Abstract
Angiogenesis plays an important role in tumor progression. Several reports have demonstrated that a disintegrin and metalloproteinase with thrombospondin motifs1 (ADAMTS1) inhibited angiogenesis via multiple mechanisms. The aim of this study was to investigate the effect of ADAMTS1 on endothelial cells in vitro and on tumor growth with regard to angiogenesis in vivo. We examined the effects of the transfection of ADAMTS1 using two constructs, full‐length ADAMTS1 (full ADAMTS1) and catalytic domain‐deleted ADAMTS1 (delta ADAMTS1). Transfection of both the full ADAMTS1 and delta ADAMTS1 gene constructs demonstrated the secretion of tagged‐ADAMTS1 protein into the conditioned medium, so we examined the effects of ADAMTS1‐containing conditioned medium on endothelial cells. Both types of conditioned media inhibited endothelial tube formation, and this effect was completely abolished after immunoprecipitation of the secreted protein from the medium. Both types of conditioned media also inhibited endothelial cell migration and proliferation. We then examined the impact of ADAMTS1 on endothelial cell apoptosis. Both conditioned media increased the number of Annexin V‐positive endothelial cells and caspase‐3 activity and this effect was attenuated when z‐vad was added. These results indicated that ADAMTS1 induced endothelial cell apoptosis. We next examined the effects of ADAMTS1 gene transfer into tumor‐bearing mice. Both full ADAMTS1 and delta ADAMTS1 significantly inhibited the subcutaneous tumor growth. Collectively, our results demonstrated that ADAMTS1 gene transfer inhibited angiogenesis in vitro and in vivo, likely as a result of the induction of endothelial cell apoptosis by ADAMTS1 that occurs independent of the protease activity.
Angiogenesis, the formation of new blood vessels from pre‐existing capillaries, is observed under various pathophysiological conditions (e.g. wound healing, diabetic retinopathy and tumor growth).1 Neovascularization consists of endothelial cell migration, proliferation and new network formation. Tumors require a substantial blood supply, and promote angiogenesis when they grow. Avascular tumors have a severely restricted growth potential. As a result, anti‐angiogenic treatments are thus considered to be potentially useful for cancer therapy.2, 3 To date, many anti‐angiogenic therapies have been designed to inhibit tumor growth as well as cancer cell dissemination.4, 5
A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) is a newly categorized MMP, which has multiple domains, including propeptide, metalloproteinase, disintegrin‐like and spacer‐region domains, and domains containing thrombospondin (TSP‐1) type Ι motifs.6 ADAMTSs have diverse functions, such as pro‐collagen processing7 and cleavage of Von Willebrand factor.8, 9 One of the best characterized biological functions of ADAMTS is proteolytic activity against extracellular matrix proteins, including proteoglycans.10, 11, 12, 13
ADAMTS1 is the first member of the ADAMTS family that was identified, and has been shown to degrade extracellular matrix proteins such as versican and aggrecan.11, 14 The recombinant ADAMTS1 protein has anti‐angiogenic properties.15 These reports supported the observed inhibitory effect of ADAMTS1 on endothelial cell growth and angiogenesis; however, whether ADAMTS1 inhibits tumor progression remains controversial.16, 17, 18, 19 Accordingly, we investigated the effect of ADAMTS1 on both endothelial cell apoptosis and tumor growth using two different constructs, a full‐length ADAMTS1 and a metalloproteinase‐deleted ADAMTS1.
Materials and Methods
ADAMTS1 expression construct and catalytic domain deletion construct
The sequence encoding ADAMTS1 was amplified by PCR using the KIAA1344 plasmid (Kazusa DNA Institute, Chiba, Japan) as a template. We designed to fuse ADAMTS1 to the COOH‐terminal v5 epitope tag present in the expression vector (pcDNA/v5‐D‐TOPO; Invitrogen, Carlsbad, CA, USA) and this construct was designated “full ADAMTS1”. The signal peptide and FLAG epitope tag containing a mammalian expression vector with the CMV promoter was a kind gift from Dr BG Hudson and Dr V Pedchenko.20 The catalytic domain deletion construct of ADAMTS1 cDNA with the FLAG epitope tag (delta ADAMTS1) was generated as detailed in Figure 1. The sequence of each construct was determined to confirm that no amino acid mutations had been caused by the PCR reaction prior to the study.
Figure 1.
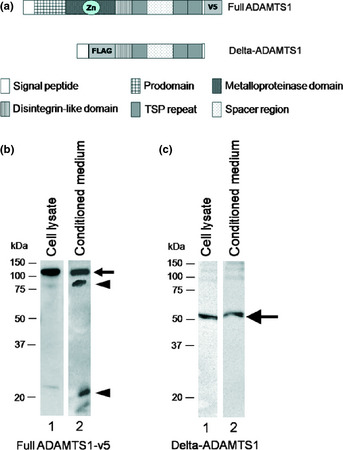
(a) Demonstration of the domain structure of full a disintegrin and metalloproteinase with thrombospondin motifs1 (ADAMTS1) and its deletion construct used in the experiments. (b) Cell lysates (lane 1) and conditioned medium (lane 2) derived from full ADAMTS1 transfectants were analyzed. The cell lysates contained pro‐ADAMTS1 protein (arrow), and conditioned medium contained two additional proteins (arrowheads). The arrowheads indicate mature ADAMTS1 and C‐terminal fragment ADAMTS1 produced by its autocatalytic activity, as previously reported. (c) The cell lysates (lane 1) from delta ADAMTS1 transfectants and conditioned medium (lane 2) derived from delta ADAMTS1 transfectants.
Cell lines and cultures
Human umbilical vein endothelial cells (HUVEC) were purchased from Kurabo (Osaka, Japan) and cultured in HuMedia‐EBM2 (Kurabo) with a kit containing 2% FBS, growth factors, 100 U/mL penicillin, and 100 μg/mL streptomycin, as previously described.5 COS‐7 cells were purchased from ECACC (Dainippon Sumitomo Pharmaceutical, Osaka, Japan). Rat smooth muscle cells (SMCs) and human skin fibroblasts (HSF) were obtained as previously reported.5 Human fibrosarcoma HT1080 cells, human prostate cancer DU145 cells, and Chinese hamster ovarian (CHO)‐K1 cells, were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells at passage 3–6 were used for all experiments. The SMC, HSF, HT1080, DU145 and CHO‐K1 cells were cultured in DMEM with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were cultured at 37°C under 5% CO2 and 20% O2 in a humidified chamber.
Transfection and preparation of conditioned media
Transient transfection was performed as previously described5, 21 with slight modifications. For the COS‐7 cells, lipofectamine 2000 (Invitrogen) was used for transfection according to the manufacturer's protocol. We renewed the DNA‐containing medium 6 and 24 h after transfection. After 48 h, the medium was changed, and we collected the conditioned medium after 72 h.
The cells were washed with PBS twice, and then proteins were extracted with cell lysis buffer as previously reported.22, 23 Alternatively, the produced tagged protein was depleted from the conditioned medium by immunoprecipitation of the tagged protein with either an anti‐v5 or anti‐FLAG M2 affinity gel (Sigma, St. Louis, MO, USA) as previously described.5 Briefly, conditioned media was incubated with affinity gel for overnight at 4°C. Supernatant after centrifugation, in which tagged‐protein was no longer detectable, was used as “conditioned medium post‐IP” in further experiments. For the transient transfection for the HUVEC, electroporation with microporator (BMS‐MP‐100; Microporator, Seoul, Korea) was performed as previously described.24 Briefly, cells were removed from the plate with trypsin, centrifuged, and dissolved in serum‐free medium. Cell and constructs were mixed into the buffer and incubated for 5 min on ice, then exposed to electricity (1000 V 30 mS, 2‐pulse). Cells were plated at a density of 1.0 × 105 cells in 96‐well dishes with 100 μL culture medium without antibiotics. After seeding, cells were allowed to recover for 24 h. The medium was then changed and cells were used for the analysis.
Western blot analysis and antibodies
The expression of full ADAMTS1 and delta ADAMTS1 was examined by a Western blot analysis, as described previously.21, 25 Briefly, 20 μL of each sample was mixed with 6× Laemmli sample buffer (60 mM Tris‐HCl [pH 6.8], 2% SDS, 5% mercaptoethanol, 10% glycerol) and subjected to SDS‐PAGE. After electrophoresis, proteins were transferred to PVDF membranes (Advantech, Tokyo, Japan) using transfer buffer, and then the membranes were blocked for 1 h in 5% nonfat dried milk in PBS. The membranes were hybridized at room temperature (RT) for 1 h with a mouse anti‐v5 antibody (Invitrogen) or an anti‐FLAG M2 monoclonal antibody (Sigma). After stringent washing with PBS containing 0.05% Tween 20 (PBS‐T) three times for 5 min each at RT, the membranes were incubated with the appropriate secondary antibodies (ICN Pharmaceuticals, Aurora, OH, USA). Following three successive washes with PBS‐T, immunoreactive bands were visualized using the enhanced chemiluminescence system (ECL; Amersham Biosciences, Piscataway, NJ, USA).
Endothelial tube formation assay
The endothelial tube formation assay was performed as previously described.5 Briefly, 100 μL of Matrigel (Becton Dickinson, Franklin Lakes, NJ, USA) was applied to 96‐well plates, and a suspension of 7.5 × 103 of HUVEC in 50 μL EBM2 was seeded into each well, and then 50 μL of each conditioned medium (n = 12 each) was added per well. The cells were incubated for 24 h at 37°C and viewed using an IX71 microscope (Olympus, Tokyo, Japan). The length of tube formations per well (magnification at 40×) was measured using the Scion Image software (Scion Corporation, Frederick, MD, USA) program by two investigators blinded to the experimental protocols, and the recorded lengths were averaged.
Cell migration assays
The HUVEC migration was assayed as described previously.5, 26 In brief, confluent cells in 48‐well plates were scratched with a sterile pipette tip. The cells were washed twice with EBM2 to remove cellular debris. After removal of the cellular debris, 100 μL EBM2 with 2% FBS and 100 μL of each conditioned medium were added to each well. The HUVEC were then incubated at 37°C for 24 h. The cell migration assays were also performed for SMC, HSF and CHO‐K1 cells using the same method. After 24 h in culture, the cells were fixed. The cells that had migrated into the denuded area were photographed with a CCD camera and counted with the Scion Image software program.
Cell proliferation assays
Cell proliferation was examined using a CellTiter AQueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. Briefly, a suspension of HUVEC, SMC, HSF and CHO‐K1 cells (1 × 104 cells/well, passages 3–5) in 50 μL EBM2 with 2% FBS was added to 96‐well plates, and supplemented with 50 μL of each conditioned medium. After incubation for 24 h or the time indicated, the cell viability was measured as described.27, 28 Z‐vad (Peptide Institute, Osaka, Japan) was used to examine whether caspase is involved in this affect. All samples were read in triplicate.
Flow cytometric analysis
An Annexin V Assay Kit (MBL, Nagoya, Japan) was used according to the manufacturer's instructions. Briefly, HUVEC, SMC, HSF and CHO‐K1 cells (5 × 105 cells/well) were seeded into 6‐well plates in 2 mL EBM2 with 2% FBS. The following day, the medium was changed to 1 mL fresh medium containing 2% FBS plus 1 mL conditioned medium. After 24 h in culture, the attached cells were trypsinized and centrifuged at 3000g for 5 min at 4°C. The cells were then washed in PBS and resuspended in binding buffer (10 mM HEPES/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Five microliters of Annexin V conjugated with biotin and propidium iodide were added, and the cells were incubated in a darkroom for 15 min. The cells were washed again in PBS and then were resuspended in binding buffer. After centrifugation at 3000g for 5 min at 4°C, the cells were fixed with 2% formaldehyde and washed with PBS. After centrifugation again, the cells were washed with PBS, and streptavidin‐conjugated FITC (DAKO, Glostrup, Denmark) was added, followed by incubation for 15 min at RT. The cells were then washed with PBS again and resuspended in 300 μL PBS. Annexin V‐FITC‐labeled cells were counted using a FACScaliber flow cytometer (Becton Dickinson). The data were then analyzed using the Cell Quest software program (Becton Dickinson).
Caspase 3/7 assay
The caspase 3/7 activity levels were measured using an assay kit (Caspase‐Glo3/7 Assay; Promega) according to the manufacturer's instructions. Briefly, HUVEC (1 × 104/well) were seeded into 96‐well plates in 100 μL EBM2 with 2% FBS. The following day, the medium was removed, and 50 μL fresh EBM2 containing 2% FBS plus 50 μL conditioned medium was added. After a 24‐h culture, the caspase 3/7 activities were measured using a plate‐reading luminometer (Dainippon Sumitomo Pharma).
In vivo tumor studies
All protocols involving experimental animals followed the local institutional guidelines for animal care, which are comparable to those described in the “Guide for the Care and Use of Laboratory Animals” published by the Institute for Laboratory Animal Research (National Institutes of Health Publication No. 85‐23, revised 1996). HT1080, DU145 and CHO‐K1 cells (1.0 × 106 cells in 50 μL serum‐free DMEM and 50 μL Matrigel) were inoculated subcutaneously into the right flank of 5‐ to 7‐week‐old male BALB/c nude mice (Charles River Japan, Yokohama, Japan) (five mice per treatment group) and each tumor growth was monitored with Vernier calipers every day, as described previously.29 The volume was calculated according to the formula (volume = length × width2 × 0.52).5, 30 When the tumors reached a mean volume of 30–60 mm3, treatment was initiated. The full ADAMTS1 cDNA‐containing plasmid (10 μg) or delta ADAMTS1 cDNA‐containing plasmid (10 μg) was mixed with 10 μL cationic lipid (DMRIEC; Invitrogen) and then dissolved in 100 μL OPTIMEM‐1 (Gibco BRL, Grand Island, NY, USA). Approximately 20 μL of the mixture was then directly injected into the tumor every 24 h for 7 days. The CMV/FLAG empty vector (10 μg) was used as a negative control. Mice were killed by cervical dislocation the day after the final treatment. The tumors were removed, snap frozen in liquid N2, and embedded in OCT compound (Sakura, Tokyo, Japan).
Immunohistochemical staining
Frozen sections (8 μm) were placed on silane‐coated glass slides (DakoCytomation, Kyoto, Japan) for histological studies, as previously described.31 Blood vessels were detected by immunohistological staining using an anti‐CD31 antibody (used at 1:100 dilution; BD Pharmingen, Franklin Lakes, NJ, USA). Immunoperoxidase staining was carried out using a VECTASTAIN ABC Kit (Vector Laboratories, Burlingame, CA, USA). The number of the vessels in the tumors was measured as described previously.5 Anti‐Cleaved Caspase‐3 (Asp175) antibody (used at 1:100 dilution; Cell Signaling Technology, Danvers, MA, USA) and anti‐CD31 antibody were used for immunofluorescent staining as previously described.32 The tumor section was incubated with two antibodies overnight at 4°C and then incubated with the secondary antibodies (Alexa Fluor 488‐conjugated anti‐rabbit IgG and Cy3‐conjugated anti‐rat IgG, respectively) at appropriate dilution. The immunofluorescent signals were observed with an LSM510 confocal microscope (Carl Zeiss Japan, Tokyo, Japan) at the Central Research Laboratory, Okayama University Medical School. All images were processed using Adobe Photoshop CS2. All experiments were repeated using three animals, in which we obtained similar results.
Statistical analysis
The statistical analysis was performed using the unpaired Student's t‐test. P < 0.05 was considered to be significant.
Results
Expression of human ADAMTS1 and its fragments
We designed two constructs, full‐length ADAMTS1 with a C‐terminal v5‐tag (full ADAMTS1) and a catalytic domain‐deleted construct (delta ADAMTS1; Fig. 1a). We first transfected these constructs into COS‐7 cells and collected the conditioned medium 72 h after transfection. The fusion proteins with full ADAMTS1 and delta ADAMTS1 were detected by a Western blot analysis (Fig. 1b,c, respectively). Transiently produced proteins from both constructs were detected in the conditioned medium, as well as in cell extracts, in our system. Full ADAMTS1 transfectants showed three major bands in the conditioned medium that consisted of the unprocessed form of ADAMTS1 and its processed forms (Fig. 1b). The lowest band (approximately 22 kDa) was considered to be a proteolytic C‐terminal fragment based on a previous reported.33 The protein in the delta ADAMTS1‐transfected conditioned medium was detected as a single band in conditioned medium and cell extracts (Fig. 1c).
Effect of the conditioned medium on endothelial tube formation
To test the effects of the transfected cell conditioned medium on endothelial cell tube formation, HUVEC were cultured on Matrigel in a mixture of fresh EB2 and conditioned medium, as described in the Materials and Methods. Representative results of the endothelial tube formation assay are shown in Figure 2. Both types of conditioned media significantly inhibited the tube formation of HUVEC compared with the control (Fig. 2a,c). These results indicated that both types of conditioned medium contained factors that effectively inhibited the endothelial cell tube formation. When we depleted v5‐ or FLAG‐tagged fragments from the conditioned medium by immunoprecipitation with an anti‐v5 or an anti‐FLAG agarose affinity gel, the inhibitory effects on tube formation were abolished (Fig. 2b,d,f). These results indicate that the effects of the conditioned media were due to the tagged proteins.
Figure 2.
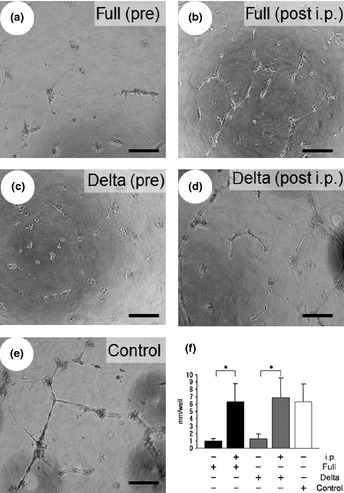
The tube formation assay was carried out with conditioned medium. The conditioned medium obtained from full a disintegrin and metalloproteinase with thrombospondin motifs1 (ADAMTS1)‐transfected cells before immunoprecipitation (a) and post‐immunoprecipitation (b). The conditioned medium obtained from delta ADAMTS1‐transfected cells before immunoprecipitation (c). The conditioned medium obtained from delta ADAMTS1‐transfected cells post‐immunoprecipitation (d) or from the control (e). (f) The length of tube branches in the lower field was measured. *P < 0.05. Each column represents the mean ± standard deviation (SD).
Inhibition of endothelial cell migration by ADAMTS1
The effects of ADAMTS1‐transfected conditioned medium on the migration of HUVEC are shown in Figure 3(a). Both types of conditioned media inhibited the migration of HUVEC, while the control (conditioned media from cells transfected with the empty vector) showed no effect on HUVEC migration. This inhibitory effect on migration appeared to be specific to endothelial cells, because neither type of conditioned medium affected the migration of SMC, HSF or CHO‐K1 cells compared with the control conditioned medium (Fig. 3b).
Figure 3.
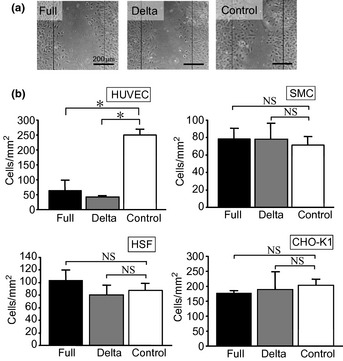
(a) The endothelial cells were incubated with conditioned medium and cell migration was monitored. (b) The inhibitory effect of each conditioned medium on cell migration was compared. Each column represents the mean ± standard deviation (SD) of triplicate samples. *P < 0.05 vs control. NS indicates that two groups were not significantly different.
Effects of ADAMTS1 on endothelial cell proliferation
We next examined the effects of the conditioned medium from ADAMTS1‐transfected cells on endothelial cell proliferation; both types of conditioned media inhibited HUVEC proliferation. As shown in Figure 4(a–d), ADAMTS1 inhibited the cell proliferation of HUVEC, but not SMC, HSF or CHO‐K1 cells. This effect was attenuated when Z‐VAD (a specific inhibitor of caspases) was added to the conditioned medium, indicating that this effect was mediated by caspases (Fig. 4e). Both types of conditioned media inhibited endothelial cell attachment to collagen I and laminin (Fig. S1). We further examined the effect of electroporation of ADAMTS1 on HUVEC proliferation. As shown in Figure 4(f), both full and delta ADAMTS1 inhibited endothelial cell proliferation.
Figure 4.
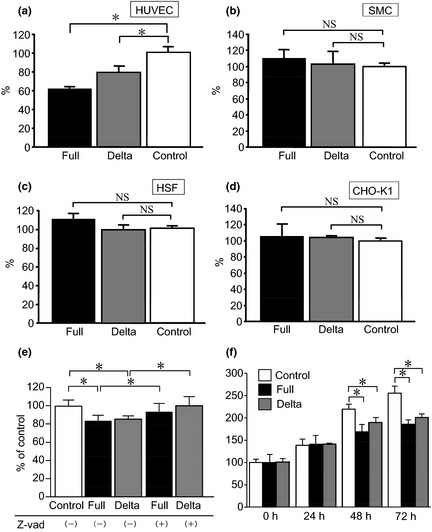
The inhibition of cell proliferation by transfected‐conditioned medium was monitored (a), human umbilical vein endothelial cells [HUVEC]; (b), smooth muscle cells [SMCs]; (c), human skin fibroblasts [HSF]; (d), Chinese hamster ovarian [CHO]‐K1). *P < 0.05 vs control. NS indicates that two groups were not significantly different. (e) The inhibitory effect of HUVEC proliferation by each conditioned medium was compared in the absence or the presence of Z‐vad. *P < 0.05. (f) The inhibition of endothelial cell proliferation by electroporation with each construct was examined. *P < 0.05 vs control. Each column represents the mean ± standard deviation (SD) of triplicate samples.
Apoptotic effects of ADAMTS1 on endothelial cells
To evaluate the apoptotic effects of ADAMTS1, we examined the Annexin V expression and caspase 3/7 activity. We found that the number of Annexin‐positive HUVEC was increased by both types of conditioned media compared to the control (Fig. 5a,b). Interestingly, both the full ADAMTS1 and delta ADAMTS1 constructs caused a distinct shift of the peak of Annexin V fluorescence after 24 h of co‐incubation only in endothelial cells, not in other types of cells, such as SMC, HSF and cancer cells (CHO‐K1 cells; Fig. 5a,b). Furthermore, both full ADAMTS1 and delta ADAMTS1 increased the caspase 3/7 activity compared with the control vector‐treated HUVEC (Fig. 5c). We also examined the effect of endothelial cell attachment incubated with each conditioned medium on extracellular matrix‐coated plates. As shown in Figure S1, both conditioned media attenuated HUVEC attachment on type I collagen and laminin, but not on fibronectin.
Figure 5.
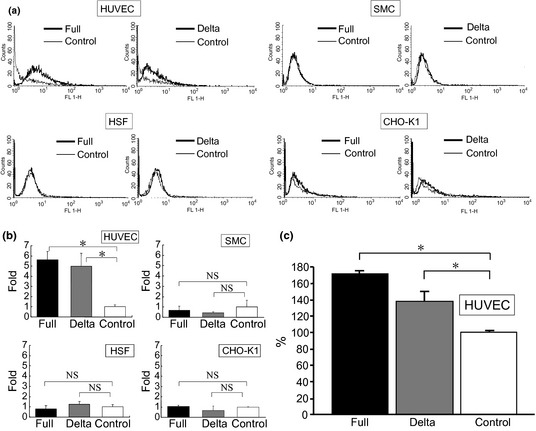
(a) The Annexin V expression was examined by fluorescence‐activated cell sorting (FACS) analysis. (b) The numbers of Annexin V‐positive cells after treatment with the conditioned medium obtained from both full a disintegrin and metalloproteinase with thrombospondin motifs1 (ADAMTS1) (full) and delta ADAMTS1 (delta)‐treated cells were compared with those after treatment with conditioned media obtained from control cells (control). *P < 0.05 vs control. NS indicates that two groups were not significantly different. (c) The caspase 3/7 activities in HUVEC treated with control medium were considered to be 100%. *P < 0.05 vs control.
Effects of ADAMTS1 and its deletion construct on tumor growth and angiogenesis
To assess the effects of ADAMTS1 gene therapy on tumor growth, HT1080, DU145 and CHO‐K1 cells were inoculated subcutaneously into nude mice. After the establishment of subcutaneous tumors, plasmids in DMRIEC were directly injected into tumors every 24 h for 7 days. An empty vector was used as a negative control. On the eighth day after starting the gene transfer, both the full ADAMTS1 and delta ADAMTS1 constructs significantly suppressed the growth of subcutaneous tumors compared with the control (Fig. 6a–f). We then examined the vessel distribution in tumors transfected with full ADAMTS1 (Fig. 7a) and delta ADAMTS1 (Fig. 7b), and compared them with cells treated with the empty vector (Fig. 7c) using an anti‐CD31 antibody. The empty vector‐treated implanted tumors were abundant in vessel formation, but the vessel formation was significantly decreased in tumors treated with the full ADAMTS1 or delta ADAMTS1 construct (Fig. 7d). Next we examined the apoptotic effect on the tumor vasculature by ADAMTS1. As shown in Figure 7(e–g), the cleaved‐caspase signals were observed in the tumor vasculature in the delta ADAMTS1‐treated mice. On the other hand, few signals for cleaved‐caspase were observed in the control mice (Fig. 7h), indicating that ADAMTS1 gene transfer induced apoptosis in the tumor vasculature in vivo. We also examined the transduced protein in tumor tissues. As shown in Figure S2(a–c), immunopositive signals for both ADAMTS1 and delta ADAMTS1 were detectable, while there was no signal in tumors treated with the empty vector (Fig. S2D). These results indicated that this tumor does not produce detectable levels of ADAMTS1, and that tumor growth could be reduced by ADAMTS1 gene transduction.
Figure 6.
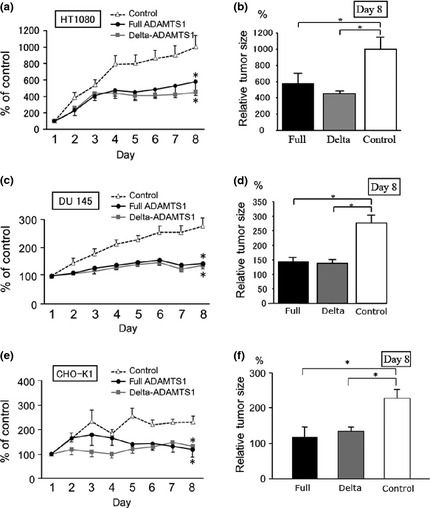
In the left column (a,c and e), the size of tumors was monitored every day until day 8 (mean ± standard error [SE]; n = 5). In the right column, the size of tumors on day 8 was compared with those in the control group (b,d and f). The size of each tumor on day 1 was considered to be 100% (a and b, HT1080; c and d, DU145; e and f, CHO‐K1.). *P < 0.05 vs control.
Figure 7.
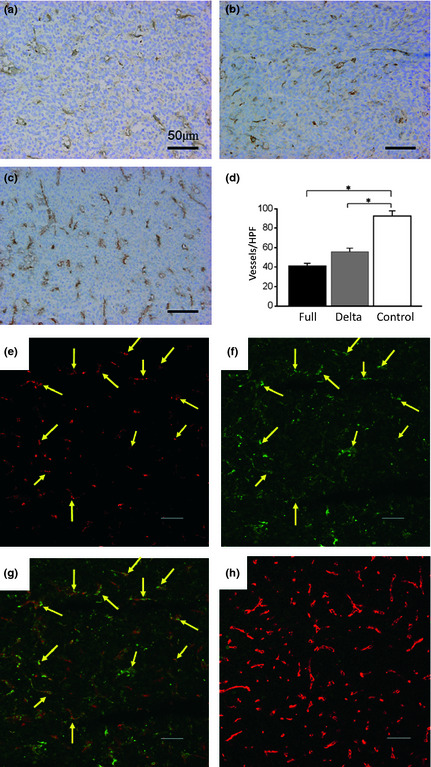
The vessel formation was examined by immunohistochemistry ([a], full‐ADAMTS1; [b], delta ADAMTS1 [a disintegrin and metalloproteinase with thrombospondin motifs1]; [c], empty vector). The number of blood vessels was counted (d). *P < 0.05 vs control. The apoptotic signals were observed in the ADAMTS1‐treated tumor vasculature (e–g). Sections of the tumor in delta ADAMTS1‐injected mice were doubly stained with anti‐CD31 antibody (Cy3; e) and anti‐Cleaved caspase‐3 antibody (fluorescein isothiocyanate [FITC]; f). (g) Merged images obtained with anti‐CD31 antibody (Cy3) and anti‐Cleaved caspase‐3 antibody (FITC). Arrows indicate the caspase‐3‐positive signals observed in the tumor vasculature. In contrast, sections of tumors injected with empty vector showed only negligible FITC signals (green) whereas Cy3 signals (red) were more abundant than in panel g. Scale bar in each panel represents 50 μm.
Discussion
In the present study, we demonstrated that ADAMTS1 inhibited angiogenesis in vitro and in vivo, which suggested that ADAMTS1 induces endothelial cell apoptosis. These inhibitory effects of ADAMTS1 could be achieved independently of its proteolytic activity.
The anti‐angiogenic properties of ADAMTS1 have been recently described. One of the most interesting properties of the gene is that it is located on chromosome 21, the locus for Down's syndrome. Reynolds et al.19 reported that tumor angiogenesis is inhibited in a mouse model of Down's syndrome that has three copies of the ADAMTS1 gene. Several reports have suggested that the proteolytic activity of the protein is key to its activity. For instance, ADAMTS1 cleaves large TSP1, and cleaved TSP‐1 demonstrates anti‐angiogenic activity.13 Iruela‐Arispe et al.34 reported that ADAMTS1 binds to the vascular endothelial growth factor (VEGF) receptor, which requires protease‐mediated activation. On the other hand, Fu et al.35 reported that the proteolytic fragment of versican produced by ADAMTS1 induced pathological angiogenesis. In addition, ADAMTS1 null mice demonstrated delayed lymphangiogenesis, thus indicating that ADAMTS1 plays a role in lymphangiogenesis.36 Interestingly, Luque et al.37 reported that the C‐terminal half of ADAMTS1 binds to VEGF and affects VEGFR2 phosphorylation and endothelial cell proliferation. Rodriguez‐Manzaneque et al.33 reported that deletion of the last two TSP1 motifs reduced the suppression of endothelial cell proliferation. Together, these data indicate that the role of ADAMTS1 in angiogenesis is diverse, and that further studies are needed to clarify the numerous roles of the protein. Our present results also demonstrated that the inhibitory effects of ADAMTS1 on endothelial cells are protease activity‐independent. We thus confirmed that ADAMTS1 has inhibitory effects on endothelial cells via a proteolytic activity‐independent mechanism, and we suggest that the C‐terminal half of ADAMTS1 has a crucial role in this activity.
We herein demonstrated that ADAMTS1 plays a role in apoptosis in endothelial cells in vitro and in vivo. As reported for other anti‐angiogenic molecules, such as TSP‐1,38, 39 ADAMTS1 induced apoptotic changes in endothelial cells, as indicated by the detection of Annexin V expression and increased caspase 3/7 activity (Fig. 5c). Furthermore, z‐vad attenuated the effect of ADAMTS1, indicating the involvement of caspase signaling (Fig. 4e). This effect was observed with both constructs, indicating that the proteolytic activity is not required for this effect. It should be noted that ADAMTS1 did not affect the viability of CHO‐K1 cells, but did inhibit the growth of tumors resulting from implanted CHO‐K1 cells. Interestingly, the inhibition of proliferation was observed in endothelial cells, but not in other types of cells, including smooth muscle cells, fibroblasts and CHO‐K1 cells. ADAMTS1 also induced apoptosis in endothelial cells, but not in CHO‐K1 cells, as indicated by the changes in Annexin V expression. We therefore believe that the tumor inhibition, which was accompanied by a decrease in vessel density that was induced by ADAMTS1 was largely due to the effect of the protein on endothelial cell apoptosis.
There is a limitation of this study. It is not clear whether there are specific receptor(s) for ADAMTS1 on endothelial cells. As shown in this study, ADAMTS1 attenuated endothelial cell attachment (Fig. S1), suggesting that ADAMTS1 induced anoikis on endothelial cells. Because of multiple functions of ADAMTS1 on inhibiting endothelial cells, apoptotic effect by ADAMTS1 is complex and we suggest that anoikis may be a part of this effect.
In summary, we demonstrated the anti‐tumorigenic effect by ADAMTS1 gene transfer that is accompanied by the inhibition of tumor angiogenesis.
Disclosure Statement
The authors have no conflict of interest.
Supporting information
Fig. S1. Inhibition of endothelial attachment by ADAMTS1.
Fig. S2. Distribution of ADAMTS1 gene transfer into tumors.
Acknowledgements
This work was supported in part by a grant‐in‐aid (23390366 and 23112511 to S.H.) for Scientific Research from the Japan Society for the Promotion of Science. The authors are grateful to Drs Toshitaka Oohasi, Tomoko Yonezawa and Satoshi Hirakawa and the other members of our department for stimulating discussions and suggestions. We thank Dr Billy G Hudson and Dr Vadim Pedchenko for providing the vector.
(Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02381.x, 2012)
References
[Correction added on 5 October 2012, after first online publication:
Reference 26 “Kim JS, Chang JH, Yu HK et al. Inhibition of angiogenesis and angiogenesis‐dependent tumor growth by the cryptic kringle fragments of human apolipoprotein(a). J Biol Chem 2003; 278: 29000–8.” was incorrect and has been replaced completely.
Reference 32 “Miyoshi T, Doi M, Hirohata S et al. Olmesartan reduces arterial stiffness and serum adipocyte fatty acid‐binding protein in hypertensive patients. Heart Vessels 2011; 26: 408–13.” has been removed.
References 33 to 40 have been re‐numbered to 32 to 39 respectively in the Reference List and in‐text citation.]
- 1. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407: 249–57. [DOI] [PubMed] [Google Scholar]
- 2. Benouchan M, Colombo BM. Anti‐angiogenic strategies for cancer therapy (Review). Int J Oncol 2005; 27: 563–71. [PubMed] [Google Scholar]
- 3. Folkman J. Angiogenesis. Annu Rev Med 2006; 57: 1–18. [DOI] [PubMed] [Google Scholar]
- 4. O'Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 1996; 2: 689–92. [DOI] [PubMed] [Google Scholar]
- 5. Miyoshi T, Hirohata S, Ogawa H et al Tumor‐specific expression of the RGD‐alpha3(IV)NC1 domain suppresses endothelial tube formation and tumor growth in mice. FASEB J 2006; 20: 1904–6. [DOI] [PubMed] [Google Scholar]
- 6. Apte SS. A disintegrin‐like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family. Int J Biochem Cell Biol 2004; 36: 981–5. [DOI] [PubMed] [Google Scholar]
- 7. Fernandes RJ, Hirohata S, Engle JM et al Procollagen II amino propeptide processing by ADAMTS‐3. Insights on dermatosparaxis. J Biol Chem 2001; 276: 31502–9. [DOI] [PubMed] [Google Scholar]
- 8. Dong JF, Moake JL, Nolasco L et al ADAMTS‐13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood 2002; 100: 4033–9. [DOI] [PubMed] [Google Scholar]
- 9. Lammle B, Kremer Hovinga JA, Alberio L. Thrombotic thrombocytopenic purpura. J Thromb Haemost 2005; 3: 1663–75. [DOI] [PubMed] [Google Scholar]
- 10. Tortorella MD, Burn TC, Pratta MA et al Purification and cloning of aggrecanase‐1: a member of the ADAMTS family of proteins. Science 1999; 284: 1664–6. [DOI] [PubMed] [Google Scholar]
- 11. Kuno K, Okada Y, Kawashima H et al ADAMTS‐1 cleaves a cartilage proteoglycan, aggrecan. FEBS Lett 2000; 478: 241–5. [DOI] [PubMed] [Google Scholar]
- 12. Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS‐1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem 2003; 278: 42330–9. [DOI] [PubMed] [Google Scholar]
- 13. Lee NV, Sato M, Annis DS et al ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J 2006; 25: 5270–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sandy JD, Westling J, Kenagy RD et al Versican V1 proteolysis in human aorta in vivo occurs at the Glu441‐Ala442 bond, a site that is cleaved by recombinant ADAMTS‐1 and ADAMTS‐4. J Biol Chem 2001; 276: 13372–8. [DOI] [PubMed] [Google Scholar]
- 15. Vazquez F, Hastings G, Ortega MA et al METH‐1, a human ortholog of ADAMTS‐1, and METH‐2 are members of a new family of proteins with angio‐inhibitory activity. J Biol Chem 1999; 274: 23349–57. [DOI] [PubMed] [Google Scholar]
- 16. Kuno K, Bannai K, Hakozaki M, Matsushima K, Hirose K. The carboxyl‐terminal half region of ADAMTS‐1 suppresses both tumorigenicity and experimental tumor metastatic potential. Biochem Biophys Res Commun 2004; 319: 1327–33. [DOI] [PubMed] [Google Scholar]
- 17. Rocks N, Paulissen G, Quesada‐Calvo F et al ADAMTS‐1 metalloproteinase promotes tumor development through the induction of a stromal reaction in vivo . Cancer Res 2008; 68: 9541–50. [DOI] [PubMed] [Google Scholar]
- 18. Casal C, Torres‐Collado AX, Plaza‐Calonge Mdel C et al ADAMTS1 contributes to the acquisition of an endothelial‐like phenotype in plastic tumor cells. Cancer Res 2010; 70: 4676–86. [DOI] [PubMed] [Google Scholar]
- 19. Reynolds LE, Watson AR, Baker M et al Tumour angiogenesis is reduced in the Tc1 mouse model of Down's syndrome. Nature 2010; 465: 813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pedchenko V, Zent R, Hudson BG. Alpha(v)beta3 and alpha(v)beta5 integrins bind both the proximal RGD site and non‐RGD motifs within noncollagenous (NC1) domain of the alpha3 chain of type IV collagen: implication for the mechanism of endothelia cell adhesion. J Biol Chem 2004; 279: 2772–80. [DOI] [PubMed] [Google Scholar]
- 21. Hirohata S, Wang LW, Miyagi M et al Punctin, a novel ADAMTS‐like molecule, ADAMTSL‐1, in extracellular matrix. J Biol Chem 2002; 277: 12182–9. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura K, Hirohata S, Murakami T et al Dynamic Induction of ADAMTS1 Gene in the Early Phase of Acute Myocardial Infarction. J Biochem (Tokyo) 2004; 136: 439–46. [DOI] [PubMed] [Google Scholar]
- 23. Demircan K, Hirohata S, Nishida K et al ADAMTS‐9 is synergistically induced by interleukin‐1beta and tumor necrosis factor alpha in OUMS‐27 chondrosarcoma cells and in human chondrocytes. Arthritis Rheum 2005; 52: 1451–60. [DOI] [PubMed] [Google Scholar]
- 24. Cilek MZ, Hirohata S, Faruk Hatipoglu O et al AHR, a novel acute hypoxia‐response sequence, drives reporter gene expression under hypoxia in vitro and in vivo . Cell Biol Int 2011; 35: 1–8. [DOI] [PubMed] [Google Scholar]
- 25. Sezaki S, Hirohata S, Iwabu A et al Thrombospondin‐1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp Biol Med (Maywood) 2005; 230: 621–30. [DOI] [PubMed] [Google Scholar]
- 26. Hatipoglu OF, Hirohata S, Cilek MZ et al ADAMTS1 is a unique hypoxic early response gene expressed by endothelial cells. J Biol Chem 2009; 284: 16325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun 1991; 3: 207–12. [DOI] [PubMed] [Google Scholar]
- 28. Nagata D, Mogi M, Walsh K. AMP‐activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem 2003; 278: 31000–6. [DOI] [PubMed] [Google Scholar]
- 29. Ito H, Kanzawa T, Miyoshi T et al Therapeutic efficacy of PUMA for malignant glioma cells regardless of p53 status. Hum Gene Ther 2005; 16: 685–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Furumatsu T, Yamaguchi N, Nishida K et al Endostatin inhibits adhesion of endothelial cells to collagen I via alpha(2)beta(1) integrin, a possible cause of prevention of chondrosarcoma growth. J Biochem 2002; 131: 619–26. [DOI] [PubMed] [Google Scholar]
- 31. Toeda K, Nakamura K, Hirohata S et al Versican is induced in infiltrating monocytes in myocardial infarction. Mol Cell Biochem 2005; 280: 47–56. [DOI] [PubMed] [Google Scholar]
- 32. Yonezawa T, Hattori S, Inagaki J et al Type IV collagen induces expression of thrombospondin‐1 that is mediated by integrin alpha1beta1 in astrocytes. Glia 2010; 58: 755–67. [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez‐Manzaneque JC, Milchanowski AB, Dufour EK, Leduc R, Iruela‐Arispe ML. Characterization of METH‐1/ADAMTS1 processing reveals two distinct active forms. J Biol Chem 2000; 275: 33471–9. [DOI] [PubMed] [Google Scholar]
- 34. Iruela‐Arispe ML, Carpizo D, Luque A. ADAMTS1: a matrix metalloprotease with angioinhibitory properties. Ann N Y Acad Sci 2003; 995: 183–90. [DOI] [PubMed] [Google Scholar]
- 35. Fu Y, Nagy JA, Brown LF et al Proteolytic cleavage of versican and involvement of ADAMTS‐1 in VEGF‐A/VPF‐induced pathological angiogenesis. J Histochem Cytochem 2011; 59: 463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brown HM, Dunning KR, Robker RL, Pritchard M, Russell DL. Requirement for ADAMTS‐1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol 2006; 300: 699–709. [DOI] [PubMed] [Google Scholar]
- 37. Luque A, Carpizo DR, Iruela‐Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem 2003; 278: 23656–65. [DOI] [PubMed] [Google Scholar]
- 38. Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis‐dependent inhibition of neovascularization by thrombospondin‐1. Nat Med 2000; 6: 41–8. [DOI] [PubMed] [Google Scholar]
- 39. Ren B, Yee KO, Lawler J, Khosravi‐Far R. Regulation of tumor angiogenesis by thrombospondin‐1. Biochim Biophys Acta 2006; 1765: 178–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Inhibition of endothelial attachment by ADAMTS1.
Fig. S2. Distribution of ADAMTS1 gene transfer into tumors.


