Abstract
Rituximab (R) plus doxorubicin, cyclophosphamide, vincristine, and prednisolone (CHOP) chemotherapy (R‐CHOP) is widely accepted as standard care for diffuse large B‐cell lymphoma (DLBCL) patients. The revised International Prognostic Index (R‐IPI) was established in 2007 after the addition of rituximab to standard DLBCL treatment. To reassess the utility of R‐IPI, we carried out a retrospective analysis of patients with DLBCL uniformly treated with standard R‐CHOP. Progression‐free survival (PFS) curves in “very good” and “good” risk groups as defined by the R‐IPI showed no statistical difference. We added soluble interleukin‐2 receptor (sIL‐2R) level to the factors comprising the R‐IPI. Five levels of sIL‐2R were weighed with respect to their impact on PFS. sIL‐2R of >2500 U/mL was determined as the most appropriate threshold. We developed a new prognostic SIL index, which includes three independent prognostic risk factors: clinical stage (S); sIL‐2R level over 2500 U/mL (I); and elevated lactate dehydrogenase level (L). This index indicates standard risk (0 or 1 risk factors, 4‐year PFS 83%, 4‐year overall survival 91%) and high risk (2 or 3 risk factors, 4‐year PFS 52%, 4‐year overall survival 67%) outcomes. The SIL index is a simple and objective prognostic index for DLBCL patients to identify candidates for experimental therapy other than R‐CHOP. (Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02331.x, 2012)
The prognosis for patients with DLBCL has dramatically improved since the introduction of rituximab, an anti‐CD20 mAb. Although R‐CHOP chemotherapy is regarded as the standard care for patients with DLBCL, approximately 30% of patients are unlikely to be cured by this treatment. To identify patients with a poor prognosis, who might be candidates for experimental therapy, the IPI was proposed in 1993, during the pre‐R era.1 The IPI depends on five negative prognostic factors: age over 60 years; advanced stage; elevated LDH level; performance status >1; and an extranodal disease number >1. Based on the number of risk factors, patients are classified into four risk groups. The IPI was based on data from patients with histologically varying, aggressive lymphomas, treated with combination chemotherapy regimens containing doxorubicin. In the R era, the R‐IPI was proposed in 2007 for patients with DLBCL who underwent R‐CHOP therapy.2 In this R‐IPI, three risk groups were identified by the redistribution of the IPI factors. Thus, the prognostic factors should be re‐evaluated under new therapeutic strategies.
Expression of sIL‐2R is induced by mononuclear cell activation.3, 4 Activated T cells, activated B cells, and some tumor cells are also known to induce sIL‐2R expression. Soluble interleukin‐2 receptor exists in healthy individuals at low levels; the upper limit of the normal range is approximately 500 U/mL. The level of sIL‐2R is elevated in most patients with B‐cell lymphoma, but the sIL‐2R‐producing cells have not been identified. The level of sIL‐2R is not of diagnostic value. It is usually measured in follow‐up patients with NHL in remission. We evaluated the sIL‐2R level in all patients with lymphomas at presentation. However, the risk‐distribution of R‐IPI did not work appropriately in our patients with DLBCL; hence, we included the sIL‐2R level in the risk factors and analyzed to establish a new prognostic index in patients with DLBCL treated with R‐CHOP. Elevated sIL‐2R level at presentation was recently reported to have prognostic value in patients with DLBCL who received R‐CHOP.5
Patients and Methods
This study was approved by the Yokohama City University Hospital Clinical Research Ethics Board. The procedures used in this study were in accordance with the Helsinki Declaration. The Yokohama City University Hematology Group, consisting of seven hospitals in Japan, has uniformly and curatively treated DLBCL patients since 2003. Treatment consists of six cycles of standard R‐CHOP therapy (50 mg/m2 doxorubicin [adriamycin] on day 1, 750 mg/m2 cyclophosphamide on day 1, 1.4 mg/m2 [maximum 2.0 mg/body] vincristine on day 1, 100 mg/body of prednisolone on days 1–5, and 375 mg/m2 R per cycle) for 21 days. We planned only R‐CHOP as first‐line therapy with curative intent and there were no patients who received autologous stem cell transplantation as consolidative therapy. Patients over 70 years of age with a Eastern Cooperative Oncology Group PS > 1 were treated with a reduced dose, and they were excluded from this study; however, if they were treated with a full dose of R‐CHOP, as decided by the attending physician, they were included. Patients who were required to reduce the initial therapy dose by more than 20% due to any major comorbidity were excluded. Consequently, all patients who were treated with a 100% dose of R‐CHOP at the first cycle were included, even if they received dose reduction or termination at subsequent cycles due to toxicity. Those with special forms of DLBCL, such as intravascular lymphoma, primary mediastinal large B‐cell lymphoma, or T‐cell‐rich B‐cell lymphoma, and those with human immunodeficiency virus infection were also excluded from this study. Up to 2009, 554 consecutive patients with DLBCL were registered and treated; 366 of these patients were enrolled in this study. All included patients were scheduled to undergo primary therapy with six cycles of full‐dose R‐CHOP, administered in seven hospitals. Clinical staging was carried out according to the Ann Arbor system, using data from the following: physical examinations; computed tomography of the neck, chest, abdomen, and pelvis; bone marrow aspiration; and biopsy. When required, data from endoscopic examination of the upper and/or lower gastrointestinal tract, lumbar puncture, magnetic resonance imaging of the brain, gallium scintigraphy, or PET were used for staging. Patients who had PR after the four initial cycles were given eight R‐CHOP cycles in total, whereas patients who did not achieve PR after the four initial R‐CHOP cycles, or those who showed disease progression at any given time, received salvage therapy. In these cases, the time point was dealt with disease progression. Additional local irradiation was also carried out in patients with PR or complete remission if deemed necessary by the attending physician. No patient received maintenance therapy with R. Patients with DLBCL who achieved complete remission but were initially at risk of CNS involvement also received methotrexate (15 mg) and hydrocortisone (25 mg) four times intrathecally for CNS prophylaxis.6 Central pathological reviews were not carried out, and only diagnoses made according to the World Health Organization classification7, 8 at individual institutions were used in subsequent analyses.
Statistical analysis
Overall survival was calculated from the date of initiation of R‐CHOP therapy to the date of last follow‐up or death. Progression‐free survival) was calculated from the date of initiation of R‐CHOP therapy to the date of disease progression, death from any cause, or last contact, whichever occurred first. Survival was estimated from Kaplan–Meier curves and compared using the log–rank test. P < 0.05 was considered statistically significant. A Cox proportional hazards model was used in multivariate analysis. Data were analyzed using spss (IBM PASW Statistics 18.0; IBM, Armonk, NY, USA).
Results
Patients and treatment
A total of 366 patients with DLBCL were identified. Clinical characteristics at diagnosis, including the distribution of the individual R‐IPI factors and sIL‐2R levels are listed and compared to the original R‐IPI report by Sehn et al.2 in Table 1. In the current study, the median age at diagnosis was 64 years (range, 18–80 years). The proportion of patients with a PS > 1 was only 15%. There was no missing data for the individual factors.
Table 1.
Patient characteristics compared with original revised International Prognostic Index (R‐IPI) report
| Characteristic | Current study | Original R‐IPI report[Link] |
|---|---|---|
| Number | 366 | 365 |
| Median age, years (range) | 64 (18–80) | 61 (16–90) |
| Male sex,% | 57 | 61 |
| R‐IPI factors | ||
| Age > 60 years, % | 62 | 51 |
| PS > 1, % | 15 | 41 |
| Elevated LDH, % | 49 | 55 |
| More than one extranodal site, % | 29 | 29 |
| Stage III/IV, % | 44 | 59 |
| sIL‐2R | ||
| >1000 | 51 | NA |
| >1500 | 38 | NA |
| >2000 | 29 | NA |
| >2500 | 24 | NA |
| >3000 | 19 | NA |
†Sehn et al.2 LDH, lactate dehydrogenase; NA, not available; PS, Eastern Cooperative Oncology Group performance status; sIL‐2R, soluble interleukin‐2 receptor.
It was planned that all patients would receive six cycles of full‐dose R‐CHOP therapy. An additional two cycles of R‐CHOP (total eight cycles) was given to patients with PR at the completion of the initial four cycles. The median number of therapy cycles was six (range, 2–8), and 91% of the patients received six cycles or more. Forty patients (11%) received radiation therapy in their primary treatment, usually to treat sites of residual masses at the end of the chemotherapy. The CNS prophylaxis was carried out in 45 patients (12%). Secondary regimens for patients with recurrence despite R‐CHOP therapy were not defined and were determined by the treating physician.
Outcome according to R‐IPI
The median follow‐up time for living patients was 43 months (range, 3–95 months). The PFS (Fig. 1A) and OS (Fig. 1B) curves are shown in Figure 1. The 4‐year PFS and 4‐year OS rates were 73% and 83%, respectively. When the PFS curve is divided into three risk groups according to the R‐IPI, there is no significant difference in PFS between the “very good” and “good” prognostic groups, although the difference between the three groups is significant (Fig. 1C). This is also true for OS, in which the survival curves between the “very good” and “good” prognostic groups showed no statistical difference (Fig. 1D).
Figure 1.
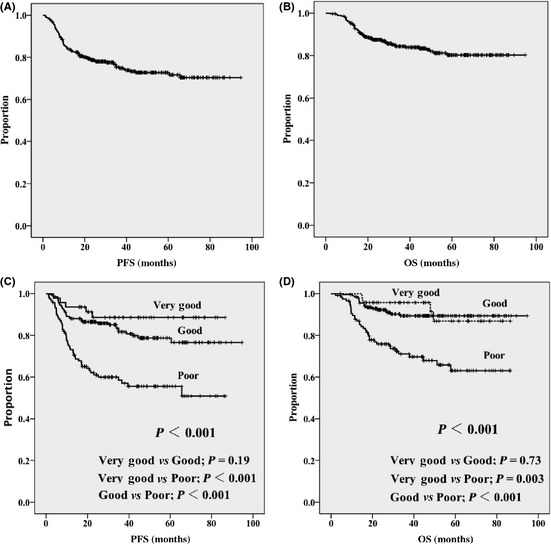
Progression‐free survival (A) and overall survival (B) in 366 patients with diffuse large B‐cell lymphoma treated with standard R‐CHOP. Progression‐free survival (C) and overall survival (D) according to the revised International Prognostic Index.
Outcome according to sIL‐2R level
To determine the appropriate cut‐off value of sIL‐2R for PFS, five levels (1000, 1500, 2000, 2500, and 3000 U/mL) were evaluated with respect to the risk factors that determine the R‐IPI by multivariate analysis (Table 2). Consequently, a sIL‐2R level of 2500 U/mL was considered to be the most useful cut‐off value. When using this cut‐off value, advanced disease (P = 0.002, RR 2.32) and elevated LDH (P = 0.017, RR 1.82) remained as adverse prognostic factors, whereas poor PS and extranodal involvement sites numbering more than 1 were no longer significant. Age of over 60 years was indeed a favorable prognostic factor (P = 0.038, RR 0.63) for PFS. This association was also observed for every other cut‐off level of sIL‐2R. When the entire cohort of 366 patients was divided into two groups based on sIL‐2R levels (above and equal or below 2500 U/mL), the resulting PFS curve was significantly inferior for patients with sIL‐2R over 2500 U/mL (Fig. 2A). Similarly, the PFS curve was significantly inferior in patients in the “very good” and “good” risk groups (Fig. 2B; P < 0.001) and in the “high” risk group (Fig. 2C; P = 0.03).
Table 2.
Correlation between soluble interleukin‐2 receptor (sIL‐2R) and factors including revised International Prognostic Index for progression‐free survival
| P‐value | RR | 95% CI | |
|---|---|---|---|
| Age > 60 years | 0.065 | 0.67 | 0.44–1.03 |
| CS ≥ 3 | <0.001 | 2.62 | 1.56–4.39 |
| LDH > N | 0.009 | 1.93 | 1.18–3.16 |
| PS ≥ 2 | 0.029 | 1.77 | 1.06–2.97 |
| EN > 1 | 0.64 | 0.89 | 0.56–1.43 |
| sIL‐2R > 1000 | 0.459 | 1.21 | 0.73–2.03 |
| Age > 60 years | 0.049 | 0.65 | 0.42–1.00 |
| CS ≥ 3 | <0.001 | 2.53 | 1.51–4.22 |
| LDH > N | 0.008 | 1.93 | 1.19–3.15 |
| PS ≥ 2 | 0.064 | 1.65 | 0.97–2.81 |
| EN > 1 | 0.636 | 0.89 | 0.56–1.43 |
| sIL‐2R > 1500 | 0.199 | 1.38 | 0.84–2.26 |
| Age > 60 years | 0.041 | 0.64 | 0.41–0.98 |
| CS ≥ 3 | 0.001 | 2.39 | I.42–4.02 |
| LDH > N | 0.011 | 1.89 | 1.16–3.08 |
| PS ≥ 2 | 0.117 | 1.54 | 0.90–2.63 |
| EN > 1 | 0.698 | 0.91 | 0.57–1.46 |
| sIL‐2R > 2000 | 0.050 | 1.64 | 1.00–2.69 |
| Age > 60 years | 0.038 | 0.63 | 0.41–0.98 |
| CS ≥ 3 | 0.002 | 2.32 | 1.38–3.93 |
| LDH > N | 0.017 | 1.82 | 0.11–2.99 |
| PS ≥ 2 | 0.171 | 1.47 | 0.85–2.53 |
| EN > 1 | 0.777 | 0.93 | 0.58–1.50 |
| sIL‐2R > 2500 | 0.028 | 1.78 | 1.06–4.44 |
| Age > 60 years | 0.064 | 0.67 | 0.43–1.02 |
| CS ≥ 3 | <0.001 | 2.67 | 1.60–4.44 |
| LDH > N | 0.007 | 1.96 | 1.20–3.20 |
| PS ≥ 2 | 0.052 | 1.72 | 1.00–2.97 |
| EN > 1 | 0.660 | 0.90 | 0.56–1.44 |
| sIL‐2R > 3000 | 0.521 | 1.19 | 0.70–2.00 |
CI, confidence interval; CS, clinical stage; EN, number of extranodal sites; LDH, lactate dehydrogenase; N, upper limit of normal range; PFS, progression‐free survival; PS, ECOG performance status; R‐IPI, revised international prognostic index; RR, relative risk; sIL‐2R, soluble interleukin‐2 receptor.
Figure 2.
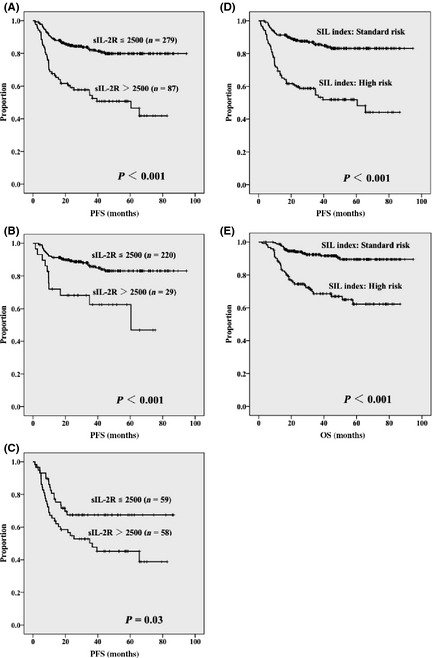
Progression‐free survival in 366 patients with diffuse large B‐cell lymphoma according to soluble interleukin‐2 receptor (sIL‐2R) ≤2500 and >2500 U/mL for the entire cohort (A), “very good” or “good” risk groups (B), and the “poor” risk group (C) according to the revised International Prognostic Index. Progression‐free survival (D) and overall survival (E) according to the SIL index, comprising stage; sIL‐2R level over 2500 U/mL, and elevated lactate dehydrogenase level.
Development of SIL index
Based on the results of multivariate analysis of individual R‐IPI risk factors and sIL‐2R levels over 2500 U/mL (Table 2), another multivariate analysis was carried out. This analysis included three risk factors related to advanced disease, sIL‐2R levels over 2500 U/mL, and elevated LDH levels to establish a new prognostic index for patients with DLBCL. The three risk factors remained significant adverse prognostic factors for PFS (Table 3). Age over 60 years, which was determined to be a favorable prognostic factor, was not included in the new multivariate analysis because it was not thought to be an appropriate factor for clinical use. After assigning 1 point for each risk factor, the standard risk group (0–1 point) and high risk group (2–3 points) were compared. The high risk group was found to be a distinct subgroup in DLBCL patients treated by standard R‐CHOP therapy (P < 0.001, RR 4.01). This index was designated the “SIL index” (S, stage; I, sIL‐2R level over 2500 U/mL; L, elevated LDH level), which is also suggestive of the term “sIL‐2R.” Patients determined to be high risk according to the SIL index had a significantly poor PFS compared to standard risk patients (P < 0.001) (Fig. 2D). The 5‐year PFS rates for patients with standard and high risk were 83% and 52%, respectively. As for OS, patients with high risk also showed inferior survival compared to patients with standard risk (P < 0.001) (Fig. 2E). Table 4 shows the distribution and outcomes of evaluating the 366 patents by IPI, R‐IPI, and SIL indices. The proportion of patients designated as “high‐intermediate” and “high” risk by IPI, “poor” risk by R‐IPI, and “high” risk by SIL index were similar (i.e. one‐third of the cohort).
Table 3.
Stage, soluble interleukin‐2 receptor, and lactate dehydrogenase (SIL) as prognostic predictors in patients with diffuse large B‐cell lymphoma
| Factors | Point | P‐value | RR |
|---|---|---|---|
| S: stage ≥ 3 | 1 | 0.001 | 2.31 |
| I: sIL‐2R ≥ 2500 U/mL | 1 | 0.010 | 1.86 |
| L: LDH ≥ N | 1 | 0.010 | 1.84 |
| SIL index | |||
| Standard | 0–1 | Referent | |
| High | 2–3 | <0.001 | 4.01 |
LDH, lactate dehydrogenase; N, upper limit of normal range; RR, relative risk; Sil‐2R, soluble interleukin‐2 receptor.
Table 4.
Distribution and outcome of patients with diffuse large B‐cell lymphoma according to each index
| Risk group | No. of factors | % Patients | 5‐year PFS, % | 5‐year OS, % |
|---|---|---|---|---|
| IPI | ||||
| Low | 0, 1 | 43 | 84 | 91 |
| Low‐intermediate | 2 | 25 | 76 | 85 |
| High‐intermediate | 3 | 16 | 62 | 80 |
| High | 4, 5 | 16 | 49 | 46 |
| R‐IPI | ||||
| Very good | 0 | 13 | 89 | 87 |
| Good | 1, 2 | 55 | 79 | 89 |
| Poor | 3, 4, 5 | 32 | 56 | 63 |
| SIL lndex | ||||
| Standard | 0, 1 | 67 | 83 | 89 |
| High | 2, 3 | 33 | 52 | 62 |
IPI, International Prognostic Index; No, number; OS, overall survival; PFS, prgression‐free survival; R‐IPI, revised international prognostic index; SIL index, stage; sIL‐2R level over 2500 U/mL; elevated lactate dehydrogenase level; sIL‐2R, soluble interleukin‐2 receptor.
Discussion
Diffuse large B‐cell lymphoma, not otherwise specified, is the substantive subject of this study. This form of lymphoma constitutes 25–30% of adult NHL in developed areas such as North America and western Europe.8 It is more common in the elderly.8 CHOP therapy has been widely used to treat aggressive NHL since the1970s. When compared to second and third generation chemotherapy, CHOP showed similar efficacy in time‐to‐treatment‐failure curves.9 Thus, CHOP was assumed to be the standard therapy from the viewpoint of simplicity, adverse effects, and cost–benefit performance. In the 21st century, the anti‐CD20 mAb rituximab has had a significant impact on the treatment of elderly DLBCL patients in advanced stages of the disease.10, 11 This is also true for young DLBCL patients.12 R‐CHOP thus became the standard therapeutic strategy for advanced stage DLBCL.13 In limited stage DLBCL, standard therapy involves three cycles of CHOP followed by involved‐field radiotherapy; this regimen is in contrast with the eight cycles of CHOP used in the pre‐R era.14 However, the effectiveness of this combined modality has not been established. The PFS and OS curves did not show a plateau in patients who received three cycles of R‐CHOP followed by involved‐field radiotherapy.15 The additional effect of R in limited stage DLBCL patients is unsatisfactory.
We adopted six cycles of R‐CHOP therapy as standard treatment for patients with limited stage DLBCL from 2003; consequently, all DLBCL patients were treated using a uniform strategy of six cycles of R‐CHOP. Although this study is not prospective, all patients were registered before initiating the primary R‐CHOP treatment. We also excluded patients who required reductions in their R‐CHOP dose for comorbid diseases. We believe that this report will aid in the determination of the outcome in DLBCL patients treated with standard R‐CHOP therapy.
Compared with the original R‐IPI report,2 the median age of our patients was slightly greater (Table 1). With regard to individual factors including R‐IPI, the proportion of patients with a PS > 1 was as low as 15% in our study, compared to 41% in the original R‐IPI report. This difference might be due to our treatment strategy that excluded DLBCL patients over 70 years of age with a PS > 1 who required R‐CHOP dose reductions. However, we included seven patients aged over 70 years with a PS > 1 because they were judged to be candidates for full‐dose R‐CHOP therapy by the attending physician (data not shown). This corresponded to 10% of the patients over age 70 in our cohort. In our patients, the R‐IPI was not necessarily predictive because the PFS and the OS curves for patients classified as “very good” or “good” risk did not show statistical significance (Fig. 1).
To establish a new and clinically more appropriate prognostic index for R‐CHOP therapy, we added sIL‐2R to the IPI as a prognostic factor. We evaluated five levels of sIL‐2R and decided that over 2500 U/mL was a risk factor. This level corresponds to an approximately fivefold elevation in sIL‐2R (based on the upper limit of the normal range). Consequently, we deleted the following three risk factors from the R‐IPI: age over 60 years; PS > 1; and extranodal involvement sites numbering more than one. Elderly patients showed unexpectedly better PFS than younger patients. This result is the opposite to that seen in previous reports.1, 2 The exclusion of patients over 70 years of age with a PS > 1, who required R‐CHOP dose reductions, might have resulted in the favorable PFS. Were this not the case, patients with more aggressive lymphoma might be included with younger patients. In developing the new prognostic index, we eliminated age as a factor. This decision is supported by changes in the upper age limit for experimental therapy, such as stem cell transplantation, to include patients older than 60 years. We hope the favorable PFS seen in elderly patients who received full‐dose R‐CHOP treatment will be shown in other patient cohorts. Regarding PS, it is possible that the determination of this subjective measure might differ between attending physicians. Deletion of the R‐IPI prognostic factor “extranodal involvement in more than one site” suggested that there was no need to count the number of extranodal sites. However, as shown in our recent report on the favorable/unfavorable involvement of sites in DLBCL,16 we do not suggest omitting the effort for searching the sites of extranodal involvement.
The SIL index is a simple prognostic index consisting of clinical stage, LDH level, and sIL‐2R level. Clinical stage reflects the degree of dissemination of tumor, LDH level reflects the amount of tumor, and sIL‐2R might reflect reactions in the microenvironment. Although consisting of only three factors, the SIL index is extremely helpful in detecting patients with poor prognosis. We were especially successful in deleting “PS” from the risk factors, as it is often subjective. We believe the SIL index plays a more active role than standard IPI or R‐IPI in DLBCL patients. We defined only two risk groups, standard and high risk. Patients in the standard risk comprised two‐thirds of the cohort and showed a 4‐year PFS rate of 83%; those in the high‐risk comprised one‐third of the cohort and showed a 4‐year PFS rate of 52%. We are gratified by the outcome of patients in the standard risk group as designed by our SIL index. The patients in the high risk group might be candidates for experimental therapy other than R‐CHOP.
In the R‐era, standard R‐CHOP therapy provides a cure in 70% and long survival in 80% of DLBCL patients. The SIL index is a simple and objective index that includes sIL‐2R levels for identifying the candidates for experimental therapy. Our results should be confirmed in an independent cohort of patients.
Disclosure Statement
The authors have no conflicts of interest.
Abbreviations
- CHOP
doxorubicin, cyclophosphamide, vincristine, and prednisolone
- CNS
central nervous system
- DLBCL
diffuse large B‐cell lymphoma
- IPI
International Prognostic Index
- LDH
lactate dehydrogenase
- NHL
non‐Hodgkin's lymphoma
- OS
overall survival
- PFS
progression‐free survival
- PR
partial remission
- PS
performance status
- R
rituximab
- R‐CHOP
doxorubicin, cyclophosphamide, vincristine, and prednisolone plus rituximab
- R‐IPI
revised International Prognostic Index
- RR
relative risk
- SIL index
S, stage; I, sIL‐2R level over 2500 U/mL; L, elevated LDH level
- sIL‐2R
soluble interleukin‐2 receptor
Acknowledgment
We thank Prof. Satoshi Morita for statistical advice.
References
- 1. Project TIN‐HsLPF . A predictive model for aggressive non‐Hodgkin's lymphoma. The International Non‐Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med 1993; 329: 987–94. [DOI] [PubMed] [Google Scholar]
- 2. Sehn LH, Berry B, Chhanabhai M et al The revised International Prognostic Index (R‐IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Blood 2007; 109: 1857–61. [DOI] [PubMed] [Google Scholar]
- 3. Smith KA. Interleukin‐2: inception, impact, and implications. Science 1988; 240: 1169–76. [DOI] [PubMed] [Google Scholar]
- 4. Voss SD, Sondel PM, Robb RJ. Characterization of the interleukin 2 receptors (IL‐2R) expressed on human natural killer cells activated in vivo by IL‐2: association of the p64 IL‐2R gamma chain with the IL‐2R beta chain in functional intermediate‐affinity IL‐2R. J Exp Med 1992; 176: 531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ennishi D, Yokoyama M, Terui Y et al Soluble interleukin‐2 receptor retains prognostic value in patients with diffuse large B‐cell lymphoma receiving rituximab plus CHOP (RCHOP) therapy. Ann Oncol 2009; 20: 526–33. [DOI] [PubMed] [Google Scholar]
- 6. Tomita N, Kodama F, Kanamori H et al Prophylactic intrathecal methotrexate and hydrocortisone reduces central nervous system recurrence and improves survival in aggressive non‐hodgkin lymphoma. Cancer 2002; 95: 576–80. [DOI] [PubMed] [Google Scholar]
- 7. Jaffe ES, Harris NL, Stein H et al Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. World Health Organization Classification of Tumours. Lyon: IARC Press, 2001. [Google Scholar]
- 8. Swerdlow SH, Campo E, Harris NL et al WHO classification of tumors of haematopoietic and lymphoid tissues. Lyon: IARC Press, 2008. [Google Scholar]
- 9. Fisher RI, Gaynor ER, Dahlberg S et al Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non‐Hodgkin's lymphoma. N Engl J Med 1993; 328: 1002–6. [DOI] [PubMed] [Google Scholar]
- 10. Coiffier B, Lepage E, Briere J et al CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med 2002; 346: 235–42. [DOI] [PubMed] [Google Scholar]
- 11. Feugier P, Van HoofA, Sebban C et al Long‐term results of the R‐CHOP study in the treatment of elderly patients with diffuse large B‐cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 2005; 23: 4117–26. [DOI] [PubMed] [Google Scholar]
- 12. Pfreundschuh M, Kuhnt E, Trümper L et al CHOP‐like chemotherapy with or without rituximab in young patients with good‐prognosis diffuse large‐B‐cell lymphoma: 6‐year results of an open‐label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011; 12: 1013–22. [DOI] [PubMed] [Google Scholar]
- 13. Non Hodgkin's lymphoma Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network, Inc. Available at: http://www.nccn.org (Accessed 24 March 2012). [DOI] [PubMed]
- 14. Miller TP, Dahlberg S, Cassady JR et al Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate‐ and high‐grade non‐Hodgkin's lymphoma. N Engl J Med 1998; 339: 21–6. [DOI] [PubMed] [Google Scholar]
- 15. Persky DO, Unger JM, Spier CM et al Phase II study of rituximab plus three cycles of CHOP and involved‐field radiotherapy for patients with limited‐stage aggressive B‐cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol 2008; 26: 2258–63. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi H, Tomita N, Yokoyama M et al Prognostic impact of extranodal involvement in diffuse large B‐cell lymphoma in the rituximab era. Cancer (in press). [DOI] [PubMed] [Google Scholar]


