Abstract
In the present study, we analyzed genomic alterations of BRCA1‐associated protein 1 (BAP1) in 23 malignant mesotheliomas (MMs), 16 epithelioid and seven non‐epithelioid, consisting of 18 clinical specimens and five established cell lines. In examining these samples for homozygous deletions and sequence‐level mutations, we found biallelic BAP1 gene alterations in 14 of 23 MMs (61%). Seven of these 14 MMs had homozygous deletions of the partial or entire BAP1 gene, another five had sequence‐level mutations, including small deletions, a nonsense mutation, and missense mutations with additional monoallelic deletions, and the remaining two had homozygous mutations without allelic loss. All but one of the 14 BAP1 gene mutations were found in the epithelioid‐type MMs; BAP1 mutations were found in 13 of 16 epithelioid‐type MMs, but in only one of seven non‐epithelioid‐type MMs (13/16 vs 1/7; P = 0.005). There was no BAP1 mRNA expression in MMs with biallelic deletion and repressed expression was confirmed in MM specimens with deletion/mutation as compared with Met5a, SV40‐transformed normal mesothelial cells. Western blot showed that seven of eight epithelioid MMs analyzed were BAP1 negative. Immunostaining with anti‐BAP1 antibody in normal lung tissues revealed clear nuclear staining of normal mesothelial cells. No nuclear staining was observed among BAP1 mutation‐positive MM tumors, whereas nuclear staining was observed among BAP1 mutation‐negative MM tumors. These results suggest that the lack of the tumor suppressor BAP1 may be more specifically involved in the pathogenesis of epithelioid MM rather than non‐epithelioid MM, and would be useful for diagnosis of epithelioid‐type MM. (Cancer Sci 2012; 103: 868–874)
Malignant mesothelioma (MM) is an asbestos‐related malignancy that arises primarily from surface serosal cells of pleural, peritoneal, and pericardial cavities. Although the use of asbestos has decreased in Western countries and Japan, the incidence of MM is expected to increase over the next few decades because of the long latency period (20–40 years) of this malignancy.1 Although the prognosis of MM is generally poor, epithelioid‐type MM has been reported to be associated with better prognosis than non‐epithelioid types of MM.2 Multiple modality approaches involving surgery with radiation, chemotherapy, or immunotherapy have generated favorable outcomes, particularly for patients with epithelioid‐type MM.3
One of the most common genetic alterations in MM is homozygous deletion of the 9p21 locus containing the CDKN2A and CDKN2B genes.4, 5 The NF2 gene is also often mutated in MM.6, 7 Conversely, mutations of the p53, Ras, and RB genes, common in many other types of tumors, are very rare in MM.8, 9 Previously, using Comparative Genomic Hybridization (CGH) array, we found that more than half of MM samples exhibit homozygous and heterozygous deletions of 3p21.1.10 Three of our MM cases, namely MM21‐P, MM34‐P, and MM14‐T, had homozygous deletions at 3p21.1. The overlapping homozygous deletion in these cases was approximately 160 kb in length, spanning nine genes and including BRCA1‐associated protein 1 (BAP1).10 Initially, BAP1 was identified as a protein that binds to the RING finger domain of BRCA1 and exhibits tumor‐suppressor activity in cancer cells.11, 12 It is one of the cysteine proteases called deubiquitinating enzymes that catalyse the removal of ubiquitin chains from ubiquitinated proteins. It has been reported recently that BAP1 is frequently mutated in metastasizing uveal melanoma, but not in low‐metastatic uveal melanoma.13 Herein, we report on genomic alterations of BAP1.
Materials and Methods
MM cells and tissue specimens.
Pleural effusions, ascites, and tumor tissues were obtained from 18 patients diagnosed with MM by pathological examination at the hospital of Hyogo College of Medicine. Matched peripheral blood was obtained from 11 patients (see Table S1), but matched normal tissues from the other seven patients were unavailable. All patients provided written informed consent. The human normal pleura transformant cell line Met5a (used as a reference) and four MM cell lines (i.e. H2052, H2452, H28, and MSTO‐211H) were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). The HMMME cell line was obtained from the Riken Bioresource Center (Tsukuba, Japan). The characteristics of these cells are given in Table S1.
Cells in pleural effusions and ascites were collected by centrifugation and cultured in α‐Minimal Essential Medium (α‐MEM; Invitrogen, Carlsbad, CA, USA) supplemented with 10% FBS (Equitech‐Bio, Ingram, TX, USA). Surgically resected tumors were cut into small pieces and used for plating on culture dishes for growth. Primary outgrowth cells were cultured in α‐MEM–10% FBS and adherent cells were expanded by several passages. These cells were termed MM primary cell cultures (MM‐Ps) and 16 MM‐Ps were used in the present study. For cases MM14 and MM29, primary cell cultures were not established; in these cases, tissue specimens (termed MM‐Ts) were used for analysis. Tumor tissues resected from all but one MM patient (17 of 18 cases; a tissue specimen was unavailable for MM34), and lung tissues of one patient with pulmonary emphysema were fixed with 10% formalin, and embedded in paraffin for subsequent immunohistochemistry.
The present study was approved by the Ethics Committee of Hyogo College of Medicine and was performed in accordance with the Declaration of Helsinki (1995) as revised in Tokyo in 2004.
Reagents.
Anti‐BAP1 antibody (C‐4; sc‐28383) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), ACTB (Sc‐74; A5316) was from Sigma‐Aldrich (St. Louis, MO, USA), and horseradish peroxidase‐conjugated secondary antibody for enhanced chemiluminescence (ECL) was from GE Healthcare (Waukesha, WI, USA).
Extraction of DNA and RNA and real‐time RT‐PCR.
In the present study, DNA and RNA were isolated from cultured cells, tissues, and peripheral blood using the AllPrep DNA/RNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. For MM cells, real‐time RT‐PCR was performed to analyze gene expression using 2 ng cDNA (reverse transcribed from total RNA) and the following primer: for BAP1, 5′‐AGGAGCTGCTGGCACTGCTGA‐3′ (sense) and 5′‐TTGTGGAGCCGGCCGATGCT‐3′ (antisense); and for GAPDH, 5′‐GCACCGTCAAGGCTGAGAAC‐3′ (sense) and 5′‐TGGTGAAGACGCCAGTGGA‐3′ (antisense). Gene expression was normalized against that of GAPDH and the expression of BAP1 in MM samples was compared with that in Met5a cells.
Screening for genomic alterations.
Genomic DNA, extracted as described above, was amplified by PCR using primers for the coding region of BAP1 (Table 1). Amplified products were analyzed for direct sequencing using Big Dye Terminator v3.1 on an Applied Biosystems 3130 Genetic Analyzer (Applied Biosystems, Foster, CA, USA). The following primer set was used to detect exon 9 in BAP1: 5′‐CTGTGACTGCAGGGAGCCCTACCA‐3′ (sense) and 5′‐AAGGGCACCTACCTGCTGCAGAG‐3′ (antisense).
Table 1.
Sequences of the primers used in the present study to amplify the 10 fragments for sequencing of the BAP1 coding region
| Fragment | Target | Primer design region | Primer sequence |
|---|---|---|---|
| 1 | Exon 1–3 | Exon 1 | Forward: 5′‐CCGTTGTCTGTGTGTGGGACTGAG‐3′ |
| Intron 3 | Reverse: 5′‐AGAGCAAGGCTGCTGCTTTCTGTG‐3′ | ||
| 2 | Exon 4 | Intron 3 | Forward: 5′‐CACCTGAGTGATGACGCAGTGCAA‐3′ |
| Intron 4 | Reverse: 5′‐TACCCACTGGATATCTGAGGACAC‐3′ | ||
| 3 | Exon 5 | Intron 4 | Forward: 5′‐TAGGAGGGTGTCTGAGTCCACTC‐3′ |
| Intron 5 | Reverse: 5′‐CAGATCTGCCCAGTTGGCTGTGAG‐3′ | ||
| 4 | Exon 6–7 | Intron 5 | Forward: 5′‐CCACCCATAGTCCTACCTG‐3′ |
| Intron 7 | Reverse: 5′‐AACAGGCCTCCAGCTCATGGTG‐3′ | ||
| 5 | Exon 8 | Exon 7 | Forward: 5′‐GGCTGAAGGTCTACCCCATTGAC‐3′ |
| Intron 8 | Reverse: 5′‐CCAGATTCACCATATGGCCTTGCA‐3′ | ||
| 6 | Exon 9–10 | Intron 8 | Forward: 5′‐GTGCCTGGCATGTATGGC‐3′ |
| Intron 10 | Reverse: 5′‐CCTCCCATGTCAGACATTAG‐3′ | ||
| 7 | Exon 11 | Intron 10 | Forward: 5′‐TTCTCTGGGAAGTGCTGGTT‐3′ |
| Intron 11 | Reverse: 5′‐GGAACCACATGGGAAAATTG‐3′ | ||
| 8 | Exon 12 | Intron 11 | Forward: 5′‐CAAGGACAGGCCATGGAAC‐3′ |
| Intron 12 | Reverse: 5′‐AGGTGCTCAACATTATCTGC‐3′ | ||
| 9 | Exon 13–14 | Intron 12 | Forward: 5′‐CATTCTGGGTACTGCTGGGT‐3′ |
| Intron 14 | Reverse: 5′‐CCACCAATCTTCACACCAAA‐3′ | ||
| 10 | Exon 15–17 | Intron 14 | Forward: 5′‐TCCTTGCCTCTAGCTGCCTAT‐3′ |
| Exon 17 | Reverse: 5′‐TACTGGGAAAAGGGGAAGTG‐3′ |
Western blotting.
Cell lysates were prepared using lysis buffer (Cell Signaling Technology, Danvers, MA, USA) and 5 μg isolated protein was electrophoresed on a 10–20% TGX gel (Bio‐Rad, Hercules, CA, USA). Proteins were transferred onto polyvinylidene difluoride membranes and blocked with 5% non‐fat powdered milk in 1× Tris‐buffered saline with 0.1% Tween‐20 (TBS‐T) for 30 min. Membranes were washed with TBS‐T, incubated with primary antibody for 2 h, followed by incubation with the secondary antibody for 1 h, and developed using an ECL Plus Western Blotting Detection System (GE Healthcare).
Immunohistochemistry.
Formalin‐fixed, paraffin‐embedded MM tissues were cut into 4‐μm sections. Sections were heated in Target Retrieval Solution (S1700; DakoCytomation, Glostrup, Denmark) at 98°C for 25 min to facilitate antigen retrieval. After blocking with goat serum, sections were incubated with mouse anti‐BAP1 antibody (1 : 50 dilution) for 30 min and then with EnVision FLEX + Mouse (LINKER) including rabbit polyclonal antibody to mouse immunoglobulin (K8022; DakoCytomation) for 15 min to increase the sensitivity of detection. This was followed by treatment with goat polyclonal antibodies to rabbit and mouse immunoglobulin for 30 min using ChemMate EnVision Kit (K5007; DakoCytomation) and visualization with 3,3′‐diaminobenzidine tetrahydrochloride. Cells were lightly stained with hematoxylin.
Statistical analysis.
Fisher's exact test was used for statistical comparisons of biallelic mutation frequencies between the two groups (epithelioid MMs versus non‐epithelioid MMs). P ≤ 0.05 was considered significant.
Results
Genomic alterations of the BAP1 gene are frequently seen in MM cells.
To examine homozygous deletion and sequence‐level mutations in the BAP1 gene, PCR was used to amplify 10 fragments, derived from the entire coding region of this gene, in 23 MM samples: 18 from patients with MM (16 MM‐Ps and two MM‐Ts) attending the hospital of Hyogo College of Medicine, four MM cell lines obtained from the ATCC, and one cell line obtained from RIKEN. These samples consisted of 16 epithelioid‐type MMs and seven non‐epithelioid MMs. We found biallelic BAP1 gene alterations in 14 of 23 MMs (61%; Table 2). For MM19‐P, MM21‐P, MM34‐P, and MM14‐T, no amplification was identified using the primer pairs described in Table 1 (Fig. 1a,b). Surprisingly sequence analysis detected many genomic alterations in both our MM cases and the commercial MM cell lines (Table 2; Fig. 1) as follows: (i) deletions of exons 1–5 causing loss of the N terminal region (MM39‐P and MM48‐P); (ii) deletion of exons 10–17 causing premature protein termination (MM57‐P); (iii) deletion of three nucleotides in exon 9 (MM29‐T); (iv) frameshift mutations causing premature protein termination due to insertion/deletion (MM56‐P) or deletion of 19 nucleotides (HMMME); (v) deletion of 23 nucleotides causing loss of a splice acceptor site (H28); (vi) nonsense mutation (MM35‐P); and (vii) missense mutation (MM62‐P and H2452). Sequence data for samples carrying these mutations were not heterogeneous (Fig. 1c,d,f–i), with the exception of MM29‐T, which likely appeared heterogeneous owing to contamination by normal tissue (Fig. 1e). Biallelic alteration of the BAP1 gene was then identified by PCR and sequencing analysis for the 14 mutated MMs. Our CGH array data10 supported these results (Table 2). Seven MM cases (i.e. MM19‐P, MM21‐P, MM34‐P, MM39‐P, MM48‐P, MM57‐P, and MM14‐T) had biallelic deletions in BAP1. For MM35‐P, MM56‐P, MM29‐T, H2452, and HMMME, biallelic alterations in this gene were caused by sequence‐level mutations and loss of the remaining allele, and both H28 and MM62‐P had biallelic mutations. Allelic changes of the BAP1 gene were deletion/deletion (six MM‐Ps, two MM‐Ts, and two MM lines), deletion/insertion and deletion (one MM‐P), deletion/base substitution (one each MM‐P and MM line), and base substitution/base substitution (one MM‐P; Fig. 2). Thus, the biallelic deletions were most frequently found for BAP1 inactivation. With the exception of the nonsense mutation at amino acid position 460 (p.S460X) in MM35‐P, the other six sequence‐level mutations occurred in the ubiquitin carboxyl terminal hydrolase domain (Fig. 2). Mutations in MM56‐P (p.R146RfsX9), H28, and HMMME (p.R150RfsX31) induced premature termination, and ones in MM29‐T (p.V234del) and MM62‐P (p.Y173S) occurred at positions close to the active site of the enzyme. A missense mutation at amino acid position 95 (p.A95D), which is known to impair deubiquitinase activity,14 was found in H2452.
Table 2.
Summary of BAP1 gene alterations in malignant mesothelioma
| Histological type | Specimen | Source | Allelic status of 3p21.1 (position: 52.4–52.6 Mb) | Genomic alterations in BAP1 | BAP1 expression | |||
|---|---|---|---|---|---|---|---|---|
| Mutation in the coding region | Somatic alteration | mRNA | Protein | Nuclear immunostaining in tumor tissue | ||||
| Epithelioid | MM19‐P | HCM | −/+ | Deletion of BAP1 coding region | + | − | − | − |
| MM21‐P | HCM | −/− | Deletion of entire BAP1 gene | + | − | − | − | |
| MM26‐P | HCM | +/+ | ND | − | ++ | ++ | + | |
| MM34‐P† | HCM | −/− | Deletion of entire BAP1 gene | + | − | N/A | N/A | |
| MM35‐P | HCM | −/+ | Nonsense mutation at aa 460 (g.52437782C>G, p.S460X) | + | ± | − | − | |
| MM39‐P | HCM | −/+ | Deletion of exons 1–5 | N/A | − | − | − | |
| MM45‐P | HCM | −/+ | ND | + | + | N/A | − | |
| MM48‐P | HCM | −/+ | Deletion of exons 1–5 | + | − | − | − | |
| MM56‐P | HCM | −/+ | Frameshift mutation at aa146(g.52441416delAinsCG, p.R146RfsX9) | N/A | ± | − | −‡ | |
| MM57‐P | HCM | −/+ | Deletion of exons 10–17 | + | − | N/A | − | |
| MM67‐P | HCM | +/+ | ND | − | + | N/A | ++ | |
| MM14‐T | HCM | −/− | Deletion of entire BAP1 gene | + | N/A | N/A | − | |
| MM29‐T | HCM | −/+ | 3‐bp deletion at aa234 (g.52440352_52440350del, p.V234del) | + | N/A | N/A | − | |
| H28 | ATCC | +/+ | 23‐bp deletion at intron 6/exon7 junction§ (g.52441351_52441329del) | N/A | ++ | N/A | N/A | |
| H2452 | ATCC | −/+ | Missense mutation at aa95 (g.52442065C>A, p.A95D) | N/A | ++ | − | N/A | |
| HMMME | RIKEN | −/+ | 19‐bp deletion (g.52441321_52441303del, p.R150RfsX31) | N/A | + | N/A | N/A | |
| Non‐epithelioid (biphasic) | MM16‐P | HCM | +/+ | ND | − | ++ | ++ | + |
| MM30‐P | HCM | +/+ | ND | − | ++ | + | + | |
| MM62‐P | HCM | +/+ | Missense mutation at aa 173§ (g.52441252A>C, p.Y173S) | N/A | ++ | N/A | − | |
| MM80‐P | HCM | +/+ | ND | − | ++ | N/A | + | |
| MSTO‐211H | ATCC | +/+ | ND | − | + | N/A | N/A | |
| Non‐epithelioid (sarcomatoid) | MM46‐P | HCM | +/+ | ND | − | ++ | ++ | + |
| H2052 | ATCC | +/+ | ND | − | ++ | N/A | N/A | |
†This specimen was unavailable for protein analysis. ‡Nuclear staining was seen in some tumor cells, but not all. §This was identified as a homozygous mutation without allelic loss by direct sequencing. The allelic status of 3p21.1 (position: 52.4–52.6 Mb), identified from our previously published Comparative Genomic Hybridization (CGH) array data,10 is shown, and biallelic and monoallelic deletion of this region is indicated by −/− and −/+, respectively. Mutations in the BAP1 coding region were identified by direct sequencing of 10 fragments, derived from the entire coding region of this gene and amplified using the primers described in Table 1. For somatic alterations, the “+” indicates mutations judged to be of somatic origin of the nine malignant mesotheliomas (MMs) for which comparisons with paired non‐tumor DNA were possible and “−” indicates no mutations in the coding region. For BAP1 expression, expression level data summarized from Figure 3 are shown as – (no expression), ±, +, or ++ (similar as that in Met5a cells). Immunohistochemistry was performed using a anti‐BAP1 antibody for primary tissue specimens and nuclear immunostaining is indicates as −, +, and ++ (similar as that in non‐tumor mesothelial cells shown in Fig. 4a). aa, amino acid; HCM, Hyogo College of Medicine; ND, not detected; N/A, not analyzed.
Figure 1.
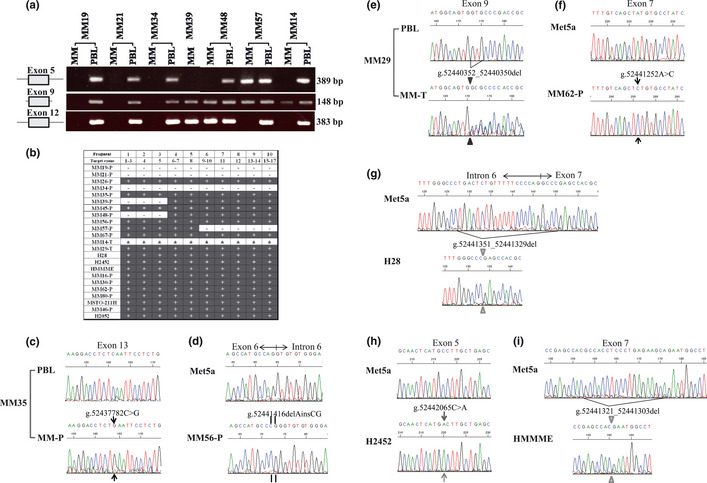
Genomic alterations of the BAP1 gene. (a) Agarose gel electrophoresis of PCR fragments amplified with the primers detecting exon 5 (fragment 3 in Table 1), exon 9, and exon 12 of BAP1 (fragment 8) from patients with malignant mesothelioma (MM19, MM21, MM34, MM39, MM48, MM57, and MM14). MM, tumor sample of malignant mesothelioma; PBL, matched normal peripheral blood leukocyte. (b) Summary of amplification of each PCR fragment using primers for the coding region of BAP1 in each sample we analyzed. +, amplified; ±, a slight amplification; −, not amplified; MM‐Ps, MM primary cell cultures; MM‐Ts, tissue specimens. (c) Electropherogram showing BAP1 nonsense mutation (g.52437782C>G) within exon 13 in MM35‐P. (d) Electropherogram showing BAP1 insertion/deletion mutation (g.52441416delAinsCG) within exon 6 in MM56‐P. (e) Electropherogram showing a 3‐bp deletion (g.52440352_52440350del) within exon 9 in MM29‐T. (f) Electropherogram showing BAP1 missense mutation (g.52441252A>C) within exon 7 in MM62‐P. (g) Electropherogram showing 23‐bp deletion (g.52441351_52441329del) at the intron 6–exon 7 boundary in the H28 cell line. (h) Electropherogram showing BAP1 misssense mutation (g.52442065C>A) within exon 5 in the MM cell line H2452. (i) Electropherogram showing a 19‐bp deletion (g.52441321_52441303del) within exon 7 in the MM cell line HMMME.
Figure 2.
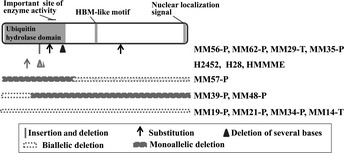
Summary of mutation sites in BAP1 for the samples from patients and cell lines showing biallelic alterations and the site of functional domain or motif. HBM, HCF‐binding motif.
All but one of 14 BAP1 gene mutations were found among epithelioid‐type MMs, and MM62‐P was the only non‐epithelioid MM showing genomic alterations in BAP1. Mutations in BAP1 were found in as many as 13 of 16 epithelioid MMs (81%). Statistical analysis indicated that the frequency of biallelic mutations in epithelioid‐type MM was significantly higher than that in non‐epithelioid‐type MM (P = 0.005).
These genomic alterations in the BAP1 gene were judged as somatic in nine cases because no such alterations were found in DNA from the available matched peripheral blood samples. However, the other three cases were unidentified because of the unavailability of matched normal tissue.
Repressed expression of the BAP1 gene in MM cells.
Real‐time RT‐PCR was conducted on 16 MM‐Ps and five commercial MM cell lines. The results indicated no expression of BAP1 mRNA in six of 16 MM‐Ps due to biallelic deletion of this gene (MM19‐P, MM21‐P, MM34‐P, MM39‐P, MM48‐P, and MM57‐P; Fig. 3a). Suppression of BAP1 mRNA expression in three MM‐Ps and one cell line was observed due to deletions/mutations (in MM35‐P, MM56‐P, and HMMME) and monoallelic deletion (in MM45‐P). Moreover, both MM67‐P and MSTO‐211H, which had no verifiable genomic changes in the coding region of BAP1, showed suppression of BAP1 expression (Fig. 3a). Western blotting showed complete absence of BAP1 protein in MM‐Ps with biallelic gene deletion (MM19‐P, MM21‐P, MM39‐P, and MM48‐P), and loss of one allele combined with mutation (MM35‐P and H2452) or combined with insertion and deletion (MM56‐P; Fig. 3B). Immunohistochemistry with anti‐BAP1 antibody showed nuclear staining in non‐tumor mesothelial cells on the lung surface (Fig. 4a) and in tumor cells of MM tissue specimens with the wild‐type BAP1 (MM26, MM67, MM16, MM30, MM80, and MM46; Fig. 4b). No nuclear staining was observed in tumor cells from which MMs with BAP1 alterations were established (MM19, MM21, MM35, MM39, MM45, MM48, MM57, MM14, MM29, and MM62; Fig. 4c). It should be noted that some tumor cells of MM56, but not all, showed nuclear staining.
Figure 3.
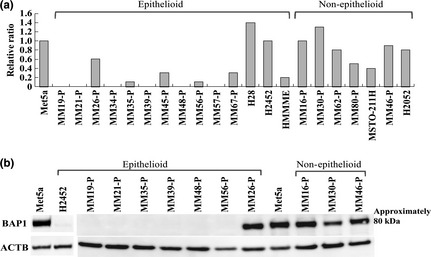
Repressed expression of BAP1 gene. (a) Gene expression of BAP1 in malignant mesothelioma (MM) cells, analyzed by real‐time RT‐PCR and normalized against GAPDH, is presented as a ratio relative to expression in Met5a cells. (b) Western blotting. Expression of BAP1 protein was examined in Met5a cells, H2452 cells, and primary cell cultures obtained from seven patients with epithelioid MM and three patients with non‐epithelioid MM. β‐actin (ACTB) was used as a positive control.
Figure 4.
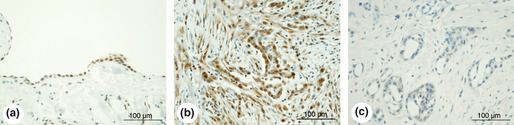
Immunostaining with anti‐BAP1 antibody. (a) A specimen of the lung surface of a patient with pulmonary emphysema. (b) A specimen of tumors from patient MM80, who had biphasic malignant mesothelioma (MM). (c) A specimen of tumors from patient MM35, who had epithelioid MM.
Discussion
In the present study, we found frequent somatic alterations in the BAP1 locus in Japanese patients with MM. Fourteen of our 23 MM samples had various types of biallelic genomic alterations in BAP1, including deletions encompassing the whole BAP1 locus or involving several exons (Table 2). Biallelic alterations are generally caused by monoallelic mutations coupled with monoallelic loss, and, although our data indicated that both H28 and MM62‐P had homozygous mutations without allelic loss, we were unable to prove this. However, the ratio of the copy numbers of exon 13 determined by quantitative real‐time PCR and of the probes designed for the BAP1 region in the CGH array suggest that these MM cells have no allelic deletions in this gene region (Table S2). No expression or decreased expression of BAP1 mRNA was frequently observed in epithelioid‐type MM cells. In addition, BAP1 gene expression was repressed in MM45‐P and MM67‐P, with monoallelic deletion of BAP1 found by real‐time PCR (Table S2). Monoallelic deletion in MM45‐P has been identified previously by paired CGH array analysis using matched peripheral blood samples;10 however, we were unable to conclusively demonstrate monoallelic loss in MM67‐P because of the unavailability of matched normal tissue.
While we were preparing this manuscript for publication, four papers were published showing that somatic and germline mutations of BAP1 predispose to MM and other types of tumors.15, 16, 17, 18 Bott et al.15 reported that 12 of 53 MM tumors (23%) collected in the US had somatic inactivating mutations of BAP1. Six of 12 had monoallelic loss of 3p21.1 with mutations in the remaining allele. Testa et al.17 found germline BAP1 mutations in two US families with a high incidence of MM. The affected members of one family had a base substitution at the intron 6–exon 7 boundary in germline DNA, whereas those of another family had a common nonsense mutation in exon 16 in germline DNA. In these affected members, somatic BAP1 alterations developed in the remaining allele, leading to biallelic inactivation of this gene. Testa et al.17 also found germline BAP1 mutations in two of 26 sporadic MMs, and these mutations showed 1‐ or 4‐bp deletions in exon 13 or 14, respectively, resulting in frameshift leading to a stop codon. Other studies reported that families with a high incidence of melanocytic tumors16 and several tumors, including MM,18 carry various somatic alterations in addition to inactivating germline mutations of BAP1. In the biallelic deletions we found, the length of deletion differed between two alleles, with the exception of H28. These results suggest that BAP1 is subject to loss‐of‐function mutations (especially two‐step alterations) because of inherent instability, although no particular mutation hotspots are apparent. Our findings reveal that BAP1 gene inactivation occurs at a very high frequency in patients with epithelioid MM, the most common form of MM, and that this may be useful for a diagnosis of MM. In contrast, Bott et al.15 could not find any significant correlation between somatic mutations of BAP1 and histological type. The rare case in the present study was MM62‐P, who had biphasic MM consisting of epithelioid and sarcomatoid types. It is interesting that, in previous reports,15, 17 BAP1 inactivation caused by deletion of a whole gene or several exons in sporadic MM was rare, but was most frequent in our patients.
In the present study, defective expression of BAP1 in MM cells with genomic alterations was confirmed by RT‐PCR, western blotting, and immunohistochemistry. The possibility of genomic and/or expression changes during cell culture was not completely eliminated. In the case of MM56, from which MM‐P cells that were western blot negative were established, some tumor cells of the MM, but not all, exhibited nuclear staining with the same anti‐BAP1 antibody. Faint staining by this antibody was detected in the cytoplasm of non‐tumor mesothelial cells. It should be noted that granular cytoplasmic staining was also seen in some MM tumor specimens having biallelic inactivation of BAP1; we are yet to determine the significance of these observations.
It is known that BAP1 regulates cell proliferation.11, 14, 19, 20 The results of the present study suggest that the lack of the tumor suppressor BAP1 may be more specifically involved in the pathogenesis of epithelioid‐type MM rather than non‐epithelioid MM. It is tempting to postulate that different pathogenic mechanisms may exert their effects depending on the histological type of MM.
In conclusion, the results of the present study indicate that the loss of BAP1 expression could be useful in the diagnosis of epithelioid‐type MM.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Table S1. Characteristics of the cells and tumor specimens used in the present study.
Table S2. Copy number ratio of BAP1 gene, as determined by CGH array and real‐time PCR.
Acknowledgments
This work was supported, in part, by a Grant‐in‐Aid for Scientific Research (KAKENHI, 20590590). The authors thank Ms Atsuko Iemoto (Hyogo College of Medicine) for technical assistance.
References
- 1. Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980; 46: 2736–40. [DOI] [PubMed] [Google Scholar]
- 2. Nojiri S, Gemba K, Aoe K et al Survival and prognostic factors in malignant pleural mesothelioma: a retrospective study of 314 patients in the west part of Japan. Jpn J Clin Oncol 2011; 41: 32–9. [DOI] [PubMed] [Google Scholar]
- 3. Neragi‐Miandoab S. Multimodality approach in management of malignant pleural mesothelioma. Eur J Cardiothorac Surg 2006; 29: 14–9. [DOI] [PubMed] [Google Scholar]
- 4. Xio S, Li D, Vijg J, Sugarbaker DJ, Corson JM, Fletcher JA. Codeletion of p15 and p16 in primary malignant mesothelioma. Oncogene 1995; 11: 511–55. [PubMed] [Google Scholar]
- 5. Prins JB, Williamson KA, Kamp MM et al The gene for the cyclin‐dependent‐kinase‐4 inhibitor, CDKN2A, is preferentially deleted in malignant mesothelioma. Int J Cancer 1998; 75: 649–53. [DOI] [PubMed] [Google Scholar]
- 6. Sekido Y, Pass HI, Bader S et al Neurofibromatosis type 2 (NF2) gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res 1995; 55: 1227–31. [PubMed] [Google Scholar]
- 7. Bianchi AB, Mitsunaga SI, Cheng JQ et al High frequency of inactivating mutations in the neurofibromatosis type 2 gene (NF2) in primary malignant mesotheliomas. Proc Natl Acad Sci USA 1995; 92: 10854–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kratzke RA, Otterson GA, Lincoln CE et al Immunohistochemical analysis of the p16INK4 cyclin‐dependent kinase inhibitor in malignant mesothelioma. J Natl Cancer Inst 1995; 87: 1870–5. [DOI] [PubMed] [Google Scholar]
- 9. Papp T, Schipper H, Pemsel H et al Mutational analysis of N‐ras, p53, p16INK4a, p14ARF and CDK4 genes in primary human malignant mesotheliomas. Int J Oncol 2001; 18: 425–33. [DOI] [PubMed] [Google Scholar]
- 10. Yoshikawa Y, Sato A, Tsujimura T et al Frequent deletion of 3p21.1 region carrying semaphorin 3G and aberrant expression of the genes participating in semaphorin signaling in the epithelioid type of malignant mesothelioma cells. Int J Oncol 2011; 39: 1365–74. [DOI] [PubMed] [Google Scholar]
- 11. Jensen DE, Proctor M, Marquis ST et al BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1‐mediated cell growth suppression. Oncogene 1998; 16: 1097–112. [DOI] [PubMed] [Google Scholar]
- 12. Nishikawa H, Wu W, Koike A et al BRCA1‐associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res 2009; 69: 111–9. [DOI] [PubMed] [Google Scholar]
- 13. Harbour JW, Onken MD, Roberson ED et al Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010; 330: 1410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ventii KH, Devi NS, Friedrich KL et al BRCA1‐associated protein‐1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res 2008; 68: 6953–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bott M, Brevet M, Taylor BS et al The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 2011; 43: 668–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wiesner T, Obenauf AC, Murali R et al Germline mutations in BAP1 predispose to melanocytic tumors. Nat Genet 2011; 43: 1018–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Testa JR, Cheung M, Pei J et al Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet 2011; 43: 1022–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdel‐Rahman MH, Pilarski R, Cebulla CM et al Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet 2011; 48: 856–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Machida YJ, Machida Y, Vashisht AA, Wohlschlegel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF‐1. J Biol Chem 2009; 284: 34179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Misaghi S, Ottosen S, Izrael‐Tomasevic A et al Association of C‐terminal ubiquitin hydrolase BRCA1‐associated protein 1 with cell cycle regulator host cell factor 1. Mol Cell Biol 2009; 29: 2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the cells and tumor specimens used in the present study.
Table S2. Copy number ratio of BAP1 gene, as determined by CGH array and real‐time PCR.


