Abstract
Multi‐walled carbon nanotubes have a fibrous structure similar to asbestos and induce mesothelioma when injected into the peritoneal cavity. In the present study, we investigated whether carbon nanotubes administered into the lung through the trachea induce mesothelial lesions. Male F344 rats were treated with 0.5 mL of 500 μg/mL suspensions of multi‐walled carbon nanotubes or crocidolite five times over a 9‐day period by intrapulmonary spraying. Pleural cavity lavage fluid, lung and chest wall were then collected. Multi‐walled carbon nanotubes and crocidolite were found mainly in alveolar macrophages and mediastinal lymph nodes. Importantly, the fibers were also found in the cell pellets of the pleural cavity lavage, mostly in macrophages. Both multi‐walled carbon nanotube and crocidolite treatment induced hyperplastic proliferative lesions of the visceral mesothelium, with their proliferating cell nuclear antigen indices approximately 10‐fold that of the vehicle control. The hyperplastic lesions were associated with inflammatory cell infiltration and inflammation‐induced fibrotic lesions of the pleural tissues. The fibers were not found in the mesothelial proliferative lesions themselves. In the pleural cavity, abundant inflammatory cell infiltration, mainly composed of macrophages, was observed. Conditioned cell culture media of macrophages treated with multi‐walled carbon nanotubes and crocidolite and the supernatants of pleural cavity lavage fluid from the dosed rats increased mesothelial cell proliferation in vitro, suggesting that mesothelial proliferative lesions were induced by inflammatory events in the lung and pleural cavity and likely mediated by macrophages. In conclusion, intrapulmonary administration of multi‐walled carbon nanotubes, like asbestos, induced mesothelial proliferation potentially associated with mesothelioma development.
Multi‐walled carbon nanotubes (MWCNT) are structurally composed of cylinders rolled up from several layers of graphite sheets. They are several to tens of nanometers in diameter and several to tens of micrometers in length. This high length to diameter aspect ratio, a characteristic shared with asbestos fibers, has led to concern that exposure to MWCNT might cause asbestos‐like lung diseases, such as lung fibrosis, lung cancer, pleural plaque and malignant mesothelioma.1, 2, 3, 4, 5, 6
Pleural plaque and malignant mesothelioma are characteristic lesions in asbestos‐exposed humans. Although fiber dimensions, biopersistence, oxidative stress and inflammation have all been implicated,7, 8, 9, 10, 11, 12 the exact mechanisms of pleural pathogenesis are unclear. According to a pathogenesis paradigm suggested by Donaldson et al.,2 asbestos fibers penetrate into the pleural cavity from the alveoli and deposit in the pleural tissue. Unlike spherical particles, fibrous materials such as asbestos are not cleared effectively from the pleural cavity, resulting in deposition of the fibers in the parietal pleura. This deposition, in turn, causes frustrated phagocytosis‐induced pro‐inflammatory, genotoxic and mitogenic responses in the deposition sites.2
Administration of MWCNT into the peritoneal cavity or scrotum in animals has been reported to induce mesothelial lesions, similar to those observed in asbestos cases.13, 14, 15 The induction of mesothelioma in the peritonum is dose dependent, and is observed with as low as 3 μg/mouse in p53 heterozygous mice.16 These studies suggest a potential risk that inhaled MWCNT might lead to pleural mesothelioma. However, actual experimental evidence demonstrating induction of pleural mesothelioma by inhaled MWCNT fibers has not yet been shown. It has been shown that inhaled MWCNT induced subpleural fibrosis with macrophage aggregates on the surface of the visceral pleura.17 Notably, some of these macrophages contained MWCNT fibers. In addition, penetration of MWCNT administrated by pharyngeal aspiration into the pleural cavity was observed,18 and intrapleural injection of 5 μg/mouse of MWCNT has been shown to lead to sustained inflammation and length‐dependent retention of MWCNT in the pleural cavity.19 Accordingly, direct interaction of MWCNT with the mesothelial tissue is postulated as an early pathogenic event.
In the present study, to examine whether MWCNT translocate into the pleural cavity and cause inflammation leading to proliferative change of the mesothelial tissue, we administered relatively high doses (five doses at 250 μg/rat) of two MWCNT samples (MWCNT‐N and MWCNT‐M) to the rat lung by intrapulmonary spraying (IPS)/intratracheal instillation; crocidolite (CRO; one kind of asbestos fiber) was used as a positive control. Intrapulmonary spraying has been shown to be an efficient method to deliver particle materials deep into the lung.20, 21, 22, 23, 24 Our results demonstrated that MWCNT, like asbestos, translocated from the lung into the pleural cavity and induced inflammatory responses in the pleural cavity and, importantly, hyperplastic visceral mesothelial proliferation. These findings are important in understanding whether MWCNT have the potential to cause asbestos‐like pleural lesions.
Materials and Methods
Animals
Eight‐week‐old male F344 rats were purchased from Charles River Japan Inc. (Kanagawa, Japan). The animals were housed in the Animal Center of Nagoya City University Medical School and maintained on a 12 h light/12 h dark cycle, and received Oriental MF basal diet (Oriental Yeast Co. Ltd, Tokyo, Japan) and water ad libitum. The study was conducted according to the Guidelines for the Care and Use of Laboratory Animals of Nagoya City University Medical School and the experimental protocol was approved by the Institutional Animal Care and Use Committee (H22M‐19).
Preparation of MWCNT and CRO suspensions
The MWCNT investigated were MWCNT‐N (Nikkiso Co., Ltd, Tokyo, Japan) and MWCNT‐7 (Mitsui Chemicals Inc., Tokyo, Japan; designated as MWCNT‐M). Crocidolite (Union for International Cancer Control grade) was from the National Institute of Health Sciences of Japan stocks. Ten milligrams of MWCNT‐N or MWCNT‐M were suspended in 20 mL of saline containing 0.1% Tween 20 and homogenized for 1 min four times at 3000 r.p.m. in a Polytron PT1600E benchtop homogenizer (Kinematika AG, Littau, Switzerland). The suspensions were sonicated for 30 min shortly before use to minimize aggregation. The CRO suspension was prepared similarly, but without homogenization. The concentration of the MWCNT and CRO suspensions was 500 μg/mL. The lengths of MWCNT and CRO in the suspensions were determined using a digital map meter (Comcureve‐9 Junior; Koizumi Sokki MFG. Co., Ltd, Nigata, Japan) on scanning electron microscope (SEM) photos. The SEM observation and length distributions of MWCNT and CRO are shown in Fig. S1A,B. To count the fiber number, 500 μg/mL suspensions of MWCNT‐N, MWCNT‐M and CRO were diluted 1:1000 with deionized water and 0.5 μL of the diluted suspensions was loaded onto clean glass slides and dried in a micro oven at 480°C for 1 min. The fiber number on the slides was counted under a polarized light microscope (PLM) (Olympus BX51N‐31P‐O PLM, Tokyo, Japan) (PLM detects all fibers longer than 200 nm). The results are shown in Fig. S1C.
Intrapulmonary spraying of MWCNT and CRO into the lung and pleural cavity lavage (PCL)
We used the intrapulmonary spraying technique that was developed in our laboratory.24 Briefly, rats were anaesthetized using isoflurane; the mouth was fully opened with the tongue gently held and the nozzle of a microsprayer (series IA‐1B Intratracheal Aerosolizer; Penn‐century, Philadelphia, PA, USA) was inserted into the trachea through the larynx and 0.5 mL suspension was sprayed into the lungs synchronizing with spontaneous respiratory inhalation. We confirmed that the dosed materials were distributed deep into the lung tissue and reached most of the terminal alveoli without causing obvious respiratory distress.
Ten‐week‐old male Fisher 344 rats were divided into four groups of six animals each and given 0.5 mL of saline containing 0.1% Tween 20 or 500 μg/mL MWCNT‐N, MWCNT‐M or CRO suspension by IPS once every other day five times over a 9‐day period. The total amount of fibers administered was 1.25 mg/rat. Six hours after the last IPS, the rats were placed under deep isoflurane anesthesia; a small incision was made in the abdominal wall, the pleural cavity was injected with 10 mL of ice cold RPMI 1640 through the diaphragm, and the lavage fluid was collected by syringe. The rats were then killed by exsanguination from the inferior vena cava and the major organs, including the lung, chest wall, brain, liver, kidney, spleen and mediastinal lymph nodes, were fixed in 4% paraformaldehyde and processed for histological examination.
Analysis of inflammatory reaction in the pleural cavity
Cells in the lavage fluid were counted using a hemocytometer (Erma Co., Ltd, Tokyo, Japan), and the cellular fraction was then isolated by centrifugation at 200g for 5 min at 4°C. Cell pellets collected from three rats were combined (generating a total of two cell pellets per group), fixed in 4% paraformaldehyde and processed for histological examination. Total protein in the supernatants of each of the lavage fluids was determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) and the supernatants were then concentrated by centrifugation in Vivaspin 15 concentrators (Sartorius Stedium Biotech, Goettingen, Germany) at 1500g for 30 min at 4°C and used for in vitro cell proliferation assays.
Light microscopy and PLM
Haematoxylin–eosin (H&E)‐stained slides of the lung tissues and cellular pellets of the PCL were used to observe MWCNT‐N, MWCNT‐M and CRO fibers with PLM at ×1000 magnification. The exact localization of the illuminated fibers was confirmed in the same H&E‐stained sections after removing the polarizing filter.
Scanning electron microscopy
The H&E‐stained slides of the lung tissue and PCL pellets were immersed in xylene for 3 days to remove the cover glass, then immersed in 100% ethanol for 10 min to remove the xylene and air‐dried for 2 h at room temperature. The slides were then coated with platinum for viewing using a scanning electronic microscope (SEM) (Model S‐4700 Field Emission SEM; Hitachi High Technologies Corporation, Tokyo, Japan) at 5–10 kV.
Immunohistochemistry and Azan–Mallory staining
CD68, proliferating cell nuclear antigen (PCNA) and mesothelin/Erc were detected using antirat CD68 antibodies (BMA Biomedicals, Augst, Switzerland), anti‐PCNA monoclonal antibodies (Clone PC10; Dako Japan Inc., Tokyo, Japan) and antirat C‐ERC/mesothelin polyclonal antibodies (Immuno‐Biological Laboratories Co., Ltd, Gunma, Japan). The CD68, PCNA and C‐ERC/mesothelin antibodies were diluted 1:100, 1:200 and 1:1000, respectively, in blocking solution and applied to deparaffinized slides. The slides were incubated at 4°C overnight and then incubated for 1 h with biotinylated species‐specific secondary antibodies diluted 1:500 (Vector Laboratories, Burlingame, CA, USA) and visualized using avidin‐conjugated horseradish peroxidase complex (ABC kit; Vector Laboratories). Azan–Mallory staining was used to visualize collagen fibers.
In vitro exposure and preparation of conditioned macrophage culture media
The induction and preparation of primary alveolar macrophages (PAM) has been described previously.24 PAM were seeded into 6 cm culture dishes at 2 × 106 cells per well in 10% FBS RPMI 1640. After overnight incubation, the culture media was refreshed and MWCNT‐N, MWCNT‐M or CRO suspensions were added to the cells to a final concentration of 10 μg/mL. The cells were then incubated for another 24 h. The conditioned macrophage culture media was then collected for in vitro cell proliferation assays.
In vitro cell proliferation assay
Human mesothelioma cells, TCC‐MESO1, derived from a patient in the Tochigi Cancer Center,25 were seeded into 96‐well culture plates at 2 × 103 cells per well in 10% FBS RPMI 1640. After overnight incubation, the cells were serum‐starved for 24 h. The media was changed to 100 μL of the concentrated supernatants of the PCL or conditioned macrophage culture media and incubated for 72 h. The relative cell number was then determined using the Cell Counting Kit‐8 (Dojindo Molecular Technologies, Rockville, MD, USA) according to the manufacturer's instruction.
Statistical analysis
Statistical analysis was performed using anova. The statistical significance was analyzed using a two‐tailed Student's t‐test. A P‐value of <0.05 was considered to be significant.
Results
Translocation of MWCNT and CRO fibers into the pleural cavity
The cell pellets of the PCL were used to examine whether the MWCNT or CRO fibers were present in the pleural cavity. We first screened the H&E‐stained PCL cell pellet slides using PLM. The exact localization of the fibers was confirmed using SEM of the same slide sections. MWCNT‐N, MWCNT‐M and CRO fibers were present in PCL cell pellets, with most of the fibers in macrophage‐like cells (Fig. 1a–c) with very few fibers located in the intercellular space or on cell surfaces (data not shown). Immunohistochemistry with CD68, a macrophage marker, showed that MWCNT and CRO fibers were mainly found in macrophages (Fig. 1d,e).
Figure 1.
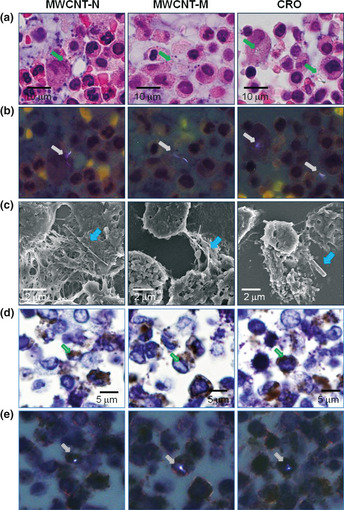
Existence of multi‐walled carbon nanotubes (MWCNT)‐N, MWCNT‐M and crocidolite (CRO) fibers in the cell pellets of the pleural cavity lavage (PCL). (a) Images of H&E‐stained slides of the cell pellets of the PCL treated with MWCNT‐N, MWCNT‐M and CRO fibers. (b) Polarized light microscope (PLM) images of the same view areas shown in (a). (c) Scanning electron microscope observation showed the existence of the MWCNT and CRO fibers in the cell pellets of the PCL. (d) CD68 immunostaining of the PCL cell pellet slides. (e) PLM observation of the same view areas shown in (d) indicate that MWCNT and CRO fibers were present in the CD68‐positive macrophages. Arrows indicate MWCNT‐N, MWCNT‐M and CRO fibers.
In tissue sections, MWCNT and CRO fibers were mainly detected in focal granulomatous lesions in the alveoli and in alveolar macrophages. Fibers were also found in the mediastinal lymph nodes, and a few fibers were detected in liver sinusoid cells, blood vessel wall cells in the brain, renal tubular cells and spleen sinus and macrophages (data not shown). We detected only a few fibers penetrating directly from the lung to the pleural cavity through the visceral pleura (Fig. S2) and did not find any fibers in the parietal pleura.
Induction of visceral mesothelial proliferation
Hyperplastic visceral mesothelial proliferation (HVMP) was clearly observed in all of the MWCNT and CRO treated groups. The HVMP lesions were composed of mesothelial cells with cuboidal appearance and increased size and density lining the visceral pleural tissue. Various degrees of lung inflammation and fibrous thickening were observed underneath the HVMP lesions (Fig. 2a, panel A). The PCNA immunostaining showed proliferating mesothelial cells within the HVMP lesions (Fig. 2a, panel B). The PCNA indices of the visceral mesothelium were increased approximately 10‐fold in all the MWCNT and CRO treated groups compared with the control group (Fig. 2b). Azan–Mallory staining showed increases in collagen fibers underneath the HVMP lesions (Fig. 2a, panel C). Fibers were not found within the HVMP lesions themselves. Alveolar macrophages with phagocytosed MWCNT or CRO fibers were frequently observed near the HVMP lesions (Fig. S3). Proliferation and other lesions of the parietal mesothelium were not observed.
Figure 2.
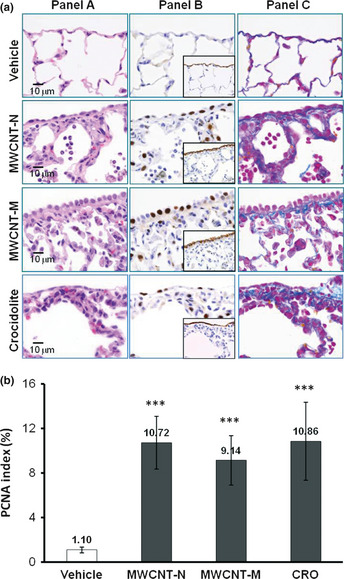
Induction of visceral mesothelial cell proliferation lesions by treatment with multi‐walled carbon nanotubes (MWCNT)‐N, MWCNT‐M or crocidolite (CRO). (a) Serial sections were prepared and stained with H&E, proliferating cell nuclear antigen (PCNA), Erc/mesothelin and Azan–Mallory's collagen staining. Panel A: increase in enlarged visceral mesothelial cells with cuboidal shapes in the MWCNT‐N, MWCNT‐M and CRO treated groups. Panel B: PCNA‐positive cells are clearly increased in the dosed groups. The inserts are immunostained with Erc/mesothelin and show the lining of the mesothelium. Panel C: Azan–Mallory's staining; sub‐pleural collagenous fibrosis is present under the mesothelial cell proliferation lesions. (b) PCNA index, expressed as the percentage of PCNA‐positive cells of the total number of visceral mesothelial cells per slide. ***P < 0.001.
Inflammatory cell infiltration in the pleural cavity
Both MWCNT and CRO treatment resulted in inflammatory reactions in the pleural cavity. The total number of cells, composed mostly of macrophages, neutrophils and lymphocytes, in the PCL in the MWCNT and CRO treated groups was significantly increased compared with the control group (Fig. 3a). As can be calculated from Fig. 3(a,b), macrophages accounted for a large part of the increase of the total cell number in the PCL, although the number of neutrophils and lymphocytes also increased. Overall, the proportion of macrophages in the cell pellets of the PCL was increased, while those of neutrophils and lymphocytes were decreased (Fig. 3b). MWCNT or CRO treatment also significantly increased the total protein level in the PLC (Fig. 3c). The proportion of cells in the PCL pellets positive for Mesothelin/Erc, a mesothelial cell marker, was 0.53–1.02%, and no intergroup difference was observed (data not shown). These data indicate that the increased cell number in the pleural cavity of the rats treated with MWCNT or CRO resulted from inflammatory cell effusion, not from mesothelial cell shedding of the pleural tissue. Many macrophages in the PCL contained MWCNT or CRO fibers.
Figure 3.
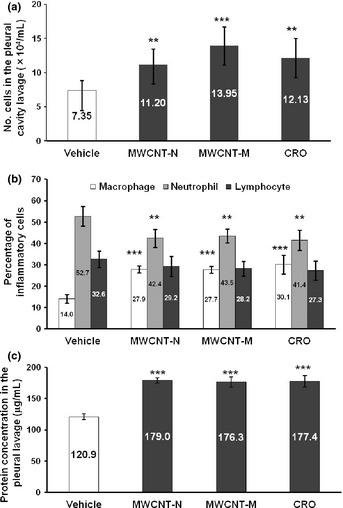
Inflammatory reaction in the pleural cavity. (a) The number of leukocytes in the pleural cavity lavage (PCL) of rats treated with multi‐walled carbon nanotubes (MWCNT) and crocidolite (CRO). (b) The proportion of macrophages, neutrophils and lymphocytes in the cell pellets of the PCL. Total cell number and cell numbers of macrophages, neutrophils and lymphocytes in 10 randomly chosen fields (×400) were counted. (c) Protein concentration in the supernatants of the PCL. **P < 0.01; ***P < 0.001.
Mesothelial cell proliferation assay in vitro
To examine whether inflammatory reactions, especially those mediated by macrophages, exert proliferative effects on mesothelial cells, we examined the effects of conditioned macrophage culture medium on mesothelial cell proliferation in vitro. The conditioned culture media of macrophages exposed to MWCNT‐N, MWCNT‐M or CRO significantly increased the proliferation of the human mesothelioma cell line TCC‐MESO1. The concentrated supernatants of the PCL taken from the rats treated with MWCNT‐N, MWCNT‐M or CRO exhibited similar effects (Fig. 4). These results indicate that factors in the PCL, possibly secreted by alveolar and pleural macrophages, are able to cause mesothelial cell proliferation.
Figure 4.
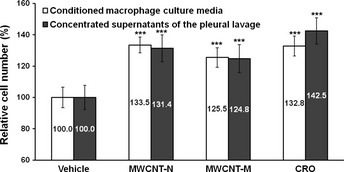
Effect of conditioned macrophage culture media and the supernatants of the pleural cavity lavage (PCL) on mesothelial cell proliferation in vitro. The conditioned culture media of macrophages treated with multi‐walled carbon nanotubes (MWCNT)‐N, MWCNT‐M or crocidolite (CRO) significantly increased the cell proliferation of TCC‐MESO1. The concentrated supernatants of the PCL from the rats treated with MWCNT‐N, MWCNT‐M or CRO had similar effects. n = 6. ***P < 0.001.
Discussion
In the present study, we compared the pleural translocation of MWCNT and CRO and examined the mesothelial lesions they induced. Our data demonstrate that after deposition in the lung, MWCNT, like CRO, translocated into the pleural cavity, mainly in pleural macrophages. Both MWCNT and CRO treatment also caused hyperplastic visceral mesothelial proliferation and marked pleural inflammation.
This is the first report to show that MWCNT administered into the rat lung causes mesothelial proliferative lesions. Adamson et al.26 reported that intratracheal instillation of asbestos in mice induced pleural mesothelial cell proliferation within several days; the degree of pleural mesothelial cell proliferation did not appear to correlate with the localization of asbestos fibers in the pleura.27 Similarly, we did not find fibers within the HVMP lesions. Thus, our findings suggest that HVMP lesions do not appear to be directly induced by the deposited MWCNT or CRO fibers. Also, in vitro exposure to MWCNT and CRO fibers did not lead to proliferation of TCC‐MESO1 cells, but rather to cell death (Fig. S4). It has been reported that macrophages play a significant role in mesothelial cell proliferation caused by asbestos exposure and surgical injury,28, 29, 30, 31 and that the conditioned medium of macrophages exposed to MWCNT promotes mesothelial cell proliferation in vitro.30 Activated macrophages secrete a panel of growth factors and cytokines to regulate cell proliferation, which can augment transformation of mesothelial cells.28, 30, 32, 33 Our observations that mesothelial cell proliferation is enhanced by conditioned macrophage culture media and by the supernatants of pleural cavity lavage are consistent with these results, although the factors that are involved need to be identified.
Translocation of asbestos34, 35 and MWCNT18 fibers from the lung to the pleural cavity has been found in rodents. This translocation also probably occurs in humans since asbestos fibers have been detected in human pleural lesions.36 However, the mechanism and route of translocation are unclear. It has been suggested that penetration through the visceral pleura, possibly driven by increased pulmonary interstitial pressure and assisted by enhanced permeability of the visceral pleura due to asbestos‐induced inflammation might be a major route.37 In the present study, only a few MWCNT and CRO fibers were observed penetrating through the visceral pleura, and a large number of the fibers in the pleural cavity was observed in macrophages. We also observed frequent deposition of MWCNT and CRO in the mediastinal lymph nodes, mostly phagocytosed by macrophages. These results suggest that a probable route of translocation of the fibers is lymphatic flow. Inflammation in the pleural cavity is probably a defense response against translocated fibers. Murphy et al.19 reported that intrapleural injection of 5 μg/mouse of long MWCNT or asbestos initiated sustained inflammation, including increased granulocyte number and protein level, in the pleural cavity. Thus, the observed proliferation of visceral mesothelial cells in the present study is probably caused by inflammatory reactions both in the lung and in the pleural cavity. In the present study, no MWCNT or crosidolite fibers or lesions were observed in the parietal pleura. This is possibly due to the short experimental period and limited amount of fibers in the pleural cavity, which would result in little inflammation in the parietal pleura.
Currently, the exposure level to MWCNT in the workplace is unknown and there are no administrational regulations for the occupational exposure limit for MWCNT. In November 2010, the National Institute of Occupational Safety and Health (NIOSH) released a non‐official carbon nanotube exposure limit for peer review. The recommended exposure limit in the air was set at 7 μg/m3.38 Previously, we used a total dose of 1.25 mg/rat of titanium dioxide over a 9‐day period and identified factors involved in titanium dioxide‐induced lung lesions.24 In the present study, we used the same protocol for the purpose of induction of observable pleural lesions and inflammation in the pleural cavity as well to ensure the presence of a detectable number of fibers in the pleural cavity after short‐term administration; this dose was higher than the NIOSH exposure limit. Time‐ and dose‐dependent experiments are needed in future studies, and further investigation is also required to elucidate cytokines and other factors that cause parietal mesothelial proliferation in animal models that are more relevant to humans.
The IPS/intratracheal instillation is a widely used method to evaluate the respiratory toxicity of particles. It should be noted that IPS/intratracheal instillation is a non‐physiological method and possibly affects the migration and distribution of particles in the lung due to the pressure from spraying. However, IPS/intratracheal instillation is relevant for identifying factors to be examined using long‐term, more physiologically relevant methods of CNT administration.
In summary, MWCNT and CRO fibers were found to translocate from the lung to the pleural cavity after intrapulmonary administration. Importantly, MWCNT and CRO treatment caused visceral mesothelial cell proliferation and inflammation in the pleural cavity. This mesothelial proliferation was plausibly induced by inflammatory events in the lung and pleural cavity and mediated primarily by macrophages. The similarity between MWCNT‐N, MWCNT‐M and CRO in translocation to the pleural cavity, induction of pleural cavity inflammation and induction of visceral pleural mesothelial proliferation suggests that MWCNT might cause asbestos‐like pleural lesions.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Characterization of multi‐walled carbon nanotubes and crocidolite fibers in the suspensions.
Fig. S2. SEM observation of multi‐walled carbon nanotubes and crocidolite fibers in the visceral pleura.
Fig. S3. Inflammation and fibrosis in the lung.
Fig. S4. Cytotoxicity of multi‐walled carbon nanotubes and crocidolite to TCC‐MESO1 cells in vitro.
Acknowledgments
This work was supported by Health and Labour Sciences Research Grants (Research on Risk of Chemical Substance 21340601) (grant numbers H19‐kagaku‐ippan‐006, H22‐kagaku‐ippan‐005).
(Cancer Sci 2012; 103: 2045–2050)
References
- 1. Bonner JC. Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc Am Thorac Soc 2010; 7: 138–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donaldson K, Murphy FA, Duffin R et al Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol 2010; 7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnston HJ, Hutchison GR, Christensen FM et al A critical review of the biological mechanisms underlying the in vivo and in vitro toxicity of carbon nanotubes: the contribution of physico‐chemical characteristics. Nanotoxicology 2010; 4: 207–46. [DOI] [PubMed] [Google Scholar]
- 4. Nagai H, Toyokuni S. Biopersistent fiber‐induced inflammation and carcinogenesis: lessons learned from asbestos toward safety of fibrous nanomaterials. Arch Biochem Biophys 2010; 502: 1–7. [DOI] [PubMed] [Google Scholar]
- 5. Pacurari M, Castranova V, Vallyathan V. Single‐ and multi‐wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A 2010; 73: 378–95. [DOI] [PubMed] [Google Scholar]
- 6. Tsuda H, Xu J, Sakai Y et al Toxicology of engineered nanomaterials – a review of carcinogenic potential. Asian Pac J Cancer Prev 2009; 10: 975–80. [PubMed] [Google Scholar]
- 7. Barrett JC. Cellular and molecular mechanisms of asbestos carcinogenicity: implications for biopersistence. Environ Health Perspect 1994; 102 (Suppl 5): 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller BG, Searl A, Davis JM et al Influence of fibre length, dissolution and biopersistence on the production of mesothelioma in the rat peritoneal cavity. Ann Occup Hyg 1999; 43: 155–66. [PubMed] [Google Scholar]
- 9. Okada F. Beyond foreign‐body‐induced carcinogenesis: impact of reactive oxygen species derived from inflammatory cells in tumorigenic conversion and tumor progression. Int J Cancer 2007; 121: 2364–72. [DOI] [PubMed] [Google Scholar]
- 10. Stanton MF, Wrench C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst 1972; 48: 797–821. [PubMed] [Google Scholar]
- 11. Walker C, Everitt J, Barrett JC. Possible cellular and molecular mechanisms for asbestos carcinogenicity. Am J Ind Med 1992; 21: 253–73. [DOI] [PubMed] [Google Scholar]
- 12. Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol 2008; 9: 147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poland CA, Duffin R, Kinloch I et al Carbon nanotubes introduced into the abdominal cavity of mice show asbestos‐like pathogenicity in a pilot study. Nat Nanotechnol 2008; 3: 423–8. [DOI] [PubMed] [Google Scholar]
- 14. Sakamoto Y, Nakae D, Fukumori N et al Induction of mesothelioma by a single intrascrotal administration of multi‐wall carbon nanotube in intact male Fischer 344 rats. J Toxicol Sci 2009; 34: 65–76. [DOI] [PubMed] [Google Scholar]
- 15. Takagi A, Hirose A, Nishimura T et al Induction of mesothelioma in p53+/− mouse by intraperitoneal application of multi‐wall carbon nanotube. J Toxicol Sci 2008; 33: 105–16. [DOI] [PubMed] [Google Scholar]
- 16. Takagi A, Hirose A, Futakuchi M et al Dose‐dependent mesothelioma induction by intraperitoneal administration of multi‐wall carbon nanotubes in p53 heterozygous mice. Cancer Sci 2012; 103: 1440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryman‐Rasmussen JP, Cesta MF, Brody AR et al Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol 2009; 4: 747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mercer RR, Hubbs AF, Scabilloni JF et al Distribution and persistence of pleural penetrations by multi‐walled carbon nanotubes. Part Fibre Toxicol 2010; 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy FA, Poland CA, Duffin R et al Length‐dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol 2011; 178: 2587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oka Y, Mitsui M, Kitahashi T et al A reliable method for intratracheal instillation of materials to the entire lung in rats. J Toxicol Pathol 2006; 19: 107–9. [Google Scholar]
- 21. Jackson P, Hougaard KS, Boisen AM et al Pulmonary exposure to carbon black by inhalation or instillation in pregnant mice: effects on liver DNA strand breaks in dams and offspring. Nanotoxicology 2012; 6: 486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morimoto Y, Hirohashi M, Ogami A et al Pulmonary toxicity of well‐dispersed multi‐wall carbon nanotubes following inhalation and intratracheal instillation. Nanotoxicology 2012; 6: 587–99. [DOI] [PubMed] [Google Scholar]
- 23. Ogami A, Yamamoto K, Morimoto Y et al Pathological features of rat lung following inhalation and intratracheal instillation of C(60) fullerene. Inhal Toxicol 2011; 23: 407–16. [DOI] [PubMed] [Google Scholar]
- 24. Xu J, Futakuchi M, Iigo M et al Involvement of macrophage inflammatory protein 1alpha (MIP1alpha) in promotion of rat lung and mammary carcinogenic activity of nanoscale titanium dioxide particles administered by intra‐pulmonary spraying. Carcinogenesis 2010; 31: 927–35. [DOI] [PubMed] [Google Scholar]
- 25. Yanagihara K, Tsumuraya M, Takigahira M et al An orthotopic implantation mouse model of human malignant pleural mesothelioma for in vivo photon counting analysis and evaluation of the effect of S‐1 therapy. Int J Cancer 2010; 126: 2835–46. [DOI] [PubMed] [Google Scholar]
- 26. Adamson IY, Bakowska J, Bowden DH. Mesothelial cell proliferation after instillation of long or short asbestos fibers into mouse lung. Am J Pathol 1993; 142: 1209–16. [PMC free article] [PubMed] [Google Scholar]
- 27. Sekhon H, Wright J, Churg A. Effects of cigarette smoke and asbestos on airway, vascular and mesothelial cell proliferation. Int J Exp Pathol 1995; 76: 411–8. [PMC free article] [PubMed] [Google Scholar]
- 28. Adamson IY, Prieditis H, Young L. Lung mesothelial cell and fibroblast responses to pleural and alveolar macrophage supernatants and to lavage fluids from crocidolite‐exposed rats. Am J Respir Cell Mol Biol 1997; 16: 650–6. [DOI] [PubMed] [Google Scholar]
- 29. Li XY, Lamb D, Donaldson K. Mesothelial cell injury caused by pleural leukocytes from rats treated with intratracheal instillation of crocidolite asbestos or Corynebacterium parvum . Environ Res 1994; 64: 181–91. [DOI] [PubMed] [Google Scholar]
- 30. Murphy FA, Schinwald A, Poland CA et al The mechanism of pleural inflammation by long carbon nanotubes: interaction of long fibres with macrophages stimulates them to amplify pro‐inflammatory responses in mesothelial cells. Part Fibre Toxicol 2012; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mutsaers SE, Whitaker D, Papadimitriou JM. Stimulation of mesothelial cell proliferation by exudate macrophages enhances serosal wound healing in a murine model. Am J Pathol 2002; 160: 681–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lechner JF, LaVeck MA, Gerwin BI et al Differential responses to growth factors by normal human mesothelial cultures from individual donors. J Cell Physiol 1989; 139: 295–300. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Faux SP, Hallden G et al Interleukin‐1beta and tumour necrosis factor‐alpha promote the transformation of human immortalised mesothelial cells by erionite. Int J Oncol 2004; 25: 173–8. [PubMed] [Google Scholar]
- 34. Choe N, Tanaka S, Xia W et al Pleural macrophage recruitment and activation in asbestos‐induced pleural injury. Environ Health Perspect 1997; 105(Suppl 5): 1257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viallat JR, Raybuad F, Passarel M et al Pleural migration of chrysotile fibers after intratracheal injection in rats. Arch Environ Health 1986; 41: 282–6. [DOI] [PubMed] [Google Scholar]
- 36. Kohyama N, Suzuki Y. Analysis of asbestos fibers in lung parenchyma, pleural plaques, and mesothelioma tissues of North American insulation workers. Ann N Y Acad Sci 1991; 643: 27–52. [DOI] [PubMed] [Google Scholar]
- 37. Miserocchi G, Sancini G, Mantegazza F et al Translocation pathways for inhaled asbestos fibers. Environ Health 2008; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. NIOSH . Occupational exposure to carbon nanotubes and nanofibers. Curr Intelligence Bull 2010; 161‐A: 1–149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Characterization of multi‐walled carbon nanotubes and crocidolite fibers in the suspensions.
Fig. S2. SEM observation of multi‐walled carbon nanotubes and crocidolite fibers in the visceral pleura.
Fig. S3. Inflammation and fibrosis in the lung.
Fig. S4. Cytotoxicity of multi‐walled carbon nanotubes and crocidolite to TCC‐MESO1 cells in vitro.


