Abstract
Angiogenesis is one of the hallmarks of tumor growth and metastasis. Identification of tumor angiogenic factors has been a critical component in understanding cancer biology and treatment. Intermedin (IMD) has been reported to promote angiogenesis in a rat ischemic model and human umbilical vascular endothelial cells. Our study sought to determine the role of IMD in human hepatocellular carcinoma tumor progression. High IMD mRNA expression levels were observed in human hepatocellular carcinoma tumors, even in early stage disease, by real‐time RT‐PCR. Immunohistochemical analysis of hepatocellular carcinoma clinical samples demonstrated that the tumor regions were significantly more immunoreactive for IMD than adjacent benign liver. Inhibition of IMD expression using RNA interference reduced cell proliferation in SK‐Hep‐1 and SNU‐398 cells. Blockage of IMD signaling using either an antagonist peptide or a neutralizing antibody inhibited growth in a dose‐dependent manner with concomitant induction of apoptosis, causing cleavage of caspase‐8 and downregulation of Gli1 and Bcl2. Conversely, addition of IMD active peptide increased the phosphorylation level of extracellular signal‐regulated kinase. Thus, IMD might play an important role in cell proliferation and survival of hepatocellular carcinoma. Our data suggests that IMD is a potential biomarker and therapeutic target for hepatocellular carcinoma. (Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02341.x, 2012)
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide, and the third leading cause of cancer death.1 The majority (>80%) of liver cancers are HCC.2 Conventional chemotherapy and hormonal therapies have minimal response rates.3 Therapeutic agents targeting angiogenesis, which is required for tumor growth and metastasis, are currently in development.4, 5 Of the known angiogenic factors, vascular endothelial growth factor (VEGF) is the most potent pro‐angiogenic factor and is secreted by nearly all solid cancers.5 Sorafenib, an inhibitor targeting the VEGF and platelet‐derived growth factor receptors, is the standard of care in patients with advanced HCC.6 Promising novel inhibitors are under investigation.7 The key to targeting angiogenesis successfully in the future may be to use a combination of multiple inhibitors against multiple targets.8
Intermedin (IMD, adrenomedullin‐2), is a secreted peptide that belongs to the calcitonin/calcitonin gene‐related peptide family.9 IMD regulates diverse physiological processes. For example, IMD is required for rat normal fetoplacental growth,10 functions as a vasodilator and reduces blood pressure in both normal and hypertensive rats.11 IMD is expressed in every endocrine organ of the hypothalamo–pituitary–adrenal axis together with its receptor, calcitonin receptor‐like receptor (CRLR).12
The signaling of IMD is mediated by the interaction of CRLR in association with receptor activity modifying protein 3 (RAMP3).13 IMD is expressed by endothelial cells, smooth muscle cells and cardiomyocytes at a basal level with enhanced expression in response to hypoxic stress14 or high blood pressure.15 In addition, IMD can be induced by hypoxia‐inducible factors (HIF) 1α and 1β in hypoxic hepatocytes.14
The calcitonin peptide family, of which IMD is a member, has a common secondary structure with an N‐terminal ring structure and an amidated carboxyl terminus. Truncation of the N‐terminal ring structure produces antagonist peptides in all family members.16 Significantly, removal of N‐terminal and C‐ terminal regions of IMD yields the IMD antagonist peptide IMD17‐47aa, which has been shown to block biological function of IMD through a receptor‐mediated mechanism.10, 17, 18
Relatively little is known about how IMD might influence cancer development. Smith et al. (19) report that IMD stimulated neovascularization of the rat ischemic hindlimb. IMD induces pro‐angiogenic responses in human vascular endothelial cells by stimulating VEGF synthesis and transactivating the VEGF receptor 2 signal pathway.20 IMD is noted to be expressed in aldosterone‐secreting adenomas and pheochromocytomas.13 Moreover, an elevated expression of IMD was observed in adrenal tumors compared with adjacent normal tissues.12 In addition, a related family member adrenomedullin (ADM) is reported to be functionally active in HCC.21 Therefore, we chose to investigate the role of IMD in HCC tumor progression. We discovered that IMD is overexpressed in human HCC tumors and cell lines and functions to regulate tumor cell growth and apoptosis.
Materials and Methods
Tissue specimens
In the present study, 50 pathologist‐verified human tissues were analyzed for gene expression using Liver Cancer cDNA Array I (OriGene Technologies, Rockville, MD, USA) or RNA from frozen tissues. Detailed pathology reports are available online: http://www.origene.com/assets/documents/TissueScan/LVRT-101.xls.
A total of 22 human HCC tissue samples in paraffin blocks were obtained through Yale Pathology database (10 cases) and US Biomax (Rockville, MD, USA) (12 cases) to perform immunohistochemistry for IMD. Three investigators, including two board‐certified pathologists, assessed the stained sections. This study was approved by the Human Investigation Committee of Yale University School of Medicine (HIC protocol No. 1003006479).
Cell culture and reagents
The primary human liver cells were purchased from Invitrogen LifeSciences (Grand Island, NY, USA). The THLE‐3, SK‐Hep‐1, SNU‐398, HepG2, Hep3B and Huh7 cell lines were purchased from the American Type Culture Collection (Manassas, VA, USA). IMD active and antagonist peptides were purchased from Phoenix Pharmaceuticals (Burlingame, CA, USA). Control rabbit IgG and rabbit antibody against IMD were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Quantitative RT‐PCR
Relative expression levels of various genes were assessed by qRT‐PCR, as described previously.22 The mRNA level of VEGF, IMD, ADM, CRLR, RAMP3, 2′‐5′‐oligoadenylate synthetase 1 (OAS1), β‐actin and 18s rRNA was assessed using the TaqMan Gene Expression real‐time PCR assays (Applied Biosystems, Carlsbad, CA, USA). The results were expressed as the threshold cycle (Ct). The relative quantification of the target transcripts normalized to the endogenous control 18s rRNA or β‐actin was determined by the comparative Ct method (ΔCt) and the 2‐ΔΔCt method was used to analyze the relative changes in gene expression between the tested cell lines according to the manufacturer's protocol (User Bulletin No. 2, Applied Biosystems).
Immunoblotting
Cell lysates were prepared and immunoblottings were performed as described.23 The membranes were hybridized with antibodies recognizing CRLR, RAMP3, GAPDH (Santa Cruz Biotechnology), Gli1, Bcl2, Caspase‐8, phospho‐ and total Erk (Cell Signaling Technologies, Danvers, MA, USA) at 1:1000 dilution. Following incubation with the horseradish peroxidase‐conjugated goat‐anti‐rabbit or mouse IgG (Zymed Laboratories, South San Francisco, CA, USA) at 1:3000 dilution, protein signals were visualized with enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA, USA).
Immunohistochemical staining of tumor tissues
The specimens were cut into 5‐μm sections and attached to glass slides. The sections were dewaxed, hydrated, and blocked in 3% hydrogen peroxide and 2.5% normal horse serum in PBS/0.1% Tween20 for 1 h followed by incubation with anti‐IMD (0.67 μg/mL) or non‐immune rabbit IgG (0.67 μg/mL) overnight at 4°C. ImmPRESS peroxidase‐anti‐rabbit IgG (Vector Laboratories, Burlingame, CA, USA) was used to detect primary antibodies. The color was developed using a Vector DAB substrate kit and counterstained with hematoxylin (Vector Laboratories). Slides were observed using an Axiovert 40 CFL microscope (Carl Zeiss Microimaging, Thornwood, NY, USA) and photographed using a Retiga Exi camera (Q Imaging, Surrey, BC, Canada). For immunofluorescent staining, tissues were treated the same as described above and then counterstaining for IMD with anti‐rabbit Alexafluor 535 nm and Ki67 with anti‐mouse fluoroscein isothiocyanate at room temperature for 1 h. After PBS washes, the slides were mounted with Prolong containing DAPI (Invitrogen LifeSciences) and examined by fluorescence microscopy. Spot software (Diagnostic Instruments) was used for picture processing.
RNA interference
An siRNA SMART pool targeting IMD and a universal negative control small interfering RNA (control siRNA) were purchased from Dharmacon (Lafayette, CO, USA). Cells were transfected with IMD siRNA or control siRNA using DharmaFECT 4 reagent (Dharmacon).
Cell proliferation assay
Cell proliferation was measured using either WST‐1 reagent (Roche Diagnostics, Indianapolis, IN, USA) or BrdU incorporation (Cell Signaling Technologies) according to the manufacturers' instruction. Absorbances were read using a microplate reader (Power Waves XS, BioTek Instruments, Winooski, VT, USA).
Apoptosis assays
Cells were stained with FITC‐labeled Annexin‐V and propidium iodide (PI),24 according to the manufacturer's instructions (Bender MedSystems, Burlingame, CA, USA). At least 20 000 events were collected on a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and analyzed using Cellquest software.
Statistical analysis
All data are expressed as mean ± SEM. Statistical differences were measured by the Student's t‐test for parametric values and by the Mann–Whitney U‐test for non‐parametric values using GraphPad Prism software (GraphPad Prism, La Jolla, CA, USA). A P‐value of < 0.05 was considered significant.
Results
Intermedin is upregulated in human hepatocellular carcinoma tumors and cell lines
To determine if IMD is present in human HCC tumor samples, we examined IMD levels in 50 specimens of HCC (of diverse AJCC stage) and adjacent benign liver parenchyma by real‐time RT‐PCR. As shown in Figure 1(A), the level of IMD expression was significantly higher (8.7‐fold, P = 0.003) in tumors as compared with non‐tumor liver tissue. Among these 50 samples, 30 samples are paired tissues (non‐tumor tissue versus tumor tissue) from the same donors. Of the 15 paired samples, 12 showed elevated IMD expression (6.5‐fold, P = 0.021) in tumor relative to non‐tumor tissues (Fig. 1B). Of note, the IMD mRNA level was elevated in all the early stage (stages I and II) tumors examined (Fig. 1B). There was also a trend between IMD gene expression and grade of disease. IMD expression was 8‐fold higher in well‐differentiated tumors, 7‐fold higher in moderately differentiated tumors, and 3.8‐fold higher in poorly differentiated tumors as compared with non‐tumor liver tissue (Table 1).
Figure 1.
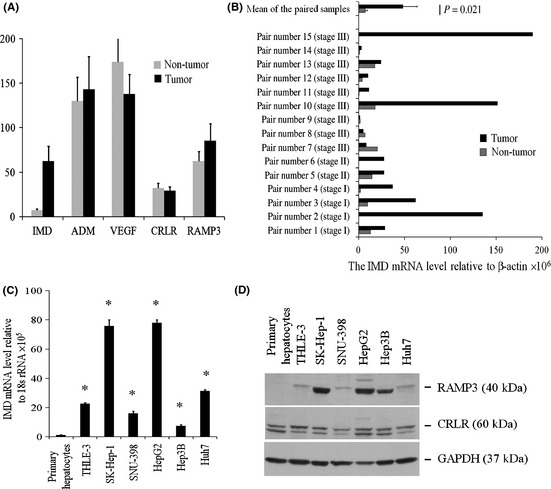
Expression of intermedin (IMD), adrenomedullin (ADM), vascular endothelial growth factor (VEGF), calcitonin receptor‐like receptor (CRLR) and receptor activity modifying protein 3 (RAMP3) in hepatocellular carcinoma (HCC) tumors and cell lines. (A) The mRNA levels in 50 human liver tissues were assessed using quantitative RT‐PCR and plotted as the level relative to β‐actin level in each tissue. (B) IMD mRNA level in 15 surgically‐resected specimens of HCC and paired non‐tumor liver tissues. (C) Relative IMD mRNA level in cell cultures. Data are shown as the mean ± SEM (n = 3). *P < 0.05. (D) Representative immunoblot revealed endogenous expression of CRLR and RAMP3 in all the HCC cell lines examined.
Table 1.
Clinicopathological characteristics of HCC tumors and IMD expression
| Characteristics | Number of patients/Total number analyzed | IMD mRNA level relative to β‐actin ×106 | Note |
|---|---|---|---|
| Median age (range) | 66 (21–86) (years) | ||
| 21–50 years old | 10/50 (20%) | 39.714 ± 23.506 | |
| 51–60 years old | 10/50 (20%) | 34.119 ± 22.069 | |
| 61–70 years old | 14/50 (28%) | 63.733 ± 17.127 | |
| 71–86 years old | 16/50 (32%) | 19.690 ± 4.693 | |
| Gender | |||
| Male | 37/50 (74%) | 26.996 ± 5.821 | |
| Female | 13/50 (26%) | 73.043 ± 22.179 | versus male, P = 0.029 |
| Tumor gradea | |||
| NT | 22/50 (44%) | 8.488 ± 1.715 | |
| AJCC G1: Well differentiated | 9/50 (18%) | 68.767 ± 29.561 | versus NT, P = 0.044 |
| AJCC G2: Moderately differentiated | 13/50 (26%) | 62.690 ± 20.156 | versus NT, P = 0.008 |
| AJCC G3: Poorly differentiated | 4/50 (8%) | 32.843 ± 11.474 | versus NT, P = 0.034 |
| Not reported | 2/50 (4%) | ||
| AJCC clinical stagea | |||
| NT | 22/50 (44%) | 8.488 ± 1.715 | |
| I | 10/50 (20%) | 67.997 ± 16.130 | versus NT, P = 0.002 |
| II | 8/50 (16%) | 61.924 ± 25.947 | versus NT, P = 0.033 |
| III | 8/50 (16%) | 71.356 ± 31.131 | versus NT, P = 0.039 |
| IV | 2/50 (4%) | 37.398 ± 15.755 | versus NT, P = 0.070 |
According to the AJCC staging system.(25 ) HCC, hepatocellular carcinoma; IMD, intermedin; NT, non‐tumor tissue.
In contrast, there is no significant difference in VEGF expression between tumor and non‐tumor tissues (Fig. 1A). We also examined the expression of the close family member ADM, and the receptors CRLR and RAMP3 in these tissues. No significant difference in gene expression of ADM, CRLR and RAMP3 was observed between tumor and non‐tumor tissues (Fig. 1A).
We examined the mRNA expression level of IMD in human primary hepatocytes, immortalized hepatocytes (THLE‐3) and HCC cell lines. As shown in Figure 1(C), IMD levels were significantly higher in both immortalized hepatocytes and HCC cell lines than in primary hepatocytes. The highest expression level was found in SK‐Hep‐1 and HepG2 cells and a relatively low level was seen in SNU‐398 and Hep3B cells. We then examined the expression of IMD receptor components by immunoblot analysis and found both CRLR and RAMP3 proteins in all five HCC lines (Fig. 1D).
To examine expression of IMD at the protein level in HCC, we performed immunohistochemical analysis on several surgically resected HCC, as well as adjacent cirrhotic liver and normal liver in cases of metastatic carcinoma. Although expression was somewhat heterogeneous within tumors, there was significant cytoplasmic positivity for IMD in the examined HCC (Fig. 2A). We also noted occasional staining of hepatocytes around vessels embedded within tumors (Fig. 2B). IMD positivity in HCC was in contrast to adjacent cirrhotic liver and non‐cirrhotic liver in patients with metastatic disease, which generally showed no staining or only localized staining. Concurrent negative controls using non‐immune rabbit IgG showed no significant labeling of hepatocytes or other liver structures. In cirrhotic liver, regenerative nodules often showed increased IMD staining relative to adjacent liver parenchyma in a “rim” pattern involving the outermost layer(s) of hepatocytes (Fig. 2C).
Figure 2.
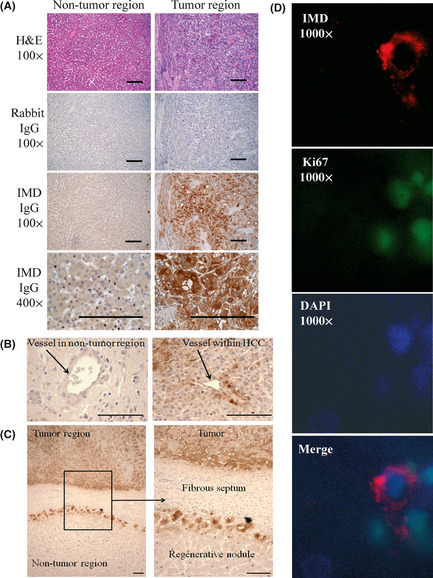
Localization of intermedin (IMD) in hepatocellular carcinoma (HCC). Sections were stained with H&E, rabbit IgG as negative control or IMD antibody. Original magnification, 100 × or 200 × or 400 × or 1000 × , as indicated; bar = 100 μm. (A) IMD was highly expressed in hepatocytes of tumor but not in adjacent non‐tumor tissue. (B) IMD was expressed in hepatocytes around vessels within HCC but not in those around vessel in non‐tumor region. (C) IMD staining was observed in a “rim” pattern involving the outermost layer(s) of hepatocytes of regenerative nodules in cirrhotic liver. (D) IMD positive cells at the periphery of regenerative nodules were often Ki67 positive cells.
As IMD expression in non‐neoplastic liver has not been previously reported, we also assessed portal tracts, vascular structures and fibrous septae for staining. There was no significant staining of normal liver structures in either cirrhotic or non‐cirrhotic liver (Fig. 2C).
To examine whether IMD is involved in tumor proliferation, we co‐stained these tissue sections with both IMD and cell proliferation marker Ki67 antibodies. There was some IMD staining at the periphery of regenerative nodules, and these IMD‐positive cells were typically also positive for Ki67 (Fig. 2D). Interestingly, the tumor regions with strong IMD staining were Ki67 negative, but were surrounded by Ki67 positive cells.
Intermedin regulates hepatocellular carcinoma cell growth
To gain insight into the function of IMD, we inhibited IMD expression using siRNA in SK‐Hep‐1 and SNU‐398 HCC cell lines, which expressed a relatively high and low amount of IMD, respectively (Fig. 1C). A 10‐nM siRNA dose was chosen as it caused minimum non‐specific stress response (data not shown). We consistently observed an approximate 90% decrease in IMD mRNA level. This reduction in IMD appeared specific as it did not significantly alter gene expression of the stress responsive gene OAS1 (data not shown).
Next, we evaluated the effect of IMD knockdown on tumor cell proliferation. The viability of SK‐Hep‐1 and SNU‐398 cells transfected with IMD siRNA was decreased significantly (30–35% decrease compared with controls, Fig. 3A). Treatment with control siRNA had no significant effect on SK‐Hep‐1 and SNU‐398 cell growth as compared to mock transfection (96.8 ± 5.1% and 92.5 ± 10.0%, respectively).
Figure 3.
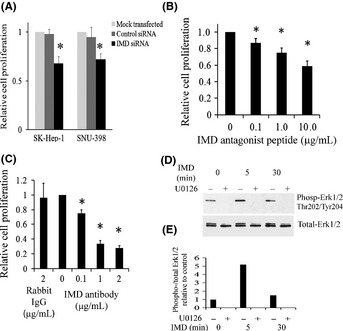
Intermedin (IMD) regulates hepatocellular carcinoma (HCC) cell proliferation. (A) SK‐Hep‐1 and SNU‐398 cells were transfected with IMD siRNA or control for 72 h. (B) SK‐Hep‐1 cells were treated with IMD antagonist peptide or vehicle (PBS) control for 72 h. (C) SK‐Hep‐1 cells were treated with control rabbit IgG or IMD antibody for 72 h. Cell proliferation was analyzed by WST‐1 assay. Data are shown as the mean ± SEM (n = 3). *P < 0.05. (D) Representative immunoblot showing that IMD increased phosphorylation of Erk1/2 in HCC cells. Sub‐confluent SK‐Hep‐1 cells were serum‐starved for 4 days, and then incubated with or without 10 μM U0126 for 24 h. IMD (10 ng/mL) was then added to the cells. Total cell lysates were harvested at 0–30 min post IMD addition and analyzed for phospho‐Erk1/2 (top) or total Erk1/2 (bottom). (E) Quantification of above immunoblot. The level of phospho‐Erk1/2 was normalized with total Erk1/2.
To verify our siRNA results, we utilized two other approaches to block IMD activity: a truncated IMD antagonist peptide and a neutralizing antibody, both of which likely block the receptor components. A significant dose‐dependent decrease (P < 0.05) in cell proliferation was observed in Sk‐Hep‐1 cells treated with IMD antagonist peptide for 72 h (Fig. 3B). Likewise, SK‐Hep‐1 cell proliferation was significantly suppressed by addition of the IMD neutralizing antibody (Fig. 3C). Similar results with slightly less effect of IMD blockage were observed using BrdU incorporation assay (Fig. S1).
In addition to knockdown and blocking studies, we examined the effect of IMD on cell growth by treating Hep3B, which expresses minimal levels of IMD, and SK‐Hep‐1 cells with IMD active peptide. As shown in Supplementary Fig. S2, IMD peptide stimulated both SK‐Hep‐1 and Hep3B cell proliferation significantly (1.22‐fold and 1.52‐fold, respectively, n = 6, P < 0.05).
To investigate the downstream effects of IMD activation, we evaluated Erk1/2, a key signaling molecule for cell proliferation.26, 27 As shown in Figure 3(D), phosphorylated Erk was increased after addition of IMD active peptide to SK‐Hep‐1 cells. This Erk activation by IMD was completely blocked when cells were pre‐incubated with U0126 (Fig. 3D).
Inhibition of intermedin induces hepatocellular carcinoma cell apoptosis
Phase‐contrast analysis showed a higher degree of nucleation in cells treated with IMD antagonist and antibody compared with the controls (Fig. S3). To determine if the observed decrease in proliferation was due to an increase in apoptosis, Annexin‐V/PI staining was performed. Early apoptotic cells (Annexin‐V positive and PI negative), late apoptotic cells (Annexin‐V positive and PI positive) and necrotic cells (Annexin‐V negative, PI positive) were counted. As shown in Figure 4(A), the IMD antagonist peptide increased late apoptotic (6%) and necrotic cells (71%) over vehicle control. Addition of the IMD neutralizing antibody or siRNA knockdown of IMD level demonstrated a similar pattern of apoptotic induction.
Figure 4.
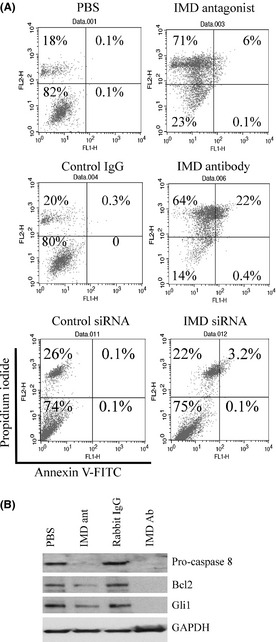
Inhibition of intermedin (IMD) induces hepatocellular carcinoma (HCC) cell apoptosis. (A) SK‐Hep‐1 cells were treated with reagent as indicated for 72 h followed by flow cytometry analysis. Numbers in the upper left quadrants of dot plots indicate the percentage of necrotic cells; numbers in the upper right quadrants, late apoptotic cells; numbers in the lower left quadrants, viable cells; numbers in the lower right quadrants, early apoptotic cells. (B) representative immunoblot of apoptotic proteins in SK‐Hep‐1 cells treated with or without IMD antagonist (ant, 1 μg/mL) or antibody (Ab, 1 μg/mL) for 48 h.
To further understand the mechanism of the observed cell death, immunoblot analysis was conducted to examine the effect of IMD inhibition on the expression of key apoptotic signaling proteins. As shown in Figure 4(B), suppression of IMD with IMD antibody dramatically decreased the expression of pro‐caspase‐8, and Bcl2 compared with control rabbit IgG treatment. This corresponded with a significant decrease in expression of Gli1 transcription factor, which is the final arbiter of Hedgehog signaling that regulates Bcl2 expression.24 Treatment with IMD antagonist peptide showed a reduction of these apoptotic proteins (Fig. 4B), although at the dose tested, it was less effective than the antibody treatment.
Discussion
This study represents the first analysis of IMD expression in liver tissues and in non‐endocrine tumors. Our results are in parallel with Takahashi's data, which show higher IMD expression in adrenocortical tumors than in non‐neoplastic adrenal cortex.12 We found that IMD protein was more strongly expressed in human HCC tumors than in non‐tumor liver tissue (Fig. 2). IMD stained malignant hepatocytes and cells surrounding vessels within tumors, but did not stain normal adjacent liver tissue. Interestingly, there was some IMD staining at the periphery of regenerative nodules, which were typically also positive for Ki67 (Fig. 2D), suggesting that IMD might be associated with proliferation of hepatocytes at the outer edge or regenerative nodules. Occasional regenerative nodules showed a greater degree of staining in more central hepatocytes, although this was highly variable and never complete. One possible explanation could be that some nodules with greater staining are actually evolving into early dysplastic foci, which can be histologically difficult to distinguish on H&E staining. Other explanations might relate to relative differences in ischemia or vascularization within different regenerative nodules, or differing rates of cellular growth/proliferation within different nodules.
More importantly, we have shown that IMD is induced at an early tumor stage in HCC (Fig. 1B). To date, diagnosis of early stage HCC can be particularly difficult and there are no clinically relevant biomarkers for the early detection of hepatocellular carcinoma.33 To test the plausibility of using IMD as a biomarker for early stage liver cancer, future studies will need to include a large‐scale immunohistochemical screening of IMD in tumor and normal tissues as well as an assessment of IMD serum levels.
Both IMD receptor components CRLR and RAMP3 were expressed in HCC tumors and cell lines (Fig. 1C,D). Furthermore, we demonstrated that IMD peptide activated Erk1/2 pathway in SK‐Hep‐1 cells (Fig. 3D). To attempt to address the influence of Erk on IMD‐mediated cell growth, the Erk inhibitor (U0126) was combined with IMD. However, U0126 pretreatment did not affect IMD growth, suggesting that either Erk inhibition by U0126 is transient and, in the absence of the inhibitor, is reactivated by IMD, or that additional cell proliferation pathways are activated by IMD. These results suggest that IMD was produced and secreted and, therefore, was able to interact with receptors in the extracellular space, including those on the cancer cells themselves in the tumor environment in an autocrine/paracrine loop mechanism.
Little information exists regarding the expression or the role of ADM in liver.32 We found no difference of ADM expression in tumor and non‐tumor HCC, suggesting that IMD and ADM have separate functions in HCC.
Intermedin is reported to be a pro‐angiogenic factor19 and could be induced by HIF in hypoxic hepatocytes.14 Thus, we hypothesized that IMD might be involved in HCC development because neovascularization is a prerequisite for tumor progression. Increased tumor growth leads to hypoxia, which induces the expression of HIF. These conditions, in turn, induce angiogenic factors, which initiate angiogenic sprouting. To test our hypothesis, we investigated the function of IMD in tumor proliferation. Our findings that downregulation of IMD inhibited HCC cell growth (Fig. 3) and addition of active IMD peptide enhanced Hep3B cell growth (Fig. S2) suggest that IMD functions as a regulator of tumor cell proliferation.
Our results provide evidence that IMD knockdown suppresses tumor cell growth (Fig. 3). We also discovered that the decrease in cell viability after treatment with IMD antagonist or antibody is likely caused by a significant induction of apoptosis (Fig. 4). Blockage of IMD function with an IMD neutralizing antibody led to a significant reduction in the caspase‐8, Bcl2 and Gli1 in SK‐Hep‐1 cells (Fig. 4B). Bcl2 is known for its ability to suppress apoptosis. Galmiche et al. (2010) report that Bcl2 family member BAD is a key regulator of apoptosis in HCC30 Bcl2 expression is regulated by the glioma‐associated oncogene 1 (Gli1) transcription factor, which plays an important role in HCC invasion and metastasis by inducing not only Bcl2 but also E‐cadherin.26, 28 Gli1 was found to be upregulated in HCC tissues and closely correlated with clinicopathological characteristics.28 It has been shown that blocking Gli1 inhibits heptoblastoma growth.31 Cytotoxic therapies currently used for cancer treatment largely depend on activation of cancer cell apoptosis. Consequently, it is likely that IMD increases HCC cell growth by preventing tumor cells from undergoing apoptotic pathways through regulation of the Hedgehog pathway, which transcriptionally controls Bcl2 expression.26 Our finding regarding inhibition of IMD‐induced apoptosis is particularly important because the progression of HCC is marked by its ability to escape programmed cell death.30
We summarize our results in Figure 5, proposing that IMD not only regulates HCC proliferation but also influences cell survival through Gli1 and Bcl2. In summary, we detected IMD in early stage HCC at the mRNA and protein level, making it a potentially useful biomarker for early disease. Unlike VEGF, IMD is expressed minimally by most normal cells and tissues (Fig. 1). Moreover, using anti‐IMD drugs alone or in combination with drugs targeting the VEGF pathway could provide a novel approach to improve outcomes in patients with HCC.
Figure 5.
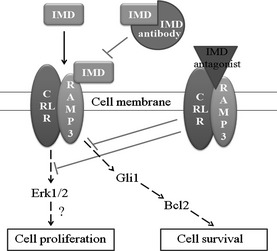
Model of regulation of tumor cell proliferation and survival by intermedin (IMD). IMD binds to receptor activity modifying protein 3 (RAMP3) and calcitonin receptor‐like receptor (CRLR), followed by activation of Erk1/2. IMD influences Gli1, which transcriptionally controls Bcl2, which controls cell survival.
Disclosure Statement
The authors have no conflict of interest to declare.
Supporting information
Fig. S1. Inhibition of intermedin (IMD) reduces BrdU incorporation. Fig. S2. Intermedin (IMD) stimulates hepatocellular carcinoma (HCC) cell proliferation. Fig. S3. Inhibition of intermedin (IMD) causes cell morphology change.
Acknowledgments
We thank Drs Chuhan Chung and Antonio Neto for kind assistance with the immunohistochemistry data analysis. This work was supported by V. A. CDA‐2 Award to C. C. and Yale Medical School Surgical OHSE Award to X. G.
References
- 1. Greten TF, Papendorf F, Bleck JS et al Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer 2005; 92: 1862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Cancer Society . Cancer Facts and Figures 2011. Atlanta, GA: American Cancer Society, 2011. Also available online: [Cited 17 November 2011.] Available from URL: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-029771.pdf [Google Scholar]
- 3. Merle P, Mornex F. Medical therapies for hepatocellular carcinoma. Cancer Radiother 2011; 15: 28–31. [DOI] [PubMed] [Google Scholar]
- 4. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007; 6: 273–86. [DOI] [PubMed] [Google Scholar]
- 5. Yoo PS, Mulkeen AL, Cha CH. Post‐transcriptional regulation of vascular endothelial growth factor: implications for tumor angiogenesis. World J Gastroenterol 2006; 12: 4937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs 2009; 69: 223–40. [DOI] [PubMed] [Google Scholar]
- 7. Worns MA, Galle PR. Novel inhibitors in development for hepatocellular carcinoma. Expert Opin Investig Drugs 2010; 19: 615–29. [DOI] [PubMed] [Google Scholar]
- 8. Elfiky AA, Rosenberg JE. Targeting angiogenesis in bladder cancer. Curr Oncol Rep 2009; 11: 244–9. [DOI] [PubMed] [Google Scholar]
- 9. Bell D, McDermott BJ. Intermedin (adrenomedullin‐2): a novel counter‐regulatory peptide in the cardiovascular and renal systems. Br J Pharmacol 2008; 153(Suppl 1): S247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chauhan M, Yallampalli U, Reed L et al Adrenomedullin 2 antagonist infusion to rats during midgestation causes fetoplacental growth restriction through apoptosis. Biol Reprod 2006; 75: 940–7. [DOI] [PubMed] [Google Scholar]
- 11. Roh J, Chang CL, Bhalla A, Klein C, Hsu SY. Intermedin is a calcitonin/calcitonin gene‐related peptide family peptide acting through the calcitonin receptor‐like receptor/receptor activity‐modifying protein receptor complexes. J Biol Chem 2004; 279: 7264–74. [DOI] [PubMed] [Google Scholar]
- 12. Morimoto R, Satoh F, Murakami O et al Expression of adrenomedullin 2/intermedin in human adrenal tumors and attached non‐neoplastic adrenal tissues. J Endocrinol 2008; 198: 175–83. [DOI] [PubMed] [Google Scholar]
- 13. Takahashi K, Morimoto R, Hirose T, Satoh F, Totsune K. Adrenomedullin 2/intermedin in the hypothalamo‐pituitary‐adrenal axis. J Mol Neurosci 2011; 43: 182–92. [DOI] [PubMed] [Google Scholar]
- 14. Copple BL, Bustamante JJ, Welch TP, Kim ND, Moon JO. Hypoxia‐inducible factor‐dependent production of profibrotic mediators by hypoxic hepatocytes. Liver Int 2009; 29: 1010–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Y, Bell D, Smith LR et al Differential expression of components of the cardiomyocyte adrenomedullin/intermedin receptor system following blood pressure reduction in nitric oxide‐deficient hypertension. J Pharmacol Exp Ther 2006; 316: 1269–81. [DOI] [PubMed] [Google Scholar]
- 16. Robinson SD, Aitken JF, Bailey RJ, Poyner DR, Hay DL. Novel peptide antagonists of adrenomedullin and calcitonin gene‐related peptide receptors: identification, pharmacological characterization, and interactions with position 74 in Receptor Activity‐Modifying Protein 1/3. J Pharmacol Exp Ther 2009; 331: 513–21. [DOI] [PubMed] [Google Scholar]
- 17. Chauhan M, Elkins R, Balakrishnan M, Yallampalli C. Potential role of intermedin/adrenomedullin 2 in early embryonic development in rats. Regul Pept 2011; 170: 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chauhan M, Yallampalli U, Dong YL, Hankins GD, Yallampalli C. Expression of adrenomedullin 2 (ADM2)/intermedin (IMD) in human placenta: role in trophoblast invasion and migration. Biol Reprod 2009; 81: 777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith RS Jr, Gao L, Bledsoe G, Chao L, Chao J. Intermedin is a new angiogenic growth factor. Am J Physiol Heart Circ Physiol 2009; 297: H1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albertin G, Sorato E, Oselladore B, Mascarin A, Tortorella C, Guidolin D. Involvement of vascular endothelial growth factor signaling in CLR/RAMP1 and CLR/RAMP2‐mediated pro‐angiogenic effect of intermedin on human vascular endothelial cells. Int J Mol Med 2010; 26: 289–94. [DOI] [PubMed] [Google Scholar]
- 21. Park SC, Yoon JH, Lee JH et al Hypoxia‐inducible adrenomedullin accelerates hepatocellular carcinoma cell growth. Cancer Lett 2008; 271: 314–22. [DOI] [PubMed] [Google Scholar]
- 22. Sadeghi MM, Esmailzadeh L, Zhang J et al ESDN is a marker of vascular remodeling and regulator of cell proliferation in graft arteriosclerosis. Am J Transplant 2007; 7: 2098–105. [DOI] [PubMed] [Google Scholar]
- 23. Guo X, Nie L, Esmailzadeh L, Zhang J, Bender JR, Sadeghi MM. Endothelial and smooth muscle‐derived neuropilin‐like protein regulates platelet‐derived growth factor signaling in human vascular smooth muscle cells by modulating receptor ubiquitination. J Biol Chem 2009; 284: 29376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martinez A, Vos M, Guedez L et al The effects of adrenomedullin overexpression in breast tumor cells. J Natl Cancer Inst 2001; 94: 1226–37. [DOI] [PubMed] [Google Scholar]
- 25. Greene FL. AJCC Cancer Staging Manual, 6th edn. New York, NY: Springer, 2002. [Google Scholar]
- 26. Mazumdar T, DeVecchio J, Shi T, Jones J, Agyeman A, Houghton JA. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res 2011; 71: 1092–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta 2010; 1802: 396–405.20079433 [Google Scholar]
- 28. Zheng X, Yao Y, Xu Q, Tu K, Liu Q. Evaluation of glioma‐associated oncogene 1 expression and its correlation with the expression of sonic hedgehog, E‐cadherin and S100a4 in human hepatocellular carcinoma. Mol Med Report 2010; 3: 965–70. [DOI] [PubMed] [Google Scholar]
- 29. Fulda S, Debatin KM. Apoptosis signaling in tumor therapy. Ann N Y Acad Sci 2004; 1028: 150–6. [DOI] [PubMed] [Google Scholar]
- 30. Galmiche A, Ezzoukhry Z, Francois C et al BAD, a proapoptotic member of the BCL2 family, is a potential therapeutic target in hepatocellular carcinoma. Mol Cancer Res 2010; 8: 1116–25. [DOI] [PubMed] [Google Scholar]
- 31. Eichenmuller M, Gruner I, Hagl B et al Blocking the hedgehog pathway inhibits hepatoblastoma growth. Hepatology 2009; 49: 482–90. [DOI] [PubMed] [Google Scholar]
- 32. Nakata T, Seki N, Miwa S et al Identification of genes associated with multiple nodules in hepatocellular carcinoma using cDNA microarray: multicentric occurrence or intrahepatic metastasis? Hepatogastroenterology 2008; 55: 865–72. [PubMed] [Google Scholar]
- 33. Meier V, Ramadori G. Clinical staging of hepatocellular carcinoma. Dig Dis 2009; 27: 131–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Inhibition of intermedin (IMD) reduces BrdU incorporation. Fig. S2. Intermedin (IMD) stimulates hepatocellular carcinoma (HCC) cell proliferation. Fig. S3. Inhibition of intermedin (IMD) causes cell morphology change.


