Abstract
Aside from the human epidermal growth factor receptor‐2 (HER2)‐targeting agent trastuzumab, molecular targeting therapy for gastric cancer (GC) has not been established. We previously reported that apoptosis signal‐regulating kinase‐1 (ASK1) was upregulated in human GC and that overexpression of ASK1 promoted GC cell proliferation. Here, we investigated the effect of ASK1 inhibitor K811 on GC cells. K811 efficiently prevented cell proliferation in cell lines with high ASK1 expression and in HER2‐overexpressing GC cells. Treatment with K811 reduced sizes of xenograft tumors by downregulating proliferation markers. These results indicate that ASK1 inhibition prevents GC cell growth in vitro and in vivo, suggesting that ASK1 inhibitors can be potent therapeutic drugs for GC.
Although the curative rate for early stage gastric cancer (GC) has been increased by improvements in endoscopic and surgical techniques, the prognosis of advanced and unresectable GC remains quite poor.1, 2, 3 Helicobacter pylori and subsequent mucosal changes appear to be critical risk factors for GC.4, 5 We previously reported that nuclear factor‐kappa B (NF‐κB) and mitogen‐activated protein kinase (MAPK) pathways play critical roles in the development of H. pylori‐induced gastritis and GC.6, 7, 8, 9, 10 However, therapies targeted to these pathways remain clinically unavailable.
Targeting vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) is now widely accepted for treatment of colorectal cancer.11 Unlike colon cancer, the benefit of complementing 5‐fluorouracil (5‐FU) or cisplatin with VEGF‐ or EGF‐targeting drugs in the treatment of GC has not been demonstrated in clinical trials.12, 13 Human epidermal growth factor receptor‐2 (HER2) is upregulated in 20% of GC, and HER2‐targeting therapy was recently established for HER2‐positive GC treatment.14 However, for the majority of patients with advanced GC whose cancer cells do not overexpress HER2, efficient therapeutic molecular targets have not been identified.
We recently demonstrated that expression of the MAP3K, apoptosis signal‐regulating kinase‐1 (ASK1), is elevated in human GC tissues, and that the ASK1‐dependent positive feedback loop controlling cyclin D1 expression is important for GC development.15 The purpose of this experiment was to investigate the effect of ASK1 inhibitor for GC treatment. We showed that ASK1 inhibitor blocked GC cell proliferation in vitro and in vivo by suppressing activation of ASK1 and downstream molecules, suggesting that ASK1‐targeting therapies might be useful for GC.
Materials and Methods
Reagents
The ASK1 inhibitor K811 is a nitrogen‐containing heterocyclic derivative compound. It was synthesized at and obtained from Kyowa Hakko Kirin Co. Ltd (Shizuoka, Japan). The company has filed a patent application for K811 (International Patent Number: WO 2012/011548 A1). Detailed information about K811 including its chemical structure is described on the World Intellectual Property Organization homepage (http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2012011548&recNum=266&docAn=JP2011066650&queryString=pa/university&maxRec=70686). K811 was dissolved in DMSO for in vitro analysis, and in 10% DMSO and 10% chremophore EL (Sigma, St. Louis, MO, USA) for in vivo analysis. Anti‐phospho‐JNK, anti‐JNK, anti‐phospho‐p38, anti‐p38, anti‐phospho‐ERK, anti‐ERK, anti‐phospho‐HER2, anti‐HER2 and anti‐phospho‐ASK1 antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti‐ASK1 and anti‐PCNA (proliferating cell nuclear antigen) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti‐cyclin D1 (AB3) was acquired from Neomarkers (Fremont, CA, USA). 5‐FU and paclitaxel (PTX) were purchased from Wako Chemical Industries (Osaka, Japan).
Cell lines
AGS, NCI‐N87, 293T and Hela cell lines were purchased from ATCC (Manassas, VA, USA), MKN45, MKN74, MKN7 and HuG1‐N cells were obtained from RIKEN (Saitama, Japan), and SCH cells were acquired from JCRB (Osaka, Japan). The 293T cells are derived from human embryonic kidney cancer cells, Hela cells are from human epitheloid carcinoma of cervix, and the others are from human gastric cancer cells. The cell lines were cultured in Ham's F‐12, DMEM or RPMI medium (Wako Chemical Industries) supplemented with 10% fetal bovine serum. Cell numbers were determined using Cell Counting Kit‐8 according to the manufacturer's protocol (Dojindo Laboratories, Kumamoto, Japan).
Xenograft model
The xenograft mouse model experiments were performed as described previously.16, 17 Nude mice were implanted with 1 × 106 SH101 or MKN45 cells subcutaneously. When tumors reached a diameter of approximately 5 mm, the animals were pair matched into K811 treatment and control groups. Each group consisted of eight mice. K811 was administered orally at a dose of 50 mg/kg per day. The control group received only the vehicle. The largest diameter of each tumor was measured every other day after tumors reached a diameter of 5 mm and tumor volumes were calculated. After treatment, the tumors were removed, fixed in 10% formalin and embedded in paraffin. The experimental protocols were approved by the Ethics Committee for Animal Experimentation and conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Graduate School of Medicine, University of Tokyo, Japan.
Plasmids and adenovirus vectors
ASK1‐expressing plasmids and adenoviruses have been described previously.18, 19 The HER2‐expressing plasmid pSV2‐neuNT was purchased from Addgene (Cambridge, MA, USA). Cells were seeded into 12‐well plates and after 24 h they were transfected with 200 ng of plasmids using Effectine Transfection Reagent (Qiagen).
Immunohistochemistry
Tissues were fixed in 10% formaldehyde, dehydrated, embedded in paraffin and sectioned as described previously.20 The sections were deparaffinized and incubated overnight at 4°C with anti‐PCNA, anti‐cyclin D1 or control antibodies. Binding of the primary antibody was detected with anti‐rabbit IgG (Vector Laboratories, Burlingame, CA, USA), followed by visualization with 3, 3′‐diaminobenzidine (Sigma‐Aldrich).
Western blotting and immunoprecipitation
Protein lysates were prepared from cells or tissues, separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). The membrane was probed with primary antibodies and then incubated with the secondary antibody. Immunocomplexes were detected using the enhanced chemiluminescence system (Amersham Biosciences, Piscataway, NJ, USA). For immunoprecipitation, samples were lysed in radioimmunoprecipitation assay buffer and immunoprecipitated with 50 μL of protein A/G Sepharose beads (Santa Cruz Biotechnology) overnight at 4°C using the indicated antibodies. The beads were washed three times with radioimmunoprecipitation buffer and then analyzed using SDS‐PAGE.
Statistical analyses
Differences between means were compared using the Student's t–test. P‐values < 0.05 were considered statistically significant.
Results and Discussion
We first analyzed the effect of the ASK1 inhibitor K811 in GC cell lines. Treatment with K811 efficiently inhibited ASK1 phosphorylation in ASK1‐overexpressing cells (Fig. 1a). It has been reported that ASK1 acts upstream of JNK and p3821, and we previously showed that ASK1 knockdown reduced cyclin D1 expression in GC.15 Levels of phosphorylated JNK, phosphorylated p38 and cyclin D1 in AGS and MKN45 cells were reduced after K811 treatment, but the level of phosphorylated ERK did not change (Fig. 1b). These results indicate that the ASK1 inhibitor K811 reduced cyclin D1 expression through inhibiting ASK1 and downstream kinases JNK and p38, but not through ERK.
Figure 1.
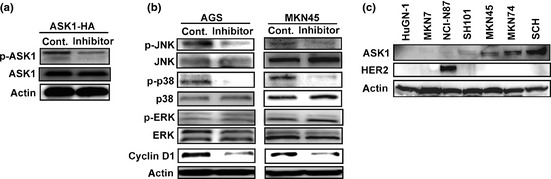
Apoptosis signal‐regulating kinase‐1 (ASK1) inhibitor K811 suppresses ASK1 phosphorylation and downstream activation. (a) AGS cells infected with ASK1‐HA adenoviral vectors were treated with 1 μM K811 or vehicle (Cont.) for 4 h. (b) AGS and MKN45 cells were treated with 1 μM K811 or vehicle (Cont.) for 4 h. (c) Western blot analysis of the indicated proteins in gastric cancer cells.
Next we tested whether treatment with K811 would inhibit GC cell proliferation. In MKN45 cells, K811 (0.5–1 μM) significantly reduced the cell number 72 h after treatment (Fig. 2a). We found that in MKN45, MKN74 and SCH cells, which express large amounts of ASK1 (Fig. 1c), treatment with K811 (0.5–3 μM) dose dependently reduced the number of cells (Fig. 2b). In contrast, K811 treatment did not affect cell migration ability (data not shown). Among MKN7, HuGN‐1 and NCI‐N87 cells, whose ASK1 expression is relatively low (Fig. 1c), K811 only inhibited cell growth in NCI‐N87 cells (Fig. S1), which overexpress HER2 protein (Fig. 1c). Hence, we hypothesized that K811 might inhibit HER2‐mediated MAPK signaling pathways. We investigated this hypothesis using AGS cells transfected with ASK1‐ and HER2‐expressing vectors (Fig. 2c). Levels of phosphorylated ASK1 were increased in cells transfected with both ASK1‐ and HER2‐expressing vectors compared with cells transfected with the ASK1‐expressing vector alone, suggesting that HER2 can activate ASK1. K811 treatment significantly inhibited phosphorylation of ASK1 in cells overexpressing both ASK1 and HER2. Similar results were obtained using non‐gastric cancer cells, Hela and 293T cells (Fig. 2c), suggesting HER2 universally contributes to ASK1 phosphorylation and downstream signaling. Indeed, in NCI‐N87 cells that overexpress HER2, immunoprecipitation analysis showed that the phosphorylated ASK1 was inhibited by K811 (Fig. S2), even though the expression level of ASK1 is low. These results indicate that K811 inhibits cell growth not only in cells that express ASK1 at high levels, but also in cells that overexpress HER2.
Figure 2.
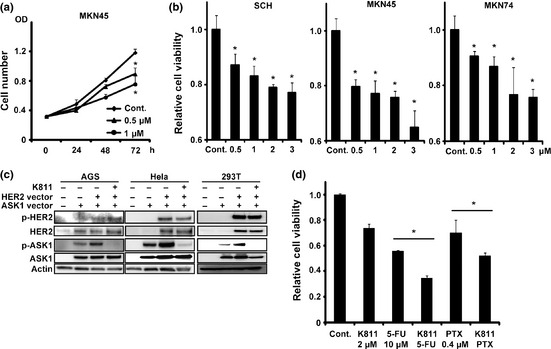
K811 prevents gastric cancer cell proliferation in vitro. (a) Numbers of MKN45 cells at 24, 48 and 72 h after treatment with K811 or vehicle (Cont.). K811 (0.5–1 μM) was administered every 24 h. (b) Relative numbers of the indicated cells 72 h after treatment with K811 (0.5–3 μM). Data show the mean ± SD. *P < 0.05 versus vehicle. (c) AGS, Hela and 293T cells were transfected with control, ASK1‐HA or mutant HER2‐expressing vectors and cultured with or without K811 (1 μM) for 4 h. (d) Relative numbers of MKN45 cells 48 h after treatment with K811 (2 μM), 5‐FU (10 μM), paclitaxel (PTX) (0.4 μM), vehicle or a combination of these drugs. The mean cell number for control cells was set to 1.0. Data show the mean ± SD. *P < 0.05.
Although the HER2‐positive rate is only 20% in GC,14 ASK1 expression was elevated in approximately 40–50% cases,15 suggesting that ASK1‐targeting therapy might be more beneficial to GC patients than HER2‐targeting therapy. In addition, as HER2 overexpression leads to cell proliferation via activation of MAPK, K811 might inhibit the growth of HER2‐positive tumors by inhibiting ASK1 and JNK activation.
Next we investigated whether K811 has additional effects on the growth of GC cells treated with other cytotoxic agents. Treatment of MKN45 cells with K811, 5‐FU or PTX alone significantly reduced the number of cells. Furthermore, K811 and 5‐FU/PTX had additive inhibitory effects on growth (Fig. 2d), suggesting that combination therapy comprising K811 and cytotoxic agents might be useful for GC treatment. However, because previous studies have shown that ASK1 plays a critical role in ROS‐dependent apoptosis,21 the combination of an ASK1 inhibitor with a ROS‐inducing drug might reduce the cytotoxic effect. Further analysis of the possible combined use of ASK1 inhibitors and ROS‐inducing drugs is needed.
To confirm the efficacy of K811 treatment in vivo, we created xenograft models using two GC cell lines, MKN45 and SH‐101 (which show moderate ASK1 expression). In both cell lines, tumors of mice treated with K811 were significantly smaller than those of mice treated with vehicle only after K811 treatment (Fig. 3). In the xenograft tumors treated with K811, phosphorylated ASK1 and phosphorylated JNK were significantly reduced compared with the control tumors (Fig. 4a). Immunohistochemical analysis showed that the number of cyclin D1‐positive and PCNA‐positive cells in the tumors of K811‐treated mice were significantly decreased (Fig. 4b,c). These experiments reveal that K811 treatment had good oral bioavailability and was effective for the treatment of GC xenografts in vivo.
Figure 3.
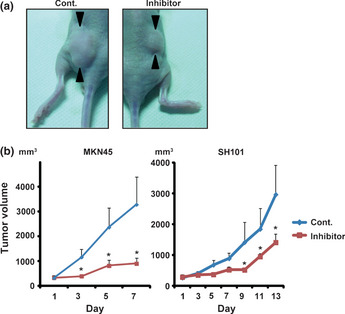
Treatment with K811 inhibits gastric cancer xenograft growth in vivo. K811 was orally given to mice daily, 9 days after s.c. injection for MKN45 cells and 7 days after injection for SH101 cells, when tumors reached a diameter of 5 mm. The starting day of K811 administration is described as day 0. (a) Day 7 MKN45 cell‐derived xenograft tumor. (b) Calculated tumor volume changes. Data show the mean ± SE. *P < 0.05.
Figure 4.
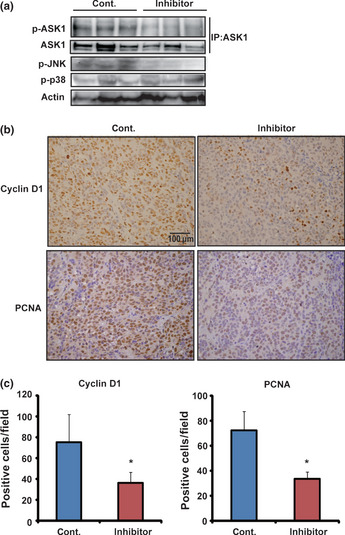
Treatment with K811 inhibits phosphorylation of ASK1 and expression of cell cycle markers in xenograft tumors. (a) Tumors were removed 4 h after the last K811 administration. Cell lysates of xenograft tumors were immunoprecipitated with anti‐ASK1 antibody and immunoblotted with anti‐phospho‐ASK1 and anti‐ASK1 antibodies. Total lysates were used for immunoblotting with phospho‐JNK, phospho‐p38 and actin. (b) Immunohistochemical staining for cyclin D1 and PCNA in MKN45 cell‐derived tumors from mice treated with or without K811. (c) Numbers of cyclin D1‐ or PCNA‐positive cells in each field. Data show the mean ± SD. *P < 0.05 versus mice treated with vehicle.
As administration of K811 at this dose for 3 weeks caused no bodyweight changes in wild‐type C57B6 mice (Fig. S3), we consider that no major side‐effect has occurred. We previously reported that ASK1 has protective functions in the development of colitis, colitis‐associated cancer22 and liver cancer.23 Thus, we observed colon and liver tissues after K811 treatment, but no histological changes were seen (Fig. S3). However, the effect of long‐term treatment with K811 against colon and liver diseases should be analyzed in future studies.
In conclusion, we demonstrated that ASK1 inhibitor treatment successfully prevents GC cell proliferation in vitro and in vivo, suggesting that ASK1‐targeting therapy is a future candidate for the treatment of advanced GC.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Effect of K811 on proliferation in apoptosis signal‐regulating kinase‐1 low‐expressing cells.
Fig. S2. K811 inhibits apoptosis signal‐regulating kinase‐1 phosphorylation in NCI‐N87 cells.
Fig. S3. Effect of K811 on bodyweight change and liver histology.
Acknowledgments
The authors thank Kyowa Hakko Kirin Co. Ltd for providing K811. Y. Hayakawa was supported by grants‐in‐aid from the Foundation for Advancement of International Science and Institute of Asahi‐Life Foundation. Y. Hirata was supported by a Grant‐in‐Aid for Scientific Research (#22590679).
(Cancer Sci 2012; 103: 2181–2185)
References
- 1. Sugimoto T, Okamoto M, Mitsuno Y et al Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: a multicenter feasible study. J Clin Gastroenterol 2012; 46: 124–9. [DOI] [PubMed] [Google Scholar]
- 2. Sano T, Sasako M, Yamamoto S et al Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para‐aortic lymphadenectomy–Japan Clinical Oncology Group study 9501. J Clin Oncol 2004; 22: 2767–73. [DOI] [PubMed] [Google Scholar]
- 3. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta‐analysis based on aggregate data. J Clin Oncol 2006; 24: 2903–9. [DOI] [PubMed] [Google Scholar]
- 4. Uemura N, Okamoto S, Yamamoto S et al Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–9. [DOI] [PubMed] [Google Scholar]
- 5. Sakitani K, Hirata Y, Watabe H et al Gastric cancer risk according to the distribution of intestinal metaplasia and neutrophil infiltration. J Gastroenterol Hepatol 2011; 26: 1570–5. [DOI] [PubMed] [Google Scholar]
- 6. Hirata Y, Maeda S, Mitsuno Y et al Helicobacter pylori activates the cyclin D1 gene through mitogen‐activated protein kinase pathway in gastric cancer cells. Infect Immun 2001; 69: 3965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirata Y, Ohmae T, Shibata W et al MyD88 and TNF receptor‐associated factor 6 are critical signal transducers in Helicobacter pylori‐infected human epithelial cells. J Immunol 2006; 176: 3796–803. [DOI] [PubMed] [Google Scholar]
- 8. Maeda S, Yoshida H, Ogura K et al H. pylori activates NF‐kappaB through a signaling pathway involving IkappaB kinases, NF‐kappaB‐inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology 2000; 119: 97–108. [DOI] [PubMed] [Google Scholar]
- 9. Mitsuno Y, Yoshida H, Maeda S et al Helicobacter pylori induced transactivation of SRE and AP‐1 through the ERK signalling pathway in gastric cancer cells. Gut 2001; 49: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakamoto K, Hikiba Y, Nakagawa H et al Inhibitor of kappaB kinase beta regulates gastric carcinogenesis via interleukin‐1alpha expression. Gastroenterology 2010; 139: 226–38.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arsene D, Galais MP, Bouhier‐Leporrier K, Reimund JM. Recent developments in colorectal cancer treatment by monoclonal antibodies. Expert Opin Biol Ther 2006; 6: 1175–92. [DOI] [PubMed] [Google Scholar]
- 12. Ohtsu A, Shah MA, Van Cutsem E et al Bevacizumab in combination with chemotherapy as first‐line therapy in advanced gastric cancer: a randomized, double‐blind, placebo‐controlled phase III study. J Clin Oncol 2011; 29: 3968–76. [DOI] [PubMed] [Google Scholar]
- 13. Lordick F, Luber B, Lorenzen S et al Cetuximab plus oxaliplatin/leucovorin/5‐fluorouracil in first‐line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer 2010; 102: 500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bang YJ, Van Cutsem E, Feyereislova A et al Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2‐positive advanced gastric or gastro‐oesophageal junction cancer (ToGA): a phase 3, open‐label, randomised controlled trial. Lancet 2010; 376: 687–97. [DOI] [PubMed] [Google Scholar]
- 15. Hayakawa Y, Hirata Y, Nakagawa H et al Apoptosis signal‐regulating kinase 1 and cyclin D1 compose a positive feedback loop contributing to tumor growth in gastric cancer. Proc Natl Acad Sci USA 2011; 108: 780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakata W, Hayakawa Y, Nakagawa H et al Anti‐tumor activity of the proteasome inhibitor bortezomib in gastric cancer. Int J Oncol 2011; 39: 1529–36. [DOI] [PubMed] [Google Scholar]
- 17. Sakamoto K, Maeda S, Hikiba Y et al Constitutive NF‐kappaB activation in colorectal carcinoma plays a key role in angiogenesis, promoting tumor growth. Clin Cancer Res 2009; 15: 2248–58. [DOI] [PubMed] [Google Scholar]
- 18. Takeda K, Shimozono R, Noguchi T et al Apoptosis signal‐regulating kinase (ASK) 2 functions as a mitogen‐activated protein kinase kinase kinase in a heteromeric complex with ASK1. J Biol Chem 2007; 282: 7522–31. [DOI] [PubMed] [Google Scholar]
- 19. Saitoh M, Nishitoh H, Fujii M et al Mammalian thioredoxin is a direct inhibitor of apoptosis signal‐regulating kinase (ASK) 1. EMBO J 1998; 17: 2596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayakawa Y, Maeda S, Nakagawa H et al Effectiveness of IkappaB kinase inhibitors in murine colitis‐associated tumorigenesis. J Gastroenterol 2009; 44: 935–43. [DOI] [PubMed] [Google Scholar]
- 21. Ichijo H, Nishida E, Irie K et al Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 1997; 275: 90–4. [DOI] [PubMed] [Google Scholar]
- 22. Hayakawa Y, Hirata Y, Nakagawa H et al Apoptosis signal‐regulating kinase 1 regulates colitis and colitis‐associated tumorigenesis by the innate immune responses. Gastroenterology 2010; 138: 1055‐67.e1–4. [DOI] [PubMed] [Google Scholar]
- 23. Nakagawa H, Hirata Y, Takeda K et al Apoptosis signal‐regulating kinase 1 inhibits hepatocarcinogenesis by controlling the tumor‐suppressing function of stress‐activated mitogen‐activated protein kinase. Hepatology 2011; 54: 185–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of K811 on proliferation in apoptosis signal‐regulating kinase‐1 low‐expressing cells.
Fig. S2. K811 inhibits apoptosis signal‐regulating kinase‐1 phosphorylation in NCI‐N87 cells.
Fig. S3. Effect of K811 on bodyweight change and liver histology.


