PubMed] [Google Scholar]
- 21. Gilles C, Polette M, Mestdagt M et al Transactivation of vimentin by beta‐catenin in human breast cancer cells. Cancer Res 2003; 63: 2658–64. [PubMed] [Google Scholar]
- 22. Korsching E, Packeisen J, Liedtke C et al The origin of vimentin expression in invasive breast cancer: epithelial‐mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J Pathol 2005; 206: 451–7. [DOI] [PubMed] [Google Scholar]
- 23. Fuyuhiro Y, Yashiro M, Noda S et al Clinical significance of vimentin‐positive gastric cancer cells. Anticancer Res 2010; 30: 5239–43. [PubMed] [Google Scholar]
- 24. Fuyuhiro Y, Yashiro M, Noda S et al Upregulation of cancer‐associated myofibroblasts by TGF‐β from scirrhous gastric carcinoma cells. Br J Cancer 2011; 105: 996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Correlation between migratory ability of gastric cancer cells and αSMA expression of fibroblasts.
Fig. S2. Correlation between invasion ability of gastric cancer cells and αSMA expression of fibroblasts.
Fig. S3. Correlation between vimentin expression and αSMA expression in gastric cancer cells.


