Abstract
Smoking is well known as a risk factor for esophageal cancer, but controversial as a prognostic factor. Moreover, evidence is scarce that a dose–response relationship exists. We conducted a retrospective study on the effect and dose–response relationship of prediagnostic smoking on the postoperative disease‐specific survival of patients with lymph node‐negative esophageal squamous cell carcinoma (ESCC). We enrolled 643 patients with lymph node‐negative ESCC who had undergone esophagectomy between 1990 and 2005 at the Department of Thoracic Surgery, Sun Yat‐sen University Cancer Center, Guangzhou, China. The patients' demographic, pathological, preoperative and cancer outcome data were obtained from medical records. These data were reviewed and analyzed using life table, Kaplan–Meier analysis and multivariate Cox regression. A significant reduction in 3‐ and 5‐year survival rates was observed in smokers with lymph node‐negative ESCC compared with those in non‐smokers. The 3‐ and 5‐year survival rates were 54% and 46% for smokers, and 67% and 64% for non‐drinkers, respectively (P < 0.05). Multivariate Cox analysis revealed that smoking was an independent prognostic factor (P = 0.008, hazard ratio = 1.404). Both log‐rank test (P = 0.065) and multivariate analysis (P = 0.091) showed no significant difference between the survival rates of light and heavy smokers. Prediagnostic smoking is an independent prognostic factor for patients with lymph node‐negative ESCC, but the dose–response relationship needs further investigation.
Esophageal cancer is the sixth leading cause of cancer mortality worldwide.1 According to the most recent global estimates, esophagus cancer causes 407 000 deaths annually.2 In China, it is the fifth most common and fourth most lethal malignant tumor, and squamous cell carcinoma prevails in terms of histology.3 Despite advances in early diagnosis and surgical treatments, overall prognosis is poor with the 5‐year survival rate rarely >20%.4
It is well known that smoking is damaging public health on a global scale by playing an important role in the development of lung, head, neck and esophageal cancers.5, 6, 7 Well‐conducted population‐based studies have reported a significantly increased risk of esophageal squamous cell carcinoma (ESCC) associated with smoking, regardless of dosage.8, 9, 10 However, the correlation between smoking and patient survival is less investigated and more controversial. Some studies reported that smokers had a worse prognosis,11, 12 while others denied the correlation.13 The present study aimed to determine whether prediagnostic smoking has an independent influence on disease‐specific survival (DSS) in patients with lymph node‐negative ESCC, and whether a dose‐dependent relationship exists.
Materials and Methods
We conducted a large‐scale retrospective study by searching the esophageal cancer database of the Department of Thoracic Surgery at Sun Yat‐sen University Cancer Center, Guangzhou, China. In total, 643 patients with lymph node‐negative ESCC who had undergone esophagectomy from 1990 to 2005 were enrolled. The mean follow‐up duration was 6.5 years (range, 1–20 years). The institutional review board of Sun Yat‐sen University Cancer Center approved the present study and granted a waiver of the informed consent process.
Clinical and pathological data were extracted from medical records. Baseline data included age, gender, surgical incision, tumor biological features, smoking, alcohol consumption and family history. Tumors were staged according to the American Joint Committee on Cancer (AJCC) Staging Manual (6th edition).14 A smoker refers to those who smoke at least once a day for more than 1 year. A drinker refers to those who drink more than 100 g/day for more than 1 year.
All statistical analyses were performed using spss version 18.0 statistical software (IBM SPSS Inc., Chicago, IL, USA). Descriptive statistics were obtained for demographic, epidemiological and clinical characteristics. Life table and Kaplan–Meier analysis were applied to calculate the 3‐ and 5‐year survival rates and compare the difference between survival rates of smokers and non‐smokers. Survival curves were generated according to smoking history (Fig. 1), and the log‐rank test was applied to determine the statistical significance of the difference between the survival curves of smokers and non‐smokers. A two‐sided P value of <0.05 was considered statistically significant. Multivariate Cox regression analysis was performed to exclude other confounding factors that might affect survival. Cases were censored on the condition that patients either died from other causes or were still alive at the end‐point of follow up to make sure that deaths were exclusively attributed to ESCC.
Figure 1.
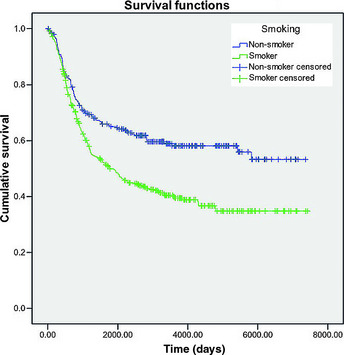
Kaplan–Meier overall survival curve for 643 lymph node‐negative esophageal squamous cell carcinoma patients grouped by history of smoking. The 3‐ and 5‐year survival rates of smokers and non‐smokers were 54% vs 46% and 67% vs 64%, respectively.
Results
Patient groups according to smoking history
The demographic data are summarized in Table 1. Smoking history was associated with male gender, alcohol consumption, tumor location and postoperative stage. No significant difference in distribution was observed for other factors such as tumor grade and surgical method.
Table 1.
Baseline characteristics grouped by smoking history
| Smoker (%) | Non‐smoker (%) | P valuea | |
|---|---|---|---|
| Gender | <0.05 | ||
| Male | 377 (96.9) | 77 (30.3) | |
| Female | 12 (3.1) | 177 (69.7) | |
| Age (years) | 0.074 | ||
| <40 | 15 (3.9) | 18 (7.1) | |
| 40–60 | 252 (64.8) | 146 (57.5) | |
| >60 | 122 (31.4) | 90 (35.4) | |
| Alcohol consumption | 111 (28.5) | 10 (3.9) | <0.05 |
| Family history | 27 (6.9) | 19 (7.3) | 0.876 |
| Tumor location | 0.021a | ||
| Upper thoracic | 49 (12.6) | 22 (8.7) | |
| Middle thoracic | 264 (67.9) | 198 (78) | |
| Lower thoracic | 76 (19.5) | 34 (13.4) | |
| Postoperative stage | 0.012a | ||
| IA | 21 (5.4) | 27 (10.6) | |
| IB | 178 (45.8) | 126 (49.6) | |
| IIA | 190 (48.8) | 101 (39.8) | |
| Tumor grade of differentiation | 0.212 | ||
| Well | 140 (36.1) | 101 (39.8) | |
| Moderately | 172 (44.3) | 95 (37.4) | |
| Poorly | 76 (19.6) | 58 (22.8) | |
| Surgical incision | 0.112 | ||
| Left thoracic | 302 (77.7) | 197 (77.5) | |
| Right thoracic | 87 (22.4) | 57 (22.4) | |
| Postoperative radiotherapy | 10 (2.6) | 3 (1.2) | 0.264 |
| Three‐field surgical dissection | 55 (14.1) | 44 (17.3) | 0.285 |
P < 0.05 was considered statistically significant.
According to the results of life table analysis, the 3‐ and 5‐year survival rates were 54% and 46% in smokers, and 67% and 64% in non‐smokers, respectively.
According to the results of Kaplan–Meier analysis and the log‐rank test, a significant difference (P < 0.01) was observed in overall survival between smokers and non‐smokers. The results of multivariate Cox regression analysis (Table 2) indicated that smoking (P = 0.008, hazard ratio [HR] = 1.404), alcohol consumption (P = 0.001, HR = 1.602), postoperative stage (P = 0.002, HR = 1.333), tumor grade (P = 0.031, HR = 1.117) and surgical incision (P = 0.011, HR = 1.111) were independent prognostic factors for survival in lymph node‐negative ESCC patients.
Table 2.
Multivariate analysis of prognostic factors related to lymph node‐negative esophageal squamous cell carcinoma
| P valuea | HR | 95.0% CI for Exp(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Smoking | 0.002 | 1.494 | 1.157 | 1.928 |
| Alcohol consumption | 0.000 | 1.702 | 1.300 | 2.227 |
| Surgery | 0.075 | 1.092 | 0.991 | 1.203 |
| Stage | 0.001 | 1.380 | 1.146 | 1.662 |
| Grade | 0.018 | 1.200 | 1.032 | 1.395 |
| Postoperative radiotherapy | 0.031 | 0.333 | 0.123 | 0.903 |
| Three‐field surgical dissection | 0.453 | 1.173 | 0.773 | 1.780 |
P < 0.05 was considered statistically significant. CI, confidence interval; Exp(B), exponentiation of the B coefficient; HR, hazard ratio.
Analysis of the dose–response relationship
Patients were classified into three subgroups according to their smoking index: non‐smokers; light smokers (smoking index, ≤500); and heavy smokers (smoking index, >500). The smoking index was defined as the number of cigarettes or pipe tobacco or cigars consumed per day multiplied by the number of years of smoking. There were 254, 158 and 231 patients in the non‐smoker, light smoker and heavy smoker groups, respectively.
Non‐smokers had better survival rates compared with light and heavy smokers (P < 0.01, Fig. 2). Pairwise comparisons in the log‐rank test showed no significant survival difference between light and heavy smokers (P = 0.065). In addition, multivariate analysis (Table 3), which eliminated the contribution from confounding factors, also showed no significance between light and heavy smokers (P = 0.091).
Figure 2.
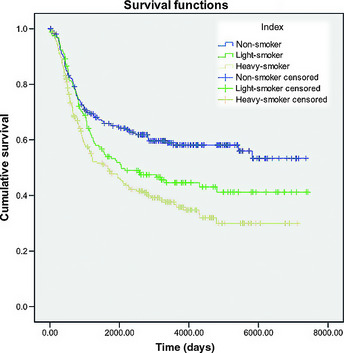
Kaplan–Meier survival curves for 643 lymph node‐negative esophageal squamous cell carcinoma patients grouped by smoking index. No significant survival difference (P = 0.065) was observed between light (n = 158) and heavy smokers (n = 231).
Table 3.
Multivariate analysis of prognostic factors among light and heavy smokers
| P valuea | HR | 95.0% CI for Exp(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 0.499 | 0.708 | 0.261 | 1.926 |
| Age | 0.325 | 0.879 | 0.680 | 1.136 |
| Alcohol consumption | 0.000 | 1.725 | 1.295 | 2.299 |
| Family history | 0.104 | 0.602 | 0.326 | 1.111 |
| Tumor location | 0.181 | 0.838 | 0.647 | 1.086 |
| Surgical incision | 0.170 | 1.089 | 0.964 | 1.231 |
| Postoperative stage | 0.013 | 1.342 | 1.065 | 1.691 |
| Tumor grade | 0.155 | 1.144 | 0.950 | 1.377 |
| Index | 0.225 | 1.188 | 0.899 | 1.569 |
| Postoperative radiotherapy | 0.011 | 0.223 | 0.070 | 0.708 |
| Three‐field surgical dissection | 0.367 | 1.263 | 0.760 | 2.099 |
P < 0.05 was considered statistically significant. CI, confidence interval; Exp(B), exponentiation of the B coefficient; HR, hazard ratio.
Discussion
At present, esophageal cancer is the fifth most common and fourth most lethal malignant tumor occurring in China. Although the incidence of esophageal adenocarcinoma in western countries is rising, it has remained unchanged in China with squamous cell carcinoma accounting for the majority of esophageal cancer cases.3, 15
Patients selected in the present study were lymph node‐negative because they were in the early stage of ESCC, where the medical treatment depends on the assessment of their postoperative prognosis. The seventh edition of the American Joint Committee on Cancer tumor–nodes–metastasis staging system for esophageal cancer has been debatable since its release in 2009.16 The new staging system has included differentiation and segment of esophageal cancer, which might affect survival. T2N0M0 and T3N0M0 ESCC, classified as stage IIa according to the sixth edition of the AJCC Cancer Staging Manual,14 is classified as stage Ib, IIa or IIb according to the seventh edition. The present study aimed to investigate whether smoking was an independent factor for the DSS of patients with lymph node‐negative ESCC by excluding other confounding factors.
While cigarette smoking is a well‐established risk factor for ESCC, the majority of epidemiological studies have yielded little or limited information on the role of cigarette smoking in the survival of lymph node‐negative ESCC patients. Some studies have examined the prognostic role of smoking among ESCC patients. According to the results of Thrift et al.12 and Sundelöf et al.,11 tobacco smoking was independently associated with decreased survival among ESCC patients. In contrast, Yu et al.17 found no association between smoking‐related survival and ESCC. In our cohort, according to the results of life table, the 3‐ and 5‐year survival rates of non‐smokers were significantly higher than those of smokers. Multivariate Cox regression analysis also indicated smoking as an independent factor influencing survival. Different outcomes might have resulted from different cases and types as well as smoking duration. Nevertheless, our findings confirmed the independent effect of smoking on the survival of lymph node‐negative ESCC patients.
To study the dose–response relationship between smoking and survival, we selected a smoking index of 500 as the cut‐off point to classify the patients into two groups: light (n = 158) and heavy smokers (n = 231). Some studies have demonstrated the dose–response relationship between smoking and cancer risk or survival.8 In the present study, although survival analysis showed no significant difference between the two groups, the Kaplan–Meier curve showed a trend of separation. Possible explanations are described below. First, a substantial number of studies have applied the Brinkman index of 400 as the threshold of heavy smoking, which we failed to find statistically significant. It is likely that differential or non‐differential exposure misclassification or biased case selection led to concealment of any important association between light and heavy smokers. Moreover, although we tried to eliminate bias from potential confounders by using Cox proportional hazard modeling, residual confounding factors cannot be completely ruled out. The impact of competing risk factors is another potential source of bias including treatment, histology and comorbidities.
Assuming the association reported here is not the result of error, then the question arises as to whether decreased survival among patients who regularly smoked is causal. Tobacco‐specific chemical compounds cause genetic or epigenetic alterations, modulate expressions of large numbers of genes that include those that encode molecules related to proliferation, invasion and metastasis,18 and interact with major treatment modalities.19 These mechanisms might explain the effect of smoking on survival in that smoking might alter the behavior of a tumor and promote its progression.
The strengths and limitations of the present study should be considered in interpreting these results. The strengths included a large sample size of consecutive patients from a well‐maintained database and an efficient recording medical system containing abundant tumor information, such as that on tumor grade and stage. In addition, postoperative radiotherapy and three‐field surgical dissection were also taken into consideration. Moreover, we explored the dose–response relationship and made an effort to control confounding factors. Furthermore, the present study showed that smoking prior to diagnosis, a well‐known risk factor for cancer development, also appeared to affect cancer outcome. This is helpful information to guide patients to improve survival by discontinuing smoking. However, the present study had all the constraints of retrospective analysis in which the comparison of smokers and non‐smokers is subject to selection bias.
In conclusion, the present study confirmed prediagnostic tobacco smoking as an independent factor for postoperative DSS in patients with lymph node‐negative ESCC, and that a history of smoking was strongly associated with poor prognosis. No dose–response relationship was established. By assessing the postoperative prognosis, doctors might be able to come up with therapy more suitable for the patients.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (No. 09YKPY51), the Science and Technology Planning Project of Guangdong Province, China (No. 2010B31500010), and the National High Technology Research and Development Program of China (863 Program) (No. 2009AA02Z421). The authors thank Enago (www.enago.cn) for English language review.
(Cancer Sci 2012; 103: 1985–1988)
References
- 1. Jemal A, Siegel R, Xu JQ, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–917. [DOI] [PubMed] [Google Scholar]
- 3. He J, Shao K. The epidemiology, current status of management, challenge and future strategy for esophageal cancer in China. Chin J Oncol 2010; 32: 401–4. [PubMed] [Google Scholar]
- 4. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349: 2241–52. [DOI] [PubMed] [Google Scholar]
- 5. Kawai H, Tada A, Kawahara M et al Smoking history before surgery and prognosis in patients with stage IA non‐small‐cell lung cancer–a multicenter study. Lung Cancer 2005; 49: 63–70. [DOI] [PubMed] [Google Scholar]
- 6. Dikshit RP, Boffetta P, Bouchardy C et al Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: a multicentric European study. Int J Cancer 2005; 117: 992–5. [DOI] [PubMed] [Google Scholar]
- 7. Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev 2011; 12: 2461–6. [PubMed] [Google Scholar]
- 8. Pandeya N, Williams GM, Sadhegi S, Green AC, Webb PM, Whiteman DC. Associations of duration, intensity, and quantity of smoking with adenocarcinoma and squamous cell carcinoma of the esophagus. Am J Epidemiol 2008; 168: 105–14. [DOI] [PubMed] [Google Scholar]
- 9. Freedmen ND, Abnet CC, Leitzmann MF et al A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol 2007; 165: 1424–33. [DOI] [PubMed] [Google Scholar]
- 10. Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 1995; 4: 85–92. [PubMed] [Google Scholar]
- 11. Sundelöf M, Lagergren J, Ye WM. Patient demographics and lifestyle factors influencing long‐term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer 2008; 44: 1566–71. [DOI] [PubMed] [Google Scholar]
- 12. Thrift AP, Nagle CM, Fahey PP. The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer 2012; 131: E759–68. [DOI] [PubMed] [Google Scholar]
- 13. Trivers KF, De Roos AJ, Gammon MD. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol 2005; 3: 225–30. [DOI] [PubMed] [Google Scholar]
- 14. American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 6th edn. New York, NY: Springer, 2002; 167–78. [Google Scholar]
- 15. Shibata A, Matsuda T, Ajiki W et al Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993–2001. Jpn J Clin Oncol 2008; 38: 464–8. [DOI] [PubMed] [Google Scholar]
- 16. Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edn. Chicago, IL: Springer Inc, 2010. [Google Scholar]
- 17. Yu GP, Ostroff JS, Zhang ZF, Tang J, Schantz SP. Smoking history and cancer patient survival: a hospital cancer registry study. Cancer Detect Prev 1997; 21: 497–509. [PubMed] [Google Scholar]
- 18. Yoshino I, Maehara Y. Impact of smoking status on the biological behavior of lung cancer. Surg Today 2007; 37: 725–34. [DOI] [PubMed] [Google Scholar]
- 19. Shitara K, Matsuo K, Hatooka S et al Heavy smoking history interacts with chemoradiotherapy for esophageal cancer prognosis: a retrospective study. Cancer Sci 2010; 101: 1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


