Abstract
Background Despite effective therapies for many conditions, patients find it difficult to adhere to prescribed treatments. Technology-mediated interventions (TMIs) are increasingly being used with the hope of improving adherence.
Objective To assess the effects of TMI, intended to enhance patient adherence to prescribed medications, on both medication adherence and clinical outcomes.
Methods A secondary in-depth analysis was conducted of the subset of studies that utilized technology in at least one component of the intervention from an updated Cochrane review on all interventions for enhancing medication adherence. We included studies that clearly described an information and communication technology or medical device as the sole or major component of the adherence intervention.
Results Thirty-eight studies were eligible for in-depth review. Only seven had a low risk of bias for study design features, primary adherence, and clinical outcomes. Eighteen studies used a TMI for education and/or counseling, 11 studies used a TMI for self-monitoring and/or feedback, and nine studies used electronic reminders. Studies used a variety of TMIs, with telephone the most common technology in use. Studies targeted a wide distribution of diseases and used a variety of adherence and clinical outcome measures. A minority targeted children and adolescents. Fourteen studies reported significant effects in both adherence and clinical outcome measures.
Conclusions This review provides evidence for the inconsistent effectiveness of TMI for medication adherence and clinical outcomes. These results must be interpreted with caution due to a lack of high-quality studies.
Keywords: eHealth, patient adherence, technology mediation, systematic review, reminder systems, outcomes
INTRODUCTION
Poor medication adherence is a major problem that undermines the benefits of health care and increases costs.1 Despite increasing numbers of efficacious self-administered therapies, medication adherence rates remain variable and low, and have not changed significantly over time.2–4 A Cochrane review by Nieuwlaat et al.5 reported no single effective, actionable, and affordable method of helping patients to follow prescribed treatments. The need for patient-centered interventions to improve adherence is apparent and the opportunity for technology in the development of these interventions is increasing.6–9 Technology-mediated interventions (TMIs) potentially require fewer (human) resources, which may be an argument for deploying these interventions to address nonadherence. However, the effectiveness of TMI on medicine adherence is unclear.8,10 To elucidate the current state of evidence, we reviewed higher quality evidence from randomized controlled trials (RCTs).
OBJECTIVE
The primary objective of this review is to assess the effects of TMI, intended to enhance patient adherence to prescribed medications for medical conditions, on both medication adherence and clinical outcomes.
METHODS
This systematic review represents an in-depth examination of the subset of studies that utilized technology as at least one component of the intervention in an updated comprehensive Cochrane review on interventions for enhancing medication adherence.5 We used a web-based database management system, developed by the Health Information Research Unit at McMaster University, to facilitate screening, data extraction, adjudication of disagreements, author review, confirmation of data, production of data tables, and production of data files for future research use. Methods for the comprehensive review are detailed in Nieuwlaat et al.,5 and are briefly summarized in the following.
Information sources and search strategy
We searched The Cochrane Library (via Wiley), MEDLINE, Embase, PsycINFO (all via Ovid), CINAHL (via EBSCO), and Sociological Abstracts (via ProQuest). We completed database searches for relevant articles on January 11, 2013, updating previous searches. All databases were searched from their start date.
Study eligibility criteria
Types of studies
Studies for this TMI review were included if they were published in English and satisfied all of the following criteria:
Were RCTs that provided unconfounded tests of interventions intended to affect adherence with prescribed, self-administered medication. A confounder is a characteristic that is extraneous to the primary question being addressed in the study, but that can influence the outcome and is unequally distributed between the treatment groups being compared.
Included patients who were prescribed medication for a medical (including psychiatric) disorder, but not for addictions (because these adherence problems are typically much more severe).
Assessed effects on measures of both medication adherence and patient outcomes.
Had at least 80% follow-up at the end of the recommended treatment period for short-term treatments, and at least 80% follow-up over six months for long-term treatments with initially positive results. For long-term regimens, negative trials with <6 months follow-up were included on the grounds that initial failure is unlikely to be followed by success.
- Although the primary review included any intervention, for this review, only interventions with any of the following components were included:
- a. information and communication technology [computers, telephones, videos, cell phones, pagers, e-mails, short messaging services (SMSs), internet]; or
- b. any medical device [electronic drug monitor (EDM) or pillbox with alarm, EDM without alarm that was used to provide feedback, home blood pressure monitors (HBPMs), telehealth devices, standalone devices, custom-made devices].
We excluded studies in which the use of technology in the intervention was ill-defined or used solely to keep the patient’s health care providers updated outside of study purposes and not as part of the intervention.
Study selection
Citations retrieved in the database searches were assessed in a three-stage review process, described in detail elsewhere.5 During all screening stages, two independent reviewers assessed eligibility, and an adjudicator resolved any disagreements.
Data extraction
Extracted data included study methods, participants, interventions, outcomes, additional notes pertaining to any of the aforementioned items, and risk of bias (e.g., allocation concealment). Two reviewers independently extracted all data, and a third extractor resolved disagreements. Primary (or corresponding) authors of included RCTs were contacted to confirm extracted data and provide missing data.
Data analysis
Reviewer agreement on the study risk of biased criteria was quantified by using the unweighted Cohen’s κ.11 All analyses were conducted using SPSS, version 20.0. When reporting results from individual studies, we cited the measures of association and P-values reported in the studies. We interpreted P < .05 as indicating statistical significance. Because we observed wide heterogeneity between the studies in terms of target diseases, interventions, technology, and outcome measures, a meta-analysis was ruled out in favor of a qualitative analysis.
Intervention effects in individual RCTs were reported for all outcomes regarding (1) adherence and (2) clinical outcomes. Adherence and clinical outcomes were considered to be primary outcomes if they (1) were indicated to be the primary outcome by the study author, (2) were used in the study power calculation, or (3) were the first outcome described in the “Results” section of each paper.
We grouped the RCTs identified in our review into three main categories based on the major function of technology in the intervention, and included interventions that employed technology to provide:
Education and/or counseling;
Self-monitoring and/or feedback; and
Electronic reminders.
For studies incorporating two or more functions of the TMI, we assigned categories based on the apparent primary purpose of the intervention. We sought to determine the degree to which adherence and clinical outcomes were determined by the characteristics of the intervention, including the type of technology and mode of delivery; and characteristics of the study, including length of study, target disease, target population, and adherence measure used. Risk of bias in the included studies was assessed by using the Cochrane risk of bias tool.12
RESULTS
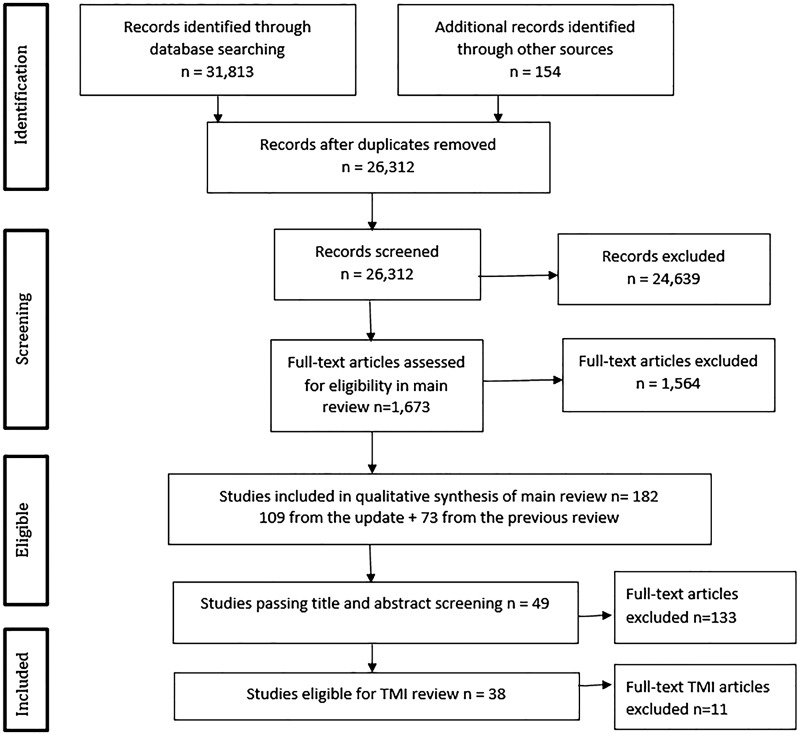
A total of 182 studies were included in the comprehensive updated Cochrane review.5 After screening the title and abstract, 133 studies were excluded because they did not meet the TMI inclusion criteria. Full-text reports of the remaining 49 studies were assessed for eligibility. Details on excluded studies and reason for exclusion are given in Supplementary Appendix A. A total of 38 studies met all inclusion criteria (Figure 1).13–50
Figure 1::
Study inclusion flowchart.
Description of included studies
Tables 1–4 summarize the characteristics of the included studies and Supplementary Appendix B provides a more detailed description of the included studies. The distribution of target diseases (Table 1) varied widely, led by HIV (11 studies, 28.9%)13,14,20,21,23,30,37,40–42,46 and cardiovascular diseases (seven studies, 18.4%).15,24,31,32,38,45,48 Three studies were nonspecific in terms of target disease, focusing on antibiotic prescriptions,16 oral contraceptives,27 and polypharmacy.49 Most studies involved adults, with eight studies (21.1%) including children and/or adolescents aged 13 years or older.13,17–19,27–29,34 All but one24 of the studies was published on or after 2005 (37 studies; 97.4%).
Table 1:
Adherence and clinical outcomes according to study characteristic (n = 38)
| Characteristic | Number of Studies | Adherence Outcome Significance, N (%) | Clinical Outcome Significance, N (%) |
|---|---|---|---|
| Overall Effectiveness of Interventions | 38 | 19 (50) | 15 (39.5) |
| Purpose of Intervention | |||
| Education and/or Counseling | 18 (47) | 7 (39) | 9 (50) |
| Self-Monitoring and/or Feedback | 11 (29) | 9 (82) | 5 (45) |
| Electronic Reminders | 9 (24) | 3 (33) | 1 (11) |
| Technology Type | |||
| Telephone (Primary) | 13 (34) | 5 (38) | 6 (46) |
| Telephone (Secondary) | 12 (32) | 7 (58) | 4 (33) |
| Telephone Total | 25 (66) | 12 (48) | 10 (40) |
| Short Messaging | 5 (13) | 1 (20) | 1 (20) |
| Internet-Dependent Computer Programs | 3 (8) | 2 (67) | 2 (67) |
| Internet-Independent Computer Programs | 3 (8) | 2 (67) | 1 (33) |
| AVRD | 6 (16) | 3 (50) | 1 (17) |
| HBPMa | 4 (11) | 3 (75) | 1 (25) |
| Telehealth Device | 4 (11) | 2 (50) | 1 (25) |
| EDM | 3 (8) | 3 (100) | 2 (67) |
| Mode of Delivery | |||
| Automated | 15 (39) | 5 (33) | 2 (13) |
| Nonautomated | 21 (55) | 12 (57) | 11 (52) |
| Self-Directed | 2 (5) | 1 (50) | 1 (50) |
| Length of Study | |||
| Short-Term (≤6 months) | 20 (53) | 10 (50) | 7 (35) |
| Long-Term (>6 months) | 18 (47) | 9 (50) | 8 (44) |
| Target Disease | |||
| HIV | 11 (29) | 3 (27) | 1 (9) |
| Cardiovascular | 7 (18) | 6 (86) | 3 (43) |
| Diabetes | 4 (11) | 1 (25) | 1 (25) |
| Depression | 4 (11) | 2 (50) | 4 (100) |
| Asthma | 4 (11) | 3 (75) | 1 (25) |
| Glaucoma | 1 (3) | 1 (100) | 0 |
| Osteoporosis | 1 (3) | 0 | 0 |
| Acne | 1 (3) | 0 | 0 |
| Rheumatoid Arthritis | 1 (3) | 1 (100) | 1 (100) |
| Cancer | 1 (3) | 1 (100) | 1 (100) |
| Polypharmacy | 1 (3) | 1 (100) | 1 (100) |
| Oral Contraceptives | 1 (3) | 0 | 0 |
| Antibiotics | 1 (3) | 0 | 0 |
| Target Population | |||
| Children or Adolescents | 8 (21) | 4 (50) | 2 (25) |
| Adherence Measure | |||
| Self-Reportb | 24 (63) | 9 (38) | 6 (25) |
| EDM | 12 (32) | 6 (50) | 2 (17) |
| Prescription Refills | 6 (16) | 2 (33) | 2 (33) |
| Pill Count | 3 (8) | 2 (67) | 1 (33) |
| Drug Metabolite | 1 (3) | 1 (100) | 1 (100) |
| High-Quality Studies | 7 (18) | 2 (29) | 2 (29) |
aTwo studies that used HBPM also used telehealth devices and were categorized in both groups.
bSeventeen studies exclusively used self-report, with seven studies using multiple measures including self-report.
Table 2:
Education and/or counseling (ranked according to type of technology and delivery method) (n = 18)
| Source | Condition | Technology Delivery Method | Intervention Description | Adherence Measures | Clinical Outcome Measures | Main Results |
|---|---|---|---|---|---|---|
| Kato et al.29 | Cancer | Computer Self-directed | Cancer-targeted video game (Re-Mission) in which goal is for players to destroy cancer cells and manage treatment-related adverse effects in a 3D environment within the body of patients with cancer (n = 197) vs. commercial video game (n = 178). | EDM, medication metabolites, self-report | Cancer-related knowledge, self-efficacy, functional status, QOL, health control, stress | At 3 months, significantly greater EDM adherence to antibiotics and greater drug metabolite concentrations. No difference in self-reported adherence. Significantly greater cancer-related knowledge and self-efficacy. |
| Both groups instructed to play for at least 1 h per week over 3 months. | No difference in functional status, QOL, perceived health control, or stress. | |||||
| Fisher et al.23 | HIV | Computer Self-directed | One session of LifeWindows, interactive HAART adherence promotion application, at each visit (n = 277) vs. control group only completed tutorial, virtual guide, and general assessment components of LifeWindows at each visit (n = 287). | Self-report | Viral load | At 18 months, no difference in adherence or viral load. Comparable proportion with undetectable viral load (79% vs. 74%, P = NS). |
| Study underpowered to detect differences in viral load. | ||||||
| Simon et al.39 | Depression | Computer/Internet Nonautomated | Three online messaging contacts from a nurse care manager to assess depression symptoms, treatment use, side effects, and follow-up as needed (n = 106) vs. UC with access to online patient-provider messaging (n = 102). | Prescription refills | Depression score, treatment satisfaction, health care utilization | At 90 days, significantly greater adherence, lower depression scores (ES = 0.29), and greater satisfaction with treatment. No differences in health care use in-person or by telephone. |
| Lester et al.30 | HIV | SMS Automated Clinician | Once weekly message from clinician to inquire about their status, then called if having problems or no reply (n = 273) vs. UC (n = 265). | Self-report | Viral load | At 12 months, significantly greater adherence (RR for nonadherence 0.81, 95% CI, 0.69–0.94). NNT to promote 95% + adherence = 9. Significantly greater viral load suppression (RR for virologic failure 0.84, 95% CI, 0.71–0.99). NNT to achieve viral load suppression = 11. |
| Both groups received in-person counseling sessions and were encouraged to disclose HIV status, find an adherence partner, and attend support groups | ||||||
| Zolfaghari et al.50 | Diabetes | SMS Automated | Six weekly messages on managing diet, exercise, stress, and diabetes (n = 38) vs. 16 20-min nurse-led diabetes counseling calls (n = 39). | Self-report | HbA1c | At 3 months, no difference in adherence to diet, exercise, or medications. No difference in HbA1c change, significant within group decrease in HbA1c. |
| Sherrard et al.38 | Postcardiac Surgery | Telephone (Telehealth) Automated | Eleven calls facilitated by an IVR system after discharge to provide education, track compliance, identify issues, and provide timely intervention by nurse (n = 137) vs. UC received automated calls on postdischarge days 3 and 10 to screen for common symptoms and no information provided (n = 143). | Self-report | Adverse events (ER visits, hospitalizations) | At 6 months, significantly greater adherence (RR 0.34 95% CI, 0.2–0.56). No difference in ER visits or hospitalizations. |
| Significant difference in composite outcome of increased adherence and decreased adverse events (RR 0.60, 95% CI, 0.37–0.96). | ||||||
| Heisler et al.26 | Diabetes | Telephone (Telehealth) Automated | Once weekly peer support calls facilitated by IVR system, with reminder every 7 days if no calls initiated (n = 126) vs. UC group received HbA1c, BP, and cholesterol levels, then attended session on care management and encouraged to contact care manager for follow-up (n = 119). | Self-report | HbA1c, BP, diabetes-related emotional distress, QOL, medication changes | At 6 months, no difference in diabetes medication adherence. |
| Significant difference in HbA1c (−0.29% vs. 0.29%; 0.58% difference, P = .004). | ||||||
| No difference in BP change. | ||||||
| No difference in diabetes-related emotional distress or QOL. | ||||||
| Significantly greater number of diabetic medication changes. | ||||||
| Simoni et al.41 | HIV | Telephone Nonautomated | Six twice-monthly group meetings to discuss shared experiences, barriers, and solutions to adherence, and peers encouraged to call support person three times per week for in-depth discussions and feedback (n = 71) vs. UC (n = 65). | EDM Self-report | Viral load, depressive symptoms | At 6 months, no difference EDM or self-reported adherence. |
| No difference in viral load or depressive symptoms. | ||||||
| Abrahams et al.13 | HIV Postexposure Prophylaxis | Telephone Nonautomated | Nine counselor-led counseling sessions over 28 days (n = 136) vs. UC received information leaflet and adherence diary (n = 138). | Pill count, pamphlet use, diary use | Depressive symptoms | At 28 days, no difference in adherence, pamphlet use, or depressive symptoms. Greater use of diary. |
| Wolever et al.47 | Diabetes | Telephone Nonautomated | Fourteen 30-min calls from health coach to provide education and discuss questions and concerns (n = 30) vs. UC (n = 26). | Self-report | HbA1c, perceived stress, or QOL | At 6 months, no difference in adherence, HbA1c, perceived stress, or QOL. If baseline HbA1c ≥7% (n = 16), significant reduction with intervention over time (−0.64%, ES = 0.34). |
| Gensichen et al.25 | Depression | Telephone Nonautomated | Thirteen calls from health care assistant to monitor depression symptoms and adherence, and to encourage self-management (n = 52) vs. UC (n = 39). | Self-report | Depression symptoms, treatment response, remission rate, QOL | At 12 months, significantly greater adherence, lower depression symptoms, and higher treatment response. No difference in remission rate or QOL. |
| Solomon et al.43 | Osteoporosis | Telephone Nonautomated | Ten calls from health educator using motivational interviewing to provide education, and identify attitudes, barriers, and solutions to medication adherence (n = 1046) vs. UC (n = 1041). | Prescription refills | Self-reported falls, fractures, general health | At 12 months, no difference in adherence, falls, fractures, or “poor” or “fair” general health. |
| Pyne et al.35 | Depression (in HIV patients) | Telephone Nonautomated | Calls every 2 or 4 weeks to monitor depression symptoms, treatment barriers, and substance abuse and treatment, and received counseling on self-management from nurse depression care manager using a web-based decision support system based on a stepped care model for depression (n = 138) vs. UC (n = 138). | Self-report | HIV symptom severity, depression severity, health status, QOL | At 12 months, no difference in adherence to antidepressant or HIV medications, depression severity, health status, or QOL. Significantly lower HIV symptom severity. |
| Howe et al.28 | Diabetes | Telephone Nonautomated | Eighteen calls over 6 months from nurse case manager who followed standardized protocol and education (n = 26) vs. One-time in-person education session (n = 21) vs. UC (n = 28). | Self-report | HbA1c, diabetes knowledge, parent–child teamwork | At 6 months, significant improvement in adherence and parent–child teamwork. No difference in HbA1c change between or within groups, or in diabetes knowledge. |
| Collier et al.21 | HIV | Telephone Nonautomated | Sixteen calls from nurse focusing on medication adherence, side effect management, and social support (n = 142) vs. UC (n = 140). | Self-report | Viral load | At 96 weeks, no difference in adherence or time to virologic failure. |
| Rickles et al.36 | Depression | Telephone Nonautomated | Three once-monthly calls from pharmacist to assess antidepressant knowledge, beliefs, concerns, goals, and adherence (n = 28) vs. UC (n = 32). | Prescription refills | Antidepressant perception, depression symptoms | At 3 months, no difference in adherence or depression symptoms. Significantly greater antidepressant knowledge, positive beliefs, and orientation toward treatment progress and significant within group reduction of depression symptoms. |
| Wu et al.49 | Polypharmacy | Telephone | Ten 15 min calls between usually scheduled visits every 2–4 months to discuss treatment regimens, side effects, follow-up appointments, importance of adherence, and self-care (e.g., diet, exercise, self-monitoring) (n = 219) vs. UC (n = 223). | Self-report | Mortality | At 2 years, for adherent patients at enrollment, more remained compliant. For nonadherent patients at enrollment, fewer remained nonadherent. |
| Screening visit involved counseling with pharmacist, then adherence assessed at enrollment. | Significant reduction in risk of mortality (41%, RR 0.59, 95% CI, 0.35–0.97). NNT = 16 to prevent one death at 2 years. Other predictors of mortality: old age, living alone, hospital admission rate, baseline adherence, number of drugs for chronic disease, and treatment with lipid lowering drugs at screening visit. | |||||
| Beaucage et al.16 | Antibiotic Prescriptions | Telephone Nonautomated | One call from pharmacist on day 3 of antibiotic treatment to discuss patient’s condition, treatment, and adherence (n = 126) vs. UC (n = 129). | Self-report | Number of infectious symptoms, infection severity | At maximum 15 days from start of treatment, no difference in adherence, number of infectious symptoms, or infection severity. |
95% CI, 95% confidence interval; BG, blood glucose; ER, emergency room; ES, effect size, reported as Cohen’s d; HAART, highly active antiretroviral therapy; mean ± standard error of mean (SEM); MEMS, medication event monitoring system; NNT, number needed to treat; NS, not significant.
Table 3:
Self-monitoring and/or feedback (ranked according to type of technology and delivery method) (n = 11)
| Source | Condition | Technology Delivery Method | Intervention Description | Adherence Measure | Clinical Outcome Measure | Main Results |
|---|---|---|---|---|---|---|
| El Miedany et al.22 | Rheumatoid Arthritis | Computer Nonautomated | Given visual feedback on computer of disease progression, discussed changes, comorbidity risks, functional disability, and QOL with MD or nurse (n = 55) vs. UC (n = 56). | Self-report | Pain score, disease activity score | At 6 months, significantly greater adherence to medication, greater improvement in pain score, and greater improvement in disease activity score. |
| Chan et al.18 | Asthma | Computer/Internet Nonautomated | Given virtual access to asthma education, transmit daily asthma symptom diary, peak flow meter, and inhaler technique videos, three in-person visits (n = 60) vs. six office-based visits (n = 60). | Asthma diary, controller refill | FEV1, PEF, rescue therapy use, health care use, asthma-related QOL | At 12 months, significantly greater asthma diary adherence. No difference in controller prescription refills, FEV1, PEF, rescue therapy use, utilization of health services, or QOL. |
| All patients had email and telephone access to case manager for feedback. | ||||||
| Van der Meer et al.44 | Asthma | Computer/Internet Nonautomated | Given internet-based treatment plan, online education, and web or telephone communication with asthma nurse. Given feedback based on ACQ, including treatment adjustment according to a predefined algorithm (n = 101) vs. daily internet-based symptom diary only (n = 99). | Self-report | FEV1, ACQ, QOL | At 12 months, no difference in adherence to daily-inhaled corticosteroid use, FEV1, ACQ, or QOL. |
| Friedman et al.24 | Hypertension | Telephone-Linked Computer (Telehealth) Automated HBPM | Once weekly calls to telephone-linked computer to submit BP reading, medication regimen, adherence, side effects, and received feedback (n = 133) vs. UC (n = 134). | Pill count | BP, health status | At 6 months, significantly greater change in adherence, no difference in decrease in SBP. Poorly adherent (<80% adherent at baseline; n = 26) patients had significant improvement in adherence and decrease in DBP, but no difference in decrease in SBP. |
| Wakefield et al.45 | Hypertension, Diabetes | Telephone-Connected Telehealth Device (Telehealth) Automated HBPM | All intervention patients transmitted daily BP, BG. High-intensity patients responded to standardized questions and received messages, calls, or mailed feedback from nurse care manager based on disease management algorithm (n = 92) vs. low-intensity patients responded to subset of standardized questions and not managed according to algorithm (n = 102) vs. UC (n = 107). | Self-report | HbA1c, SBP | At 12 months, no difference in adherence or HbA1c change. |
| Significant SBP reduction in high-intensity vs. UC (−4.92 vs. 3.34; P = .006), but no difference vs. low-intensity (−4.92 vs. 0.76; P = .08). | ||||||
| Marquez Contreras et al.31 | Hypertension | Telephone Nonautomated | Three telephone calls from a nurse to discuss treatment and feedback on adherence (n = 184) vs. three mailings providing education, reinforcing compliance, and reminding of clinical visits (n = 172) vs. UC (n = 182). | Pill count | BP | At 6 months, telephone and mail groups had significantly greater adherence vs. UC, and within each group, there was a significant reduction in BP compared to baseline. Telephone group, but not mail group, had significant reduction in SBP and DBP vs. UC. |
| Marquez-Contreras et al.32 | Hypertension | HBPM Nonautomated | Given HBPM, manual, summary of functions, and card on which to record BP 3 days/week (n = 100) vs. UC (n = 100) | EDM | BP | At 24 weeks, significantly greater adherence. No difference in SBP or DBP. Significantly greater decrease in DBP, but not SBP. Significant BP reduction within each group. |
| Antonicelli et al.15 | Heart Failure | Telephone Nonautomated EKG Transmission HBPM | Once weekly call from team member to obtain symptoms, adherence, previous 24-h BP, heart rate, weight, urine output, and EKG transmission. Therapeutic modifications made and follow-up arranged accordingly (n = 28) vs. monthly calls for data collection and three clinic visits (n = 29). | Self-report | Mortality or hospital admission | At 12 months, significantly greater adherence and significantly lower incidence of mortality or hospital admission (0.043 vs. 0.107 events/patient/month; P = .006). |
| Significant reduction in SBP and heart rate within each group compared to baseline. | ||||||
| Otsuki et al.34 | Asthma | EDM Nonautomated | AMF group received objective feedback on EDM measured adherence, goal-setting, reinforcement of goals, self-monitoring strategies during five home visits from an asthma educator (n = 83) vs. ABC group received five home visits from an asthma educator (n = 84) vs. UC (n = 83). | Self-report, controller refill | Asthma morbidity measures | At 12 months, no difference across all groups in adherence, but significant within group increase in controller refills and lower ED visit rate. No difference in AMF vs. ABC across all outcomes. AMF + ABC vs. UC had faster increase in controller refills (IRR 0.93, 95% CI, 0.86–1.00; P = .05), faster decrease in ED visits (IRR 0.88, 95% CI, 0.78–0.99) and less steroid use (IRR 0.83, 95% CI, 0.73–0.95). No differences in asthma symptom frequency or hospitalization. |
| AMF vs. UC had significantly greater controller refills (IRR 1.52, 95% CI, 1.05–2.19) and greater reduction in ED visits (IRR 0.85, 95% CI, 0.74–0.97). No difference in self-reported adherence, asthma symptoms, steroid use, or hospitalizations. | ||||||
| Sabin et al.37 | HIV | EDM Nonautomated | Patients <95% adherent were provided with summarized EDM data that was discussed with an MD or nurse (n = 34) vs. UC, had EDM data collected, adherence counseling only if <95% self-reported adherence (n = 34). | EDM | Viral load, CD4 count | At 12 months, significantly greater adherence. No difference in viral load suppression or CD4 count. |
| Wu et al.48 | Heart Failure | EDM Nonautomated Nurse | Plus group received feedback on adherence + Lite (n = 27) vs. Lite group received two in-person and two calls from nurse to provide education and counseling based on theory of planned behavior (n = 27) vs. UC (n = 28). | EDM | Cardiac event-free survival, QOL | At 9 months, Plus and Lite each had significantly greater adherence vs. UC, but no difference in adherence between Plus and Lite. Plus + Lite vs. UC had significantly longer cardiac event-free survival [HR 4.22 (Wald 7.677), adjusted for sociodemographic and clinical factors]. |
| No difference between all groups in QOL. |
ACQ, asthma control questionnaire; DBP, diastolic blood pressure; FEV1, forced expiratory volume; HR, hazard ratio; mean ± SEM; MD, physician; NS, not significant; PEF, peak expiratory flow; QOL, quality of life; r2, regression coefficient; RR, risk ratio; SBP, systolic blood pressure; SMS, short message service; UC, usual care
Table 4:
Electronic reminders (ranked according to type of technology and delivery method) (n = 9)
| Source | Condition | Technology Delivery Method | Intervention Description | Adherence Measure | Clinical Outcome Measure | Main Results |
|---|---|---|---|---|---|---|
| Hou et al.27 | Birth Control Pill | SMS Automated | Once daily reminder to take pill at time specified by patient. No response required (n = 41) vs. UC (n = 41). | Self-report, EDM | Pregnancy | At 3 months, no difference in adherence. No pregnancies reported in study, despite missed pills across both groups. |
| Boker et al.17 | Acne | SMS Automated | Twice daily customized reminder. Response required (n = 19) vs. UC (n = 21). | EDM | Acne severity, QOL | At 12 weeks, no difference in adherence, objective, or self-reported acne severity, or QOL. |
| Simoni et al.42 | HIV | Two-way Pager System Automated | Received two-way pager system providing minimum 3/day customized reminders, educational material, adherence assessments, and entertainment. Response page required (n = 56) and pager + peer support via 6 × 2/month educational meetings and weekly calls from peers (n = 56) vs. UC (n = 57). | Self-report, EDM | Viral load, CD4 count | At 9 months, messaging and messaging + peer support vs. UC had no difference in adherence, viral load, or CD4 count. |
| Andrade et al.14 | HIV | AVRD Automated | Daily, timed voice messages with blinking light reminders to take HAART. Response required on device push-button plus adherence counseling (n = 32) vs. once monthly 30-min adherence counseling session by pharmacist (n = 32). | EDM | Viral load, CD4 count | At 24 weeks, no difference in adherence, undetectable viral load, or CD4 count. Significantly greater reduction in viral load. Among patients with memory impairment (14 reminder and 17 counseling-only), significantly higher adherence in reminder group. No difference in adherence in memory-intact patients. |
| Wang et al.46 | HIV | AVRD Automated | Received pillbox with audiovisual alarm and nurse delivered home visits every 2 months and calls every 2 weeks (n = 58) vs. UC (n = 58). | Self-report | QOL, symptoms of depression | At 8 months, significantly greater 100% adherence and taking all pills on time. Significantly greater QOL and lower depression symptoms. |
| Chung et al.20 | HIV | AVRD Automated | Received digital pocket alarm that beeped and flashed twice daily for 6 months after HAART initiation, could not be reprogrammed or disabled (n = 100), and alarm and counseling (n = 100) vs. received three counseling sessions at HAART initiation (n = 100) and UC (n = 100). | Prescription refill | Viral failure, CD4 count, mortality | At 18 months, no difference in adherence, time to viral failure, CD4 count, or mortality. |
| Simoni et al.40 | HIV | AVRD Automated | Received alarm device only (n = 8) or alarm + three nurse-led counseling sessions (in-person or via telephone) (n = 18) vs. UC (n = 34). | Self-report, EDM | Viral load, CD4 count | At 25 weeks, no difference in adherence, viral load, or CD4 count. |
| Charles et al.19 | Asthma | AVRD Automated | Received smart inhaler with twice-daily alarm that beeped and light that turned from green to red once dose was taken (n = 55) vs. Smart inhaler only. No audiovisual alarm (n = 55). | EDM | ACQ, PEF | At 24 weeks, significantly increased adherence. No difference in ACQ or PEF. |
| Okeke et al.33 | Glaucoma | AVRD Automated | Received dosing aid with once-daily audiovisual alarm, educational video, review of current barriers to drop-taking, and reminder calls (n = 35) vs. UC (n = 31). | EDM | Intraocular pressure | At 3 months, significantly increased adherence. No difference in intraocular pressure. |
PEF, Peak expiratory flow
The most common primary information and communication technology used to deliver the intervention was the telephone (13 studies, 34.2%).13,15,16,21,25,28,31,35,36,41,47,49 In 12 studies, the telephone was used secondarily as a cointervention with SMS,30,42 telehealth devices,24,26,38,45 internet-dependent computer programs,18,44 EDM,48 and electronic audiovisual reminder devices (AVRDs).33,40,46 Five studies (13.2%) used short messaging through pagers or cellphones,17,27,30,42,50 three studies (7.9%) used internet-independent computer programs,22,23,29 and three studies (7.9%) used internet-dependent computer programs.18,39,44 The most common medical device used was an AVRD (e.g., pillbox with light that flashes and alarm that sounds at specified times to take medications; six studies, 15.8%).14,19,20,33,40,46 Four studies (10.5%) used an HBPM.15,24,32,45 In two of these studies, participants were required to transmit their blood pressure (BP) measurements using a telehealth device;24,45 the other studies used telephone15 or in-person32 sharing of BP measurements. Two studies used an interactive voice response (IVR)26,38 system, which we classified as a telehealth device; thus, four studies (10.5%) used telehealth devices.24,26,38,45 Three studies (7.9%) used EDMs.34,37,48 In terms of the mode of delivery of the intervention, in two studies (5.3%), the intervention was self-directed, in that participants interacted with a computer software program,23,29 and in 15 studies (39.5%), the intervention was automated,14,17,19,20,24,26,27,30,33,38,40,42,45,46,50 with a majority of these studies using electronic reminders (Table 3). The interventions in the remaining 21 studies (55.3%) were nonautomated and delivered by individuals often mediated by technology (e.g., nonautomated telephone calls or in-person feedback on EDM data).
Most studies (30 studies, 78.9%) used just one measure of adherence. The most common measure was self-report (24 studies, 63.2%), which was used exclusively in 17 studies (44.7%).15,16,21–23,25,26,28,30,35,38,44–47,49,50 Twelve studies (31.6%) used electronic monitoring of medication intake,14,17,19,27,29,32,33,37,40–42,48 with four of these studies also using self-report.27,40–42 Adherence was measured by using prescription refills in six studies (15.8%),18,20,34,36,39,43 with two of these studies of asthma additionally using self-report.18,34 Pill count was used exclusively in three studies (7.9%).13,24,31 One study of cancer used self-report, electronic monitoring, and measured chemotherapy metabolites.29 A majority of studies (26 studies, 68.4%) used multiple individual measures for clinical outcomes.
The most commonly used measures of clinical outcome were self-reported (22 studies, 57.9%). For example, self-reported outcome measures included quality of life (QOL),17,18,25,26,29,35,44,46–48 health status,14,24,29,35,43 symptom or disease severity,13,16,17,19,22,25,35,36,39,41,44,46 disease-related knowledge,28,29 stress,26,29,47 and self-efficacy.29 Twenty-seven studies (71.1%) used objective outcome measures grouped according to the target disease under study. From the 11 studies of individuals with HIV, five studies used both viral load and CD4 cell count,14,20,37,40,42 and four studies used viral load only21,23,30,41 as outcome measures, with the remaining two studies using self-reported quality of life46 and symptoms of depression.13,46 All four studies of hypertension24,31,32,45 used blood pressure as a clinical outcome measure, with one study including patients with diabetes45 also measuring changes in glycosylated hemoglobin. Two studies of congestive heart failure,15,48 one study of patients with polypharmacy,49 and one study of HIV20 used mortality as an outcome measure. All four studies of diabetes26,28,47,50 used glycosylated hemoglobin as an outcome measure, with one study also using blood pressure.26 All four studies of asthma18,19,34,44 used objective measures of pulmonary function as outcomes. One study targeting glaucoma33 measured intraocular pressure as a clinical outcome. Health care utilization (e.g., unscheduled clinic visits, emergency department visits, or hospitalizations) was used in seven studies, including target diseases of postcardiovascular surgery,38 asthma,18,34,44 osteoporosis,43 heart failure,15 and depression.39
Risk of bias
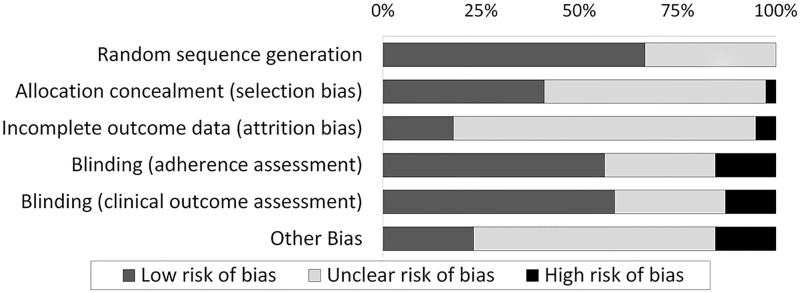
We assessed the methodological quality of all 38 included studies by using the Cochrane risk of bias tool (Figure 2).12 Seven studies (18.4%)16,20,29,41–43,49 were classified as high-quality studies because they had low risk of bias for study design (i.e., low risk of bias for random sequence generation and allocation concealment) and for primary adherence and clinical outcome assessment. The remaining studies were of lower quality (Supplementary Appendix C).
Figure 2::
Risk of bias graph: review of authors’ judgments about each risk of bias item, presented as percentages across all included studies.
Effectiveness of interventions
Overall, 50% (19/38) of studies found improvements in at least one measure of adherence and 39.4% (15/38) of studies found improvements in at least one clinical outcome measure, with 36.8% (14/38) of studies finding improvements in both adherence and clinical outcome. Table 1 summarizes the effectiveness of interventions based on characteristics of the intervention, including the purpose of the intervention, technology used and mode of delivery, and characteristics of the study, including length of study, target disease, target population, adherence measure used, and study quality. Supplementary Appendix D provides the corresponding study references for those outlined in Table 1.
Education and/or counseling
Eighteen studies (47.4%) examined education and/or counseling interventions (Table 2).13,16,21,23,25,26,28–30,35,36,38,39,41,43,47,49,50 Educational interventions typically involved the provision of information, either audiovisual or verbal, about the target disease, symptoms, comorbid diseases and conditions, rationale for treatment, treatment benefits, side effects, and benefits of adherence. In addition to education, most interventions assessed target disease symptoms, knowledge, attitudes, and barriers to treatment, and counseled on overall goal setting and adherence promoting activities. Additional counseling was provided on the importance of self-care (e.g., diet, exercise, sleep, and self-monitoring), peer and social support, and follow-up. All studies examined repeated exposure to education and/or counseling (e.g., none of the studies examined the impact of a single education and/or counseling session).
In two of 18 studies, the intervention was self-directed and participants received the intervention by interacting with an internet-independent computer program.23,29 In another study, participants received structured online messaging regarding depression via an internet-dependent computer.39 Four studies delivered automated interventions, two using SMS30,50 and two using IVR systems.26,38 The remaining 11 studies used nonautomated telephone calls to provide education and/or counseling. The education and/or counseling was provided by a variety of individuals, including peers,26,41 nurses,21,28,35,39,50 clinicians,30 counselors,13 health coaches,47 health educators,43 health care assistants,25 and pharmacists.16,36,49 Seven of these 18 studies reported significant improvements in adherence, with all of these studies reporting improvement in at least one clinical outcome measure.25,28–30,38,39,49 An additional two studies reported an improvement in at least one clinical outcome, with no improvement in adherence.26,35 A subjective (e.g., self-reported) outcome measure was used in six of nine studies with improvement in at least one clinical outcome measure, and in most of these studies, not all clinical outcome measures were significantly improved.25,28,29,38,35,39 Three studies showed improvement in an objective primary outcome measure.26,30,49 Heisler et al.26 reported a significant glycosylated hemoglobin (HbA1c) decrease in patients with diabetes who received peer support facilitated by an IVR system, compared to patients who did not receive such support. Interestingly, self-reported adherence was not significantly different between groups. Lester et al.30 reported significantly improved self-reported adherence and greater viral load suppression in Kenyan patients with HIV who received a weekly text message in Swahili that read “Mambo?,” which translates to “How are you?” If patients responded with having problems or did not reply in 48 h, study personnel contacted them. Wu et al.49 found that brief telephone calls from pharmacists in patients with polypharmacy significantly improved adherence and decreased mortality. Of the seven high-quality studies, five studies16,29,41,43,49 used education and/or counseling interventions, with only two studies finding significant effects: Wu et al.49 (described above) and Kato et al.,29 who found that a cancer-targeted video game intervention improved knowledge, self-efficacy, and EDM-measured antibiotic and drug metabolite-measured adherence. The remaining three high-quality studies all provided education and/or counseling using nonautomated telephone calls and found no differences in adherence or clinical outcomes.16,41,43
Self-monitoring and/or feedback
Eleven studies (28.9%) assessed technology-mediated self-monitoring and/or feedback interventions (Table 3).15,18,22,24,31,32,34,37,44,45,48 All of these studies were of poor methodological quality, with only three studies21,22,35 having low risk of bias for study design (i.e., low risk of bias for random sequence generation and allocation concealment), but high risk of bias for blinding of adherence and clinical outcome assessment (Supplementary Appendix C). The self-monitoring behaviors in six of 11 studies involved patients keeping track of asthma symptoms by using a diary18,44 or measuring blood pressure.15,24,32,45 In all but one32 of these studies, patients were provided with feedback on their self-monitoring. In five of 11 studies, patients received feedback on either disease progression22 or medication adherence, measured by using self-report interviews31 or EDMs.34,37,48 Two studies used automated telehealth devices and HBPM in patients with hypertension.24,45 The remaining nine studies used nonautomated technologies, including internet-independent computer programs,22 internet-dependent computer programs,18,44 telephone,31 HBPMs,15,32 and EDMs.34,37,48 The intervention was delivered by a case manager,18 physician or nurse,22,31,37,44,45,48 health educator,34 or member of a specialized team.15
Nine of 11 studies reported increased adherence according to at least one measure, with three studies using self-report15,18,22 and the other studies using objective measures of adherence, including prescription refill rate,34 pill count,24,31 and EDM data.32,37,48 Of the nine studies reporting increased adherence, five studies found significant improvements in at least one clinical outcome measure. El Miedany et al.22 provided patients with visual feedback of their rheumatic disease progression on a computer, and found increased self-reported adherence and lower disease activity and pain scores. Marquez Contreras et al.31 found that three nurse-initiated telephone calls over six months to discuss treatment regimen and adherence improved adherence measured by pill count and resulted in a significantly lower mean reduction in systolic blood pressure compared to usual care, but not compared to a group receiving three mailed communications for patients with hypertension. Otsuki et al.34 provided asthma patients with five asthma-related home visits and objective feedback on medication adherence measured by EDM, and found a significant improvement in controller refill rate and in all asthma morbidity outcomes compared to usual care, but not compared to patients who only received the home visits. Two studies of patients with heart failure found significantly greater adherence, measured by self-report15 or EDM,48 and lower incidence of hospitalization and mortality.
Electronic reminders
Nine studies utilized electronic reminders as the intervention, alone17,19,27 or in combination with another intervention (Table 4).14,20,33,40,42,46 Two studies had low risk of bias.20,42 The electronic reminders in all studies were automated. Three studies provided automated short messages to personal cellular phones17,27 or to a two-way pager device provided to patients.42 Six studies implemented AVRDs.14,19,20,33,40,46 Across all studies, reminders were sent once daily in two studies,27,33 twice daily in three studies,17,19,20 and three times daily in one study;42 in three studies of patients with HIV,14,40,46 the frequency of reminders was unspecified, but can be assumed to be multiple times daily because they targeted multiple antiretroviral medications. Three of nine studies revealed a significant improvement in adherence,19,33,46 and only one of nine studies found a significant improvement in at least one clinical outcome measure.46 Wang et al.46 provided HIV patients with an electronic pillbox with an audiovisual alarm in addition to nurse-delivered home visits and telephone calls. They found a significantly greater proportion of patients reporting 100% adherence, a greater number of patients taking pills on time, greater QOL, and lower symptoms of depression in intervention patients compared to usual care. In the other two studies reporting a significant improvement in adherence, patients received a multifunction device that was not only an AVRD, but also an EDM, which was physically attached to the mediation dispenser: an asthma inhaler19 or topical ophthalmic solution bottle.33 Andrade et al.14 found that an AVRD that provided daily, timed programmed voice message reminders with a blinking light and required a response for patients with HIV on antiretrovirals did not increase EDM measured adherence. Although they did not find a difference in undetectable viral load or CD4 count between groups, they did find a significantly greater reduction in viral load (secondary outcome) in the AVRD groups compared to counseling alone. In a subgroup analysis of patients with memory impairment, they found a significantly higher mean adherence in the AVRD group.14
Both of the high-quality studies (low risk of bias) used electronic reminders for patients with HIV.20,42 Chung et al.20 did not find a significant improvement in adherence, viral failure, CD4 count, or mortality by providing patients with a digital pocket alarm that beeped and flashed twice daily, compared to usual care or counseling only. Simoni et al.42 used a two-way pager messaging system that provided dosing reminders, education, adherence assessments, and entertainment, along with peer support, but did not find differences in adherence, viral load, or CD4 count.
DISCUSSION
Our primary objective was to conduct an in-depth analysis of RCTs that examined the effects of TMI on both medication adherence and clinical outcomes. Methods were relatively weak in many studies, with only seven studies having low risk for bias.16,20,29,41–43,49 Only half of the included studies used an objective method to measure adherence (e.g., EDM, prescription refills, pill count, or serum medication metabolite concentration), with the remainder using self-report. Self-reported measures are less reliable and tend to overestimate adherence, resulting in high baseline and potentially final adherence, making it difficult to detect the impact of intervention. This was the case in three studies that found no difference in adherence, but found an improvement in clinical outcomes, although two studies additionally used self-reported clinical outcome measures.16,26,27 On the other hand, in most studies, patients were not blinded to treatment allocation, potentially leading to high self-reported adherence, resulting in a treatment effect where there was actually none. Future studies should incorporate objective measures of medication adherence when possible.
Results of this review revealed a diverse range of interventions, with varied adherence and clinical outcomes. In most instances, technology was buried within complex interventions, and therefore, isolating the sole effect of technology was not possible.
Overall, approximately half of studies found improvement in medication adherence, with over one-third of all studies finding improvement in clinical outcomes. This theme of greater significance in adherence compared to clinical outcomes was a consistent trend across all functions of technology, categories of technologies, modes of delivery of intervention, target diseases, and adherence measures, but again may simply reflect the use of self-report in unblinded trials.
Given the relatively low success rate in terms of a positive impact on both adherence and clinical outcomes, and a lack of common intervention characteristics, it appears as though no consistent evidence exists indicating that the use of a single technology to deliver all or part of intervention can lead to increased adherence and positive clinical outcomes. Certainly, there were no studies showing substantive improvements in patient-important outcomes.
Despite the low number of high-quality studies, the future of TMIs for medication adherence remains promising because the application of rigorous health research methodologies to test the effectiveness of TMIs is a relatively recent development, as witnessed by all but one of the included studies being published on or after 2005.
Educating patients about their disease, reinforcing the importance of adhering to prescribed treatment, and the provision of psychosocial support are considered cornerstones of approaches toward increasing medication adherence across disease spectrums. Not surprisingly, the largest subset of studies we identified sought to address this “knowledge-motivation” deficit. Although grouped together based on the primary purpose of the intervention, these studies were diverse in terms of the technology used, mode of delivery, target disease, and adherence measures. An interesting feature of this group of studies was the wide spectrum of technology and that the predominant technology in use was the telephone. Although all adherence interventions should probably incorporate an educational component, the effect of education alone, whether delivered with or without a TMI, should be questioned.
The next most common purpose of technology in the intervention was as an instrument of active or passive self-monitoring, and/or as a medium for communicating feedback on adherence. It is postulated that feedback interventions work on the basis of theory of planned behavior, which addresses an individual’s intention to engage in a behavior at a specific time and place in terms of motivation (intention) and behavioral control. Achieving long-term medication adherence can be difficult in the absence of perceived symptoms that explicitly provide cues to the patient regarding missed medication, which is common in many chronic conditions (e.g., hypertension, depression). Technology can be used to provide external feedback to help patients achieve positive adherence behavior. Interestingly, a majority of the studies identified in this category found an improvement in adherence, with just under half additionally finding an improvement in clinical outcomes. This may be considered the most successful category of interventions; however, there remained a lack of methodological and operational homogeneity among these studies. All of the “successful” studies from this category were nonautomated, with a component of the intervention relying on person-to-person communication. In some cases, the contents of this communication were explicitly stated, but often, it was broadly stated as “counseling,” “feedback,” or “discussion” and likely involved some form of education and/or counseling. The content and circumstances around these conversations and their impact on subsequent adherence is immeasurable and not reproducible, making it difficult to determine the true contribution of technology to the impact of the intervention.
A commonly stated reason for nonadherence is “forgetting.” Whereas nine studies targeted this issue by providing electronic reminders, only three found any impact on adherence outcomes and only one study found an improvement in clinical outcomes. This is in contrast with a previous study, which found that SMS reminders, more than AVRDs, are effective at increasing medication adherence.8 However, this study did not examine clinical outcomes. Similar to these authors,8 most of the studies using electronic reminders in our study were <6 months in duration. Future studies should examine electronic reminders for longer durations. Some lessons can be learned from the general failure of reminder interventions to improve either outcome. It is likely that forgetfulness is only one of many facets of the complex multifactorial problem of medication nonadherence, and addressing the issue as a one-dimensional problem is ineffective. From the three studies in this group that reported improvement in adherence, two studies also provided telephone calls to participants, which involves going beyond simplistic automatic reminding by means of a novel medication administration device. Further, the addition of visual cues might have acted as a persistent reinforcement to the audible reminders. Perhaps the visual dimension might have functioned as direct feedback, potentially adding one more mechanism of action for these interventions. It is also plausible that individualized reminder messages from a known member of the care team might be more efficacious than mass standard reminder messages, even though the latter might be easier and less resource-intensive to execute. Further research examining the influences of customized messages, source, content, and frequency of delivery is critical.
From a technology perspective, mobile telephone penetration is very high in both developed and developing countries, making direct-to-patient SMS interventions very appealing. Aside from reminders, SMS was used as an educational intervention in two studies,30,50 with the study by Lester et al.30 finding improvements in both adherence and clinical outcomes through a once-weekly SMS that asked patients with HIV how they were doing. There are now several reviews examining the impact of SMS on adherence in specific target conditions,51,52 attendance at healthcare appointments,53 self-management of long-term illnesses,54 communication of results of medical investigations,55 and preventative health care.9 Overall, results indicate that SMS has the potential to improve many facets of healthcare, but the field is young and in need of high-quality, longitudinal studies.
Another feature of successful interventions was that they were more likely to be nonautomated, and thus required person-to-person communication. In fact, of the six automated studies that found an improvement in adherence, the three studies that also found improvement in clinical outcomes had a nonautomated component involving communication either in-person or via telephone. In these cases, automated interventions often served as a screening tool to identify patients likely to be nonadherent and may need further assessment and support. This was the case in studies by Lester et al.30 and Sherrard et al.38
Challenges of TMI
Our review was able to identify certain challenges of using technology in adherence interventions. In general, evaluations of TMIs carry all the drawbacks of trials of conventional adherence interventions, including reporting bias and difficulty in blinding patients in addition to those delivering the intervention. Technology overlap can dilute intervention fidelity. Another concern with using commonplace technology (e.g., telephone) is that patients may use the intervention outside of the framed protocol. Heisler et al.26 found that patients who were assigned to a reciprocal peer support intervention reported being in contact with their peers outside of the custom-built interactive system, which makes it difficult to determine the impact of only the study communication. Whereas some TMIs are associated with lower costs due to their wide market penetration (e.g., telephone, mobile phones, SMS, computers, internet), novel TMIs may require custom built software, websites, video games, or mobile applications, which can increase costs. Additionally, TMIs face many obstacles owing to the complexities of the health care system including privacy, security, cost, and provider reimbursement.56
Limitations
The quality of the included studies varied greatly; only seven studies were identified as having low risk of bias in study design and outcome assessment,16,20,29,41–43,49 with only two studies finding significant improvements in adherence and clinical outcomes.29,49 Thus, a majority of studies were of low methodological quality. Our electronic database search strategy identified 11 studies using a TMI, which were excluded from detailed review for various reasons (Supplementary Appendix A). The most common reason for exclusion was that studies only reported on the overall effect of a complex intervention, and not on the independent contribution of the technology-mediated component and its impact on adherence or clinical outcomes. Because these studies did not contribute to the review, we may be missing important results. It is possible that despite extensive searching, we may have missed certain trials that met all of our criteria because the literature on patient adherence is not well indexed. This is because the number of studies is quite small and scattered across traditional disease boundaries. Furthermore, technological components are often buried deep within interventions, making them difficult to identify in the literature.
Directions for future research
It was difficult to accurately assess the contributions made by various components of the included interventions, a problem that was magnified when multiple technologies were involved in a single intervention (e.g., SMS and telephone) or when a single intervention had multiple technology components (e.g., AVRD that has an audio alarm and visual cue). Future research should assess complex interventions involving technology through the modification and application of known frameworks for the development and evaluation of complex interventions.57
In addition, future studies should target high-risk (unintentionally nonadherent) patients and aim to identify which TMI would be most beneficial for which patients. For example, the current pediatric population can be considered digital natives;58 as such, novel TMIs (e.g., videogame-based interventions, smart-watch interventions, wearable technology) may appeal to their digitally immersive lifestyles, with the potential to be applied across wide spectra of diseases. Future research should also address the specific psychological mechanisms by which TMIs affect health behaviors, and identify the scope of behavioral processes that are particularly suitable for change by means of technology. Several operational questions remain unanswered. For example, are TMIs delivered by family or peers, as opposed to clinicians, more effective? Does feedback have a persistent effect after discontinuation, or must it be continued indefinitely? Finally, in addition to RCTs, future reviews of TMIs could include quasi-experimental study designs (e.g., interrupted time series) because they are strong alternatives to RCTs, pragmatic, and useful for the investigation of mediating factors and secular trends.59,60
E-health and the use of technology to improve medication adherence are in their infancy. As technology evolves, we will likely see the extinction of older technologies and adoption of new technologies and novel interventions to attain improvements in medication adherence.
CONCLUSIONS
This review shows the limited effectiveness of TMI for improving patient adherence and ultimately influencing clinical outcomes, primarily due to a lack of high-quality studies. The methodology for testing TMI for this purpose is generally suboptimal at present; strong, currently available methods need to be applied. Technology will also need to improve if clinically important effects are to be realized.
AUTHOR CONTRIBUTIONS
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Mistry, Keepanasseril, Wilczynski, Nieuwlaat, Haynes.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Mistry, Keepanasseril.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Mistry, Keepanasseril.
Administrative, technical, or material support: Mistry, Keepanasseril, Wilczynski.
Study supervision: Haynes.
COMPETING INTERESTS
None.
FUNDING
KRS 262115, Knowledge Synthesis Grant, Canadian Institutes of Health Research, Government of Canada.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ADDITIONAL CONTRIBUTIONS
Patient Adherence Review Team contributed to data acquisition.
Supplementary Material
Acknowledgments
We thank Dr Ann McKibbon for critically reviewing an earlier version of this manuscript.
REFERENCES
- 1. Cutrona SL, Choudhry NK, Fischer MA, et al. Modes of delivery for interventions to improve cardiovascular medication adherence. Am J Manag Care. 2010;16(12):929–942. [PMC free article] [PubMed] [Google Scholar]
- 2. Sackett DL. Methods for compliance research. In: Hayes RB, Taylor DW, Sackett DL, eds. Compliance in Health Care. Baltimore, MD; Johns Hopkins University Press; 1979:323–333. [Google Scholar]
- 3. Naderi SH, Bestwick JP, Wald DS. Adherence to drugs that prevent cardiovascular disease: meta-analysis on 376,162 patients. Am J Med. 2012;125(9):882–887. [DOI] [PubMed] [Google Scholar]
- 4. Gialamas A, Yelland LN, Ryan P, et al. Does point-of-care testing lead to the same or better adherence to medication? A randomised controlled trial: the PoCT in General Practice Trial. Med J Australia. 2009;191(9):487–491. [DOI] [PubMed] [Google Scholar]
- 5. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane DB Syst Rev. 2014, Issue 2 Art. No.: CD000011. doi:10.1002/14651858.CD000011.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeVito Dabbs A, Song MK, Hawkins R, et al. An intervention fidelity framework for technology-based behavioral interventions. Nurs Res. 2011;60(5):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrer L, Gulliver A, Chan JK, et al. Technology-based interventions for mental health in tertiary students: systematic review. J Med Internet Res. 2013;15(5):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vervloet M, Linn AJ, van Weert JC, et al. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. Am Med Inform Assoc. 2012;19(5):696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vodopivec-Jamsek V, de Jongh T, Gurol-Urganci I, et al. Mobile phone messaging for preventive health care. Cochrane DB Syst Rev. 2012, Issue 12 Art. No.: CD007457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Linn AJ, Vervloet M, van Dijk L, et al. Effects of eHealth interventions on medication adherence: a systematic review of the literature. J Med Internet Res. 2011;13(4):e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleiss J. Statistical Methods for Rates and Proportions. 2nd ed. New York, NY: Wiley-Interscience; 1981. [Google Scholar]
- 12. Higgins JPT, Altman DG, Sterne JAC, eds. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane-handbook.org. Accessed June 16, 2013.
- 13. Abrahams N, Jewkes R, Lombard C, et al. Impact of telephonic psycho-social support on adherence to post-exposure prophylaxis (PEP) after rape. AIDS Care. 2010;22(10):1173–1181. [DOI] [PubMed] [Google Scholar]
- 14. Andrade ASA, McGruder HF, Wu AW, et al. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clin Infect Dis. 2005;41(6):875–882. [DOI] [PubMed] [Google Scholar]
- 15. Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14(6):300–305. [DOI] [PubMed] [Google Scholar]
- 16. Beaucage K, Lachance-Demers H, Ngo TT, et al. Telephone follow-up of patients receiving antibiotic prescriptions from community pharmacies. Am J Health-Syst Pharm. 2006;63(6):557–563. [DOI] [PubMed] [Google Scholar]
- 17. Boker A, Feetham HJ, Armstrong A, et al. Do automated text messages increase adherence to acne therapy? Results of a randomized, controlled trial. J Am Acad Dermatol. 2012;67(6):1136–1142. [DOI] [PubMed] [Google Scholar]
- 18. Chan DS, Callahan CW, Hatch-Pigott VB, et al. Internet-based home monitoring and education of children with asthma is comparable to ideal office-based care: results of a 1-year asthma in-home monitoring trial. Pediatrics 2007;119(3):569–578. [DOI] [PubMed] [Google Scholar]
- 19. Charles T, Quinn D, Weatherall M, et al. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. J Allergy Clin Immunol. 2007;119(4):811–816. [DOI] [PubMed] [Google Scholar]
- 20. Chung MH, Richardson BA, Tapia K, et al. A randomized controlled trial comparing the effects of counseling and alarm device on HAART adherence and virologic outcomes. Public Library Sci. 2011;8(3):e1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collier AC, Ribaudo H, Mukherjee AL, et al. A randomized study of serial telephone call support to increase adherence and thereby improve virologic outcome in persons initiating antiretroviral therapy. J Infect Dis. 2005;192(8):1398–1406. [DOI] [PubMed] [Google Scholar]
- 22. El Miedany Y, El Gaafary M, Palmer D. A controlled pilot assessment of the utility of visual feedback in the treatment of early rheumatoid arthritis. Rheumatol Int. 2012;32(10):3061–3068. [DOI] [PubMed] [Google Scholar]
- 23. Fisher JD, Amico KR, Fisher WA, et al. Computer-based intervention in HIV clinical care setting improves antiretroviral adherence: the LifeWindows Project. AIDS Behav. 2011;15(8):1635–1646. [DOI] [PubMed] [Google Scholar]
- 24. Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens. 1996;9(4 part 1):285–292. [DOI] [PubMed] [Google Scholar]
- 25. Gensichen J, von Korff M, Peitz M, et al. Case management for depression by health care assistants in small primary care practices: a cluster randomized trial. Ann Intern Med. 2009;151(6):369–378. [DOI] [PubMed] [Google Scholar]
- 26. Heisler M, Vijan S, Makki F, et al. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hou MY, Hurwitz S, Kavanagh E, et al. Using daily text-message reminders to improve adherence with oral contraceptives: a randomized controlled trial. Obstet Gynecol. 2010;116(3):633–640. [DOI] [PubMed] [Google Scholar]
- 28. Howe CJ, Jawad AF, Tuttle AK, et al. Education and telephone case management for children with type 1 diabetes: a randomized controlled trial. J Pediatr Nurs. 2005;20(2):83–95. [DOI] [PubMed] [Google Scholar]
- 29. Kato PM, Cole SW, Bradlyn AS, et al. A video game improves behavioral outcomes in adolescents and young adults with cancer: a randomized trial. Pediatrics 2008;122(2):e305–e317. [DOI] [PubMed] [Google Scholar]
- 30. Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTelKenya1): a randomised trial. Lancet 2010;376(9755):1838–1845. [DOI] [PubMed] [Google Scholar]
- 31. Marquez Contreras E, Vegazo Garcia O, Claros NM, et al. Efficacy of telephone and mail intervention in patient compliance with antihypertensive drugs in hypertension: ETECUM-HTA Study. Blood Pressure 2005;14(3):151–158. [DOI] [PubMed] [Google Scholar]
- 32. Marquez-Contreras E, Martell-Claros N, Gil-Guillen V, et al. Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM-HTA study. J Hypertens. 2006;24(1):169–175. [DOI] [PubMed] [Google Scholar]
- 33. Okeke CO, Quigley HA, Jampel HD, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmology 2009;116(12):2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otsuki M, Eakin MN, Rand CS, et al. Adherence feedback to improve asthma outcomes among inner-city children: a randomized trial. Pediatrics 2009;124(6):1513–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pyne JM, Fortney JC, Curran GM, et al. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Arch Intern Med. 2011;171(1):23–31. [DOI] [PubMed] [Google Scholar]
- 36. Rickles NM, Svarstad BL, Statz-Paynter JL, et al. Pharmacist telemonitoring of antidepressant use: effects on pharmacist-patient collaboration. J Am Pharm Assoc. 2005;45(3):344–353. [DOI] [PubMed] [Google Scholar]
- 37. Sabin LL, DeSilva MB, Hamer DH, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS Behav. 2010;14(3):580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sherrard H, Struthers C, Kearns SA, et al. Using technology to create a medication safety net for cardiac surgery patients: a nurse-led randomized control trial. Can J Cardiovasc Nurs. 2009;19(3):9–15. [PubMed] [Google Scholar]
- 39. Simon GE, Ralston JD, Savarino J, et al. Randomized trial of depression followup care by online messaging. J Gen Intern Med. 2011;26(7):698–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simoni JM, Chen WT, Huh D, et al. A preliminary randomized controlled trial of a nurse-delivered medication adherence intervention among HIV-positive outpatients initiating antiretroviral therapy in Beijing, China. AIDS Behav. 2011;15(5):919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Simoni JM, Pantalone DW, Plummer MD, et al. A randomized controlled trial of a peer support intervention targeting antiretroviral medication adherence and depressive symptomatology in HIV-positive men and women. Health Psychol. 2007;26(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simoni JM, Huh D, Frick PA, et al. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;52(4):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solomon DH, Iversen MD, Avorn J, et al. Osteoporosis telephonic intervention to improve medication regimen adherence: a large, pragmatic, randomized controlled trial. Arch Intern Med. 2012;172(6):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van der Meer V, Bakker MJ, van den Hout WB, et al. Internet-based self-management plus education compared with usual care in asthma: a randomized trial. Ann Intern Med. 2009;151(2):110–120. [DOI] [PubMed] [Google Scholar]
- 45. Wakefield BJ, Holman JE, Ray A, et al. Effectiveness of home telehealth in comorbid diabetes and hypertension: a randomized, controlled trial. Telemed J E-Health 2011;17(4):254–261. [DOI] [PubMed] [Google Scholar]
- 46. Wang H, Zhou J, Huang L, et al. Effects of nurse-delivered home visits combined with telephone calls on medication adherence and quality of life in HIV-infected heroin users in Hunan of China. J Clin Nurs. 2010;19(3–4):380–388. [DOI] [PubMed] [Google Scholar]
- 47. Wolever RQ, Dreusicke M, Fikkan J, et al. Integrative health coaching for patients with type 2 diabetes: a randomized clinical trial. Diabetes Educ. 2010;36(4):629–639. [DOI] [PubMed] [Google Scholar]
- 48. Wu JR, Corley DJ, Lennie TA, et al. Effect of a medication-taking behavior feedback theory-based intervention on outcomes in patients with heart failure. J Card Fail. 2012;18(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu JY, Leung WY, Chang S, et al. Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. Brit Med J. 2006;333(7567):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zolfaghari M, Mousavifar SA, Pedram S, et al. The impact of nurse short message services and telephone followups on diabetic adherence: which one is more effective? J Clin Nurs. 2012;21(13–14):1922–1931. [DOI] [PubMed] [Google Scholar]
- 51. Horvath T, Azman H, Kennedy GE, et al. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane DB Syst Rev. 2012, Issue 3 Art. No.: CD009756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lavender T, Richens Y, Milan SJ, et al. Telephone support for women during pregnancy and the first six weeks postpartum. Cochrane DB Syst Rev. 2013, Issue 7 Art. No.: CD009338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, et al. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane DB Syst Rev. 2013, Issue 12 Art. No.: CD007458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, et al. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane DB Syst Rev. 2012, Issue 12 Art. No.: CD007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, et al. Mobile phone messaging for communicating results of medical investigations. Cochrane DB Syst Rev. 2012, Issue 6 Art. No.: CD007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steinhubl SR, Muse ED, Topol EJ. Can mobile health technologies transform health care? JAMA 2013;310(22):2395. [DOI] [PubMed] [Google Scholar]
- 57. Shepperd S, Lewin S, Straus S, et al. Can we systematically review studies that evaluate complex interventions? PLoS Med. 2009;6(8):e1000086 doi:10.1371/journal.pmed.1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pensky M. Digital natives, digital immigrants: a new way to look at ourselves and our kids. On the Horizon 2001;9:1–6. http://www.marcprensky.com/writing/Prensky%20-%20Digital%20Natives,%20Digital%20Immigrants%20-%20Part1.pdf. Accessed September 23, 2014. [Google Scholar]
- 59. Ramsey CR, Matowe L, Grilli R, et al. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behaviour change strategies. Int J Technol Assess Health Care 2003;19(4):613–623. [DOI] [PubMed] [Google Scholar]
- 60. Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. New York, NY: Houghton Mifflin Company; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.