Abstract
Identification of small molecules that safely inhibit cancer progression is critical for cancer therapeutics. Saponins exhibit cytostatic and cytotoxic activity against various cancer cells, but the mechanism is not well understood. Here, we investigated whether saponin D (designated SB365), an active component isolated from Pulsatilla koreana, could inhibit the progression of hepatocellular carcinoma (HCC) and considered its mechanism. SB365 strongly suppressed the growth of HCC cells in a dose‐dependent manner and induced apoptosis by increasing the proportion of sub G1 apoptotic cells from 8% to 21% through induction of expression of Bax and cleaved caspase‐3. In addition, SB365 exhibited potent anti‐angiogenic activity and decreased the expression of hypoxia‐inducible factor‐1α (HIF‐1α) and vascular endothelial growth factor, a key molecule for angiogenesis. Furthermore, SB365 suppressed the tube formation and migration of HUVEC, as well as in vivo neovascularization in a mouse Matrigel plug assay. In vivo study showed that SB365 significantly inhibited tumor growth in an HCC xenograft model, inducing apoptosis by increasing the expression of the cleaved caspase‐3 and DNA fragmentation. The expressions of vascular endothelial growth factor and CD34 in the tumor tissue were decreased by SB365 treatment. In examining its mechanism, SB365 was found to effectively suppress the phosphorylation of PI3K downstream factors, such as Akt, mTOR and p70S6K both in vitro and in vivo. Our study demonstrates that SB365 not only induces apoptosis but also inhibits cell growth and angiogenesis through modulation of the PI3K/Akt/mTOR pathway in human HCC. We suggest that SB365 may be a new chemotherapeutic candidate against HCC.
Hepatocellular carcinoma (HCC) is the most frequently occurring primary liver cancer, with an incidence rate of half a million cases per year in the world.1 Although surgery remains the treatment of choice for HCC, tumor size and hepatic functional reservation might restrict surgical ablation.2 Conventional therapies such as transcatheter arterial embolization, microwave coagulation therapy, radiotherapy and percutaneous ethanol injection therapy are generally not prescribed for advanced HCC due to their low efficacy.3 In clinical trials, first‐line drugs used for HCC contain sorafenib, doxorubicin, fluorouracil and cisplatin, for example. However, the response of HCC to current chemotherapy remains inconsistent because of significant side effects and low drug response. Hence, the discovery of new anti‐cancer agents with high efficacy and low toxicity is critical.
Recent study indicates that HCC cell activation increases phosphatidylinositol 3‐kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signalling.4 In the process of carcinogenesis, the PI3K family is involved in various cellular functions, including cell growth, proliferation and survival.5 Akt is one of the most commonly activated protein kinases in human cancers and is associated with malignant transformation and apoptotic resistance.6 mTOR, located downstream of Akt, is phosphorylated in response to stimuli that activates the PI3K/Akt pathway.7 Finally, mTOR positively regulates the phosphorylation of ribosomal p70S6 kinase (p70S6K) and eukaryotic initiation factor 4E (Eif4E) binding protein 1 (4EBP1).7 Indeed, PI3K/Akt/mTOR signalling is activated in 30–50% of HCC patients and p70S6K is aberrantly activated in 50% of HCC.9 For this reason, the PI3K/Akt/mTOR pathway has emerged as an attractive target for therapeutic intervention in HCC.
Ingredients from many herbs have been used as chemopreventive and pharmacological medicine for HCC and other cancers.10 Among them, saponins have been demonstrated to possess a wide range of properties beneficial to health and are used as precursors for drugs in the pharmaceutical industry.11, 12 It has been reported that several saponins have anti‐cancer properties, inhibiting cell growth through cell cycle arrest and apoptosis in various cancer cell types.13, 14, 15 Most importantly, the efficacy of saponins differs according to their structure and source. Therefore, we first isolated various saponins from root of Pulsatilla koreana to discover new saponin varieties with high efficacy. The roots of P. koreana have been widely used in traditional herbal medicine in the treatment of disease including malaria and amoebic dysentery in Korea.16 In the present study, Pulsatilla saponin D (here after designated SB365) was selected among the many kinds of saponins isolated from P. koreana. Because of the critical role of the PI3K/Akt/mTOR pathway in cancer cell survival, apoptosis and angiogenesis, and its expression in various cancer cells, we investigate whether SB365 exhibits anti‐cancer activity and mediate its effects through the modulation of the PI3K/Akt/mTOR pathway. The present study reveals that SB365 induces apoptosis as well as inhibits proliferation and angiogenesis by modulating the PI3K/Akt/mTOR pathway in HCC cells.
Material and Methods
Cells and materials
Human HCC cell lines Huh‐7 and HepG2 were purchased from the Japanese Collection of Research Bioresources (Osaka, Japan) and ATCC (Manassas, VA, USA), and normal liver cell line HL‐7702 was purchased from the Shanghai Institute of Cell Biology (Shanghai, China). FBS, media, penicillin–streptomycin and all other agents used in the cell culture studies were purchased from Invitrogen (Carlsbad, CA, USA). Cultures were maintained at 37°C in a CO2 incubator with a controlled humidified atmosphere composed of 95% air and 5% CO2. HUVEC were grown in a gelatin coated 75‐cm2 flask in M199 medium containing 3 ng/mL basic fibroblast growth factor (bFGF), 5 U/mL heparin and 20% FBS at 37°C in a humidified atmosphere of 5% CO2/95% air.
Preparation of SB365
SB365 was isolated from roots of P. koreana collected from Kyeryong Mountain near Daejeon, Korea. Powdered roots of P. koreana (50 g) were extracted three times with 50% aqueous ethanol (500 mL), and the resulting extracts were combined and concentrated in vacuo to yield a light brown residue. The fraction was chromatographed using a Sephadex LH‐20 column (200 g, 60 × 4 cm) with an 80:20 mixture of methanol and H2O, to give four fractions: SPX1 (139 mg, 24.8%), SPX2 (344 mg, 61.4%), SPX3 (61 mg, 10.9%) and SPX4 (15.7 mg, 2.8%). After heating, the chromatogram was sprayed with 10% H2SO4. The third fraction, which exhibited the most potent activity, was again chromatographed by solid phase HPLC (solid phase, RP‐C18, 250 × 10 mm; mobile phase, MeOH‐H2O [82:20] as the mobile phase; UV wavelength, 210 nm; flow rate, 1 mL/min) to yield three major fractions. Among them, the third fraction (SPX3), which exhibited the most potent activity, was purified by HPLC to yield saponin D, SB365. For more precise analysis of purified SB365, we also used mass spectrometry and NMR spectroscopy.
Measurement of cell proliferation
Cell viability was measured by MTT assay. Briefly, Huh‐7 and HepG2 cells were plated at a density of 3–5 × 103 cells/well in 96‐well plates. The following day, the medium was removed and cells were treated with either DMSO as a control or various concentrations of SB365. The final concentration of DMSO in the medium was ≤0.1% (v/v). After the cells were incubated for 72 h, 20 μL MTT solution (2 mg/mL) was added to each well for another 4 h at 37°C. The formazan crystals that formed were dissolved in DMSO (200 μL/well) by constant shaking for 5 min. The plate was then read on a microplate reader at 540 nm. Three replicate wells were used for each analysis. The median inhibitory concentration (IC50, the drug concentration at which cell growth was inhibited by 50%) was assessed from the dose–response curves.
Tube formation assay
For the tube formation assay, 10 mg/mL (200 μL) of Matrigel (BD Biosciences, San Diego, CA, USA) was polymerized for 30 min at 37°C. HUVEC were suspended in M199 (2% FBS) medium at a density of 2.5 × 105 cells/mL, and 0.2 mL of cell suspension was added to each well coated with Matrigel, together with or without the indicated concentrations of SB365 and vascular endothelial growth factor (VEGF) (50 ng/mL) for 14 h. The morphological changes of the cells and tubes formed were observed under a phase‐contrast microscope and photographed at 200× and 400× magnification.
Wound migration assay
HUVEC plated on 60 mm‐diameter culture dishes at 90% confluence were wounded with a razor blade score 2 mm in width and marked at the injury line. After wounding, the peeled‐off cells were removed with a serum‐free medium and further incubated in M199 with 2% FBS, 1 mM thymidine (Sigma‐Aldrich, St. Louis, MO, USA), SB365 (0.1–10 μM) and/or VEGF (50 ng/mL). HUVEC were allowed to migrate for 24 h and were rinsed with a serum‐free medium, followed by fixing with absolute alcohol. Migration was quantitated by counting the number of cells that moved beyond the reference line.
Matrigel plug assay
Animal care and experimental procedures were conducted in accordance with the Guide for Animal Experiments of the Korean Academy of Medical Sciences. Male 6‐week‐old BALB/c mice were obtained from ORIENT‐BIO Laboratory Animal Research Center (Gyeonggi‐do, Kapyung, Korea). The mice were subcutaneously injected with 500 μL of Matrigel containing concentrated VEGF (50 ng/mL), and either SB365 (10 μM) or PBS (10 μL). After 7 days, mice were killed and the Matrigel plugs were removed. H&E staining was performed to identify the formation and infiltration of new, functional microvessels. Functional vessels with intact red blood cells were quantified manually using a microscope (high‐power field ×200).
Tumor xenograft study
Male nude mice were obtained from Central Laboratory Animal (Seoul, Korea). Animal care and all experimental procedures were conducted in accordance with the approval and guidelines of the INHA Institutional Animal Care and Use Committee (INHA IACUC) of the Medical School of Inha University (approval ID: 101228‐74). Male 6‐week‐old nude mice were randomly divided into three groups (control, SB365 10 mg/kg and SB365 30 mg/kg). The Huh‐7 human HCC cells were inoculated into one flank of each nude mouse (5 × 106 Huh‐7 cells). When the tumors had reached a volume of approximately 50–70 mm3, mice were given a daily oral dose of 10 and 30 mg/kg of SB365 or the vehicle (200 μL PBS, control group), for 15 days. The tumor volume was calculated using the formula: V = length × width2 × 0.5.
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed using anova and an unpaired Student's t‐test. A P‐value of 0.05 or less was considered statistically significant. Statistical calculations were performed using SPSS software for Windows Version 10.0 (SPSS, Chicago, IL, USA).
Results
SB365 inhibited cell growth in hepatocellular carcinoma cells
To identify the anticancer effect of SB365, we first compared cell growth in two HCC cell lines (Huh‐7 cell and HepG2) after treatment with SB365, sorafenib and doxorubicin (DOX). HCC cells were treated with four concentrations of each compound (1–10 μM) for 72 h. As shown in Figure 1, SB365 had the most inhibitory effect on Huh‐7 cell growth in a dose‐dependent manner. SB365 induced reduction of cell growth rate at a starting dose of 1 μΜ in Huh‐7 cells, and it strongly inhibited up to 90% of cell growth at 5 and 10 μM. In HepG‐2 cells, the inhibitory effect of SB365 was lower than that of DOX at 1–2 μM but cell growth at 5–10 μM was more inhibited by SB365 compared to DOX. To predict their side effects, we also treated each compound to HL‐7702 normal liver cell line. DOX showed high cytotoxicity in normal liver cells from a starting dose of 1–10 μM, although it strongly inhibited the growth of HCC cells, whereas SB365 and sorafenib exhibited low cytotoxicity in HL‐7702 normal liver cells. However, inhibition of cell growth by sorafenib was lower than that of SB365. Accordingly, these results reveal that, compared to sorafenib or doxorubicin, SB365 is more effective in HCC cells with less cytotoxicity.
Figure 1.
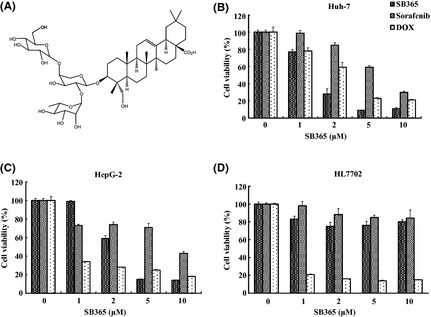
Chemical structure of SB365 (Pulsatilla saponin D) and its effect on the proliferation of human hepatocellular carcinoma cells and normal liver cells. (A) Chemical structure of SB365, (B and C) cytotoxic effects of SB365, sorafenib and doxorubicin (DOX) on HCC cells (Huh‐7 and HepG2) and (D) normal liver cell line (HL‐7702) were measured by MTT assay. Results are expressed as percent cell proliferation relative to the proliferation of control. Data represents mean ± SD from the triplicate wells.
SB365 induced apoptosis in Huh‐7 HCC cells
We performed flow cytometric analysis to determine changes in the cell cycle profile induced by SB365. Flow cytometry data revealed that the SB365 treatment increased the sub G1 phase by 8–21%, indicating apoptosis (Fig. 2A). In addition, induction of apoptosis by SB365 was evaluated by DAPI and TUNEL staining to characterize the nuclear morphology. As shown in Figure 2(B), the cells treated with 10 μM SB365 present the morphological features of apoptotic cells, such as bright nuclear condensation and perinuclear apoptotic bodies (by DAPI staining). Apoptosis by SB365 was confirmed by TUNEL staining, which detected DNA fragmentation. In addition, the expression of Bcl‐2, Bax and cleaved caspase‐3 was investigated by western blotting after SB365 treatment. As expected, SB365 increased the expression of the cleaved caspase‐3 and Bax, and decreased the expression of Bcl‐2 in Huh‐7 HCC cells (Fig. 2C). These results show that SB365 can induce cell apoptosis in Huh‐7 HCC cells.
Figure 2.
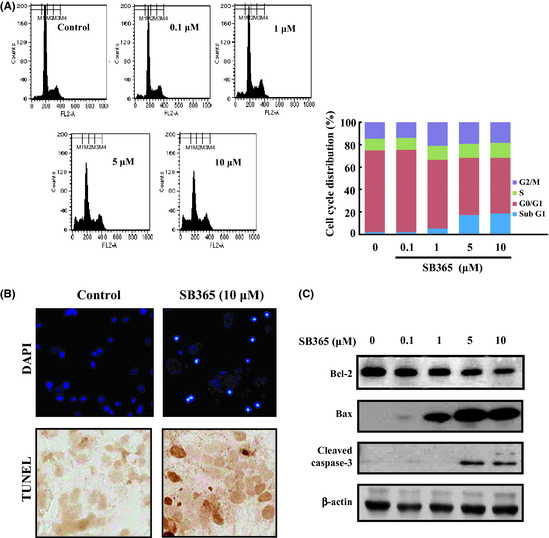
Effect of SB365 on apoptosis of Huh‐7 cells. (A) Huh‐7 cells were treated with SB365 (0, 0.1, 1, 5 and 10 μM) for 24 h, stained with propidium iodide (PI) and analyzed on a FACSCalibur flow cytometer. (B) The induction of apoptosis by SB365 was conducted by DAPI and TUNEL staining, which were photographed at ×100 and ×400 magnification. (C) The expression of Bcl‐2, Bax and cleaved caspase‐3 were determined by western blotting in cells treated with SB365 at the indicated doses for 24 h.
SB365 inhibited angiogenesis in Huh‐7 hepatocellular carcinoma cells and HUVEC
To investigate the effect of SB365 on vascular endothelial cells, we first analyzed the viability of HUVEC exposed to SB365. As shown in Figure 3(A), treatment with 1–10 μM of SB365 for 48 h reduced cell viability by 50–80% compared to untreated control cells (P < 0.01). Hypoxia‐inducible factor‐1α (HIF‐1α) is the main transcriptional modulator of angiogenic factors such as VEGF. Thus, it was appropriate to investigate the effect of SB365 on the expression of hypoxia‐induced HIF‐1α and VEGF. Huh‐7 cells were treated with various concentrations of SB365 (0.1–10 μM) under hypoxia mimic condition induced by 100 μM CoCl2 for 6 h. As shown in Figure 3(B) and C, SB365 inhibited hypoxia‐induced HIF‐1α expression and VEGF production in a dose‐dependent manner (P < 0.05). In addition, the anti‐angiogenic potential of SB365 was examined using HUVEC. From an in vitro tube formation assay, we observed that SB365 significantly inhibited VEGF‐induced tube formation of vessel‐like structures, consisting of the elongation and alignment of the cells in a dose‐dependent manner (P < 0.05, Fig. 3D). In addition, we carried out a wounding migration assay to examine the effect of SB365 on VEGF‐induced endothelial cell migration. The migration was definitely reduced in the SB365‐treated group compared to the control group (P < 0.05, Fig. 3E). These results indicate that SB365 could prevent VEGF‐induced tube formation and migration of endothelial cells.
Figure 3.
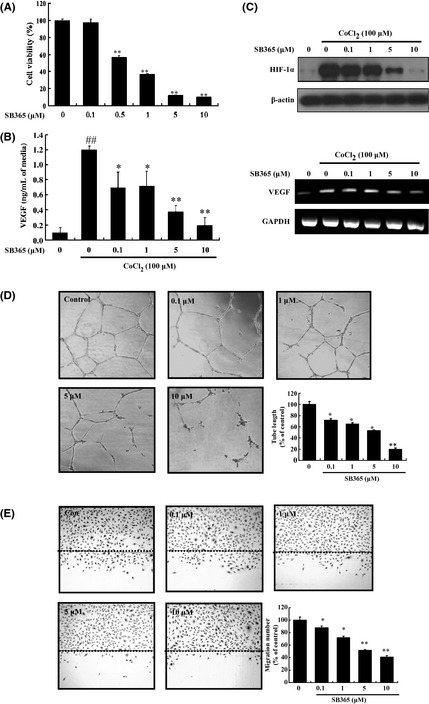
Effect of SB365 on angiogenesis of Huh‐7 hepatocellular carcinoma cells and HUVEC. (A) Inhibition of HUVEC proliferation by SB365. **P < 0.01, compared to control. (B) Production of vascular endothelial growth factor (VEGF) by SB365 in hypoxia‐induced Huh‐7 cells. Data represent mean ± SD from the triplicate wells. (C) Expression of hypoxia‐inducible factor‐1α (HIF‐1α) and VEGF by SB365 in hypoxia‐induced Huh‐7 cells (CoCl2, 100 μM). (D) Effects of SB365 on tube formation in vitro. (E) Effects of SB365 on migration in vitro. Data represent mean ± SD from three independent experiments. ## P < 0.01 compared to control. *P < 0.05 and **P < 0.01 compared to CoCl2.
SB365 inhibited angiogenesis in vivo
To confirm whether SB365 possesses anti‐angiogenic activity, we performed a Matrigel plug assay in vivo. As shown in Figure 4(A), blood vessels were rarely observed in Matrigel plugs without VEGF. VEGF strongly induced neovessels containing intact red blood cells inside the Matrigel, which were clearly inhibited by 10 μM SB365 treatment. We performed H&E staining to quantify the amount of functional vasculature in matrigel plugs and found that SB365 dramatically inhibited VEGF‐induced vessel formation in vivo (P < 0.01) For histological analysis, each section of the Matrigel plug was stained with H&E and CD34. The stained Matrigel sections showed that the plug with SB365 treatment had fewer vessels within the gels than the VEGF‐induced Matrigel plug. Expression of CD34 was also decreased by SB365 treatment in the VEGF‐induced Matrigel plug. These results confirmed that SB365 produced a potent anti‐angiogenic activity in vivo (Fig. 4B).
Figure 4.
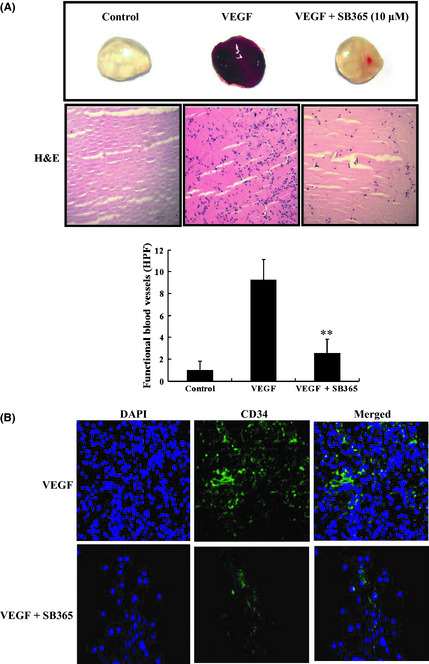
Effect of SB365 on Matrigel plug assay in vivo. (A) Effect of SB365 on Matrigel plug assay in vivo. Matrigel plugs were implanted into mice with vascular endothelial growth factor (VEGF) (50 ng/mL) and/or SB365 (10 μM). The plugs were sectioned and stained with H&E. Infiltrating microvessels with intact red blood cells were qualified by manual counting. **P < 0.01 compared to VEGF. (B) Endothelial cells in the plug were immunostained with the CD34. A stained plug was observed by microscopy at ×200 and ×400 magnifications.
SB365 inhibited PI3K/Akt/mTOR signaling pathway in Huh‐7 hepatocellular carcinoma cells
PI3K/Akt/mTOR plays an important role in regulating critical cellular functions, including cell growth and metabolism.18 Therefore, we investigated the effects of SB365 on the PI3K/Akt/mTOR pathway in Huh‐7 HCC cells. When HCC cells were treated with various concentrations of SB365 for 24 or 48 h, phosphorylation levels of Akt and its downstream factor mTOR were effectively suppressed (Fig. 5A). mTOR activation results in phosphorylation of effectors such as p70S6K, which subsequently leads to mTOR‐dependent gene transcription that regulates cell proliferation and protein synthesis.19 Therefore, we further identified the effect of SB365 on the expression of p70S6K. As expected, SB365 inhibited phosphorylation of p70S6K in a dose‐dependent manner. These results were confirmed using a fluorescence imaging system (Fig. 5).
Figure 5.
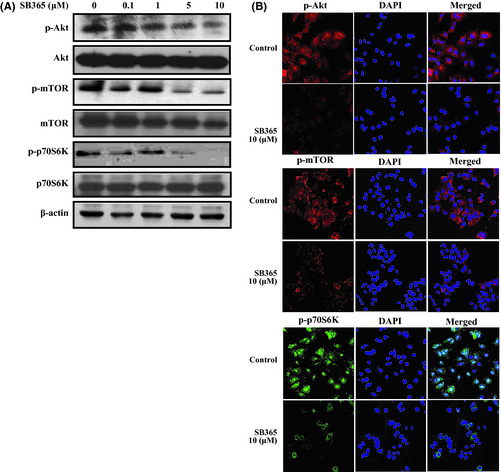
Effect of SB365 on the PI3K/Akt/mTOR pathway signalling in Huh‐7 hepatocellular carcinoma cells. (A) Cells were treated with SB365 with various doses (0.1–10 μM). Western blotting experiment for p‐Akt, p‐mTOR and p‐p70S6K were performed. (B) Immunofluorescent imaging for target proteins of PI3K/Akt/mTOR pathway after SB365 treatment (10 μM). For labelling, anti‐rabbit antibodies against p‐Akt, p‐mTOR, and p‐70S6K were used (×400 magnification).
SB365 inhibited tumor growth in hepatocellular carcinoma xenograft model
Strong anticancer efficacy of SB365 led us to investigate the in vivo efficacy of SB365 against the Huh‐7 cell xenograft in nude mice. As shown in Figure 6(A), SB365 suppressed tumor growth at doses of 10 and 30 mg/kg for 15 days compared with the control group. SB365 administration resulted in significant reduction of tumor volume in the nude mice (77% and 88% at 10 and 30 mg/kg, respectively). There was no difference in body weight in SB365 treatment groups compared with the control group. In addition, low toxicity of SB365 was confirmed by the values of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and bilirubin (Table 1). These results show that SB365 is not particularly toxic to mice at the curative dose (Fig. 6B). Consistent with this observation, the weight of tumors isolated from the SB365‐treated groups was significantly decreased, by 80% and 89% at SB365 doses of 10 and 30 mg/kg, respectively, compared with the control (Fig. 6C, P < 0.05). Our results demonstrate that SB365 had anti‐tumor efficacy against HCC xenograft models.
Figure 6.
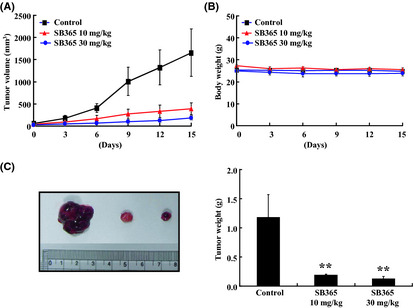
In vivo effect of SB365 in the hepatocellular carcinoma (HCC) mouse xenograft model. (A) Tumor growth of Huh‐7 xenograft in nude mice. (B) Average body weight of nude mice. All mice received subcutaneous injection of Huh‐7 cells (5 × 106 cells/200 μL PBS) on the flank. (C) Tumor size and weight in Huh‐7 mouse xenograft. Data represented mean ± SD (n = 6). *P < 0.05 and **P < 0.01 compared to control.
Table 1.
Effects of SB365 on serum parameters with respect to liver functions of hepatocellular carcinoma xenograft mouse model
| Parameter | Groups | ||
|---|---|---|---|
| Con | SB365 10 mg/kg | SB365 30 mg/kg | |
| ALT (IU/L) | 58.2 ± 2.8 | 60.5 ± 8.5 | 62.5 ± 4.7 |
| AST (IU/L) | 108.5 ± 15.5 | 110.5 ± 26 | 124.1 ± 17.1 |
| Total bilirubin (mg/dL) | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 |
Values are expressed as mean ± SD (n = 6). ALT, alanine aminotransferase; AST, aspartate aminotransferase.
SB365 inhibited angiogenesis and proliferation with induction of apoptosis in a hepatocellular carcinoma xenograft model
In a histopathological analysis, we observed that there was a greater degree of tumor apoptosis and necrosis in the SB365‐treated group compared with the control group by H&E staining (Fig. 7). Furthermore, SB365 decreased the expression of Ki‐67, a cell proliferation marker, as well as both VEGF and CD34 in the SB365‐treated group compared with the control group. In addition, the apoptotic effect of SB365 on HCC tumor tissues was identified by expression of the cleaved caspase‐3 and DNA fragment by TUNEL assay. Furthermore, SB365 decreased the phosphorylation of Akt and mTOR, regulating many different events involved in cell survival and proliferation.
Figure 7.
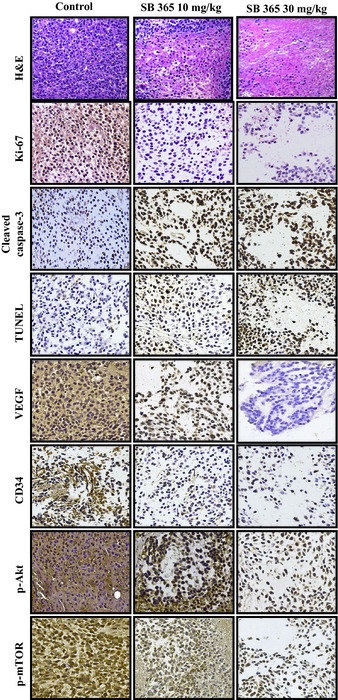
In vivo effect of SB365 on proliferation, apoptosis and angiogenesis of hepatocellular carcinoma xenograft. Tumors were excised and processed for immunostaining for Ki‐67, cleaved caspase‐3, TUNEL, vascular endothelial growth factor (VEGF), CD34, p‐Akt and p‐mTOR, including H&E staining (×400 magnification).
Discussion
Despite the recent advances in the understanding of the nature of HCC carcinogenesis, the increasing incidence and relative alleviation rate of chemotherapy have spurred efforts to use innovative approaches to establish more effective treatment regimens. For several reasons, including safety and minimizing toxicity and side effects, the use of medicinal compounds from herbal/natural sources is becoming increasingly popular over synthetic drugs, and provides alternative treatment options for patients.20 In the present study, we obtained SB365, saponin D, from P. koreana, and explored its anti‐cancer effects against HCC cells. SB365 effectively suppressed the PI3K/Akt/mTOR pathway, which might lead to the inhibition of cell growth and angiogenesis together with the induction of apoptosis in HCC cells. This is the first report that clearly characterizes the anti‐tumor properties of SB365 and its mechanism in both HCC cells and a tumor xenograft model.
Inhibiting cell growth is a critical action of anti‐cancer drug‐induced cell death. Apoptosis, another important strategy for overcoming cancer, can contribute to cell growth inhibition in cancer cells.21, 22 In the present study, SB365 inhibited 40–80% of the growth of HCC cells at concentrations from 2 to 10 μM. Even though SB365 strongly inhibited the growth of HCC cells, it did not affect the viability of normal liver cells, which was confirmed by the result that SB365 also did not change the levels of ALT, AST and bilirubin (Table 1) as well as body weight (Fig. 6B) in vivo. However, sorafenib showed low effect (Average IC50 of sorafenib, SB365, and doxorubicin; 6, 1.8, and 0.9 μM) in HCC cells and doxorubicin showed 80% cell cytotoxicity in cell viability starting at 1 μM in normal liver cells. Although IC50 of doxorubicin is higher than SB365, effect of doxorubicin is likely to be cell toxicity because of showing high cell cytotoxicity in normal liver cells. The inhibitory effect of saponins on HCC cell growth showed the distinction in the different species and sources of saponins.23, 24, 25 Interestingly, SB365 had a lower IC50 level than other saponins (IC50, 3 μΜ) in HCC cells. This capacity of SB365 led to not only inhibition of cell growth but also apoptosis. These events were supported by in vivo results, showing that SB365 treatments of both 10 and 30 mg/kg increased the expression of cleaved caspase‐3 and led to inhibited tumor growth in HCC xenograft models. These results indicate that induction of apoptosis and inhibition of cell growth by SB365 might contribute to the suppression of tumor growth.
Given the importance of tumor angiogenesis in the growth of HCC, inhibition of angiogenic pathways is an alternative to targeting cancer cell proliferation. Recent clinical research has reported that HIF‐1α and VEGF might be useful in a molecular prediction model for lymph node metastasis of HCC.26 In the present study, SB365 obviously inhibited the expression of HIF‐1α and VEGF under hypoxia and also decreased the secretion of VEGF in Huh‐7 cells, indicating that SB365 inhibited hypoxia‐induced angiogenesis. In addition, SB365 inhibited not only the tube formation and migration of HUVEC but also the formation of neovessels in Matrigel plugs. Its anti‐angiogenic effect was supported by decreased expression of CD34 in VEGF‐mediated Matrigel plugs and decreased VEGF in the tumor tissue of xenograft animal models. From our in vitro and in vivo results, we suggest that SB365 exhibits potent anti‐angiogenic activity by decreasing VEGF. Indeed, these results are similar to previous reports that various saponins inhibit angiogenesis by inhibiting the growth and new vessel development of endothelial cells and decreasing the expression of VEGF in cancer cells.27, 28, 29, 30
The PI3K/Akt/mTOR pathway is frequently over‐activated in HCC and is believed to contribute to their aggressive phenotype and resistance to chemotherapy.31 Recent clinical study reports that activation of the PI3K/Akt/mTOR pathway is correlated with tumor progression and reduced survival of patients.32 Thus, the inhibitors of PI3K, Akt and mTOR would have strong anticancer effects against human cancers. Although many individual saponins have been isolated from natural/herbal plants, studies on the anticancer mechanisms of these compounds are inadequate. Thus, we attempted to investigate the effect of SB365 on the PI3K/Akt/mTOR pathway in HCC cells. As expected, SB365 inhibited the phosphorylation of Akt and mTOR in a dose‐dependent manner. In addition, SB365 effectively inhibited phosphorylation of p70S6K, which is an effector of mTOR in HCC cells. Overall, our results indicate that not only inhibition of cell growth but also apoptosis by SB365 may be regulated through the PI3K/Akt/mTOR pathway.
In conclusion, the present study demonstrates that SB365 inhibits the PI3K/Akt/mTOR pathway and invokes strong anti‐cancer activity against HCC by inducing apoptosis as well as inhibiting cell growth/proliferation and angiogenesis. We suggest that the anti‐cancer effect of SB365 could be regulated through PI3K/Akt/mTOR signaling, thereby implying that SB365 is as a potential therapeutic agent against HCC.
Disclosure statement
The authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by the National R&D Program for Cancer Control (1020250), the Ministry of Health & Welfare, the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012‐0002988), and Inha University Grant and SB Pharmaceutical Co.
(Cancer Sci 2012; 103: 1929–1937)
References
- 1. El‐Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008; 134: 1752–63. [DOI] [PubMed] [Google Scholar]
- 2. Kerr SH, Kerr DJ. Novel treatments for hepatocellular cancer. Cancer Lett 2009; 286: 114–20. [DOI] [PubMed] [Google Scholar]
- 3. Lin DY, Lin SM, Liaw YF. Non‐surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 1997; 12: S319–28. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol 2011; 7: 1149–67. [DOI] [PubMed] [Google Scholar]
- 5. Wymann MP, Zvelebil M, Laffargue M. Phosphoinositide 3‐kinase signalling–which way to target? Trends Pharmacol Sci 2003; 24: 366–76. [DOI] [PubMed] [Google Scholar]
- 6. Vivanco I, Sawyers CL. The phosphatidylinositol 3‐Kinase AKT pathway in human cancer. Nat Rev Cancer 2002; 2: 489–501. [DOI] [PubMed] [Google Scholar]
- 7. Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004; 4: 335–48. [DOI] [PubMed] [Google Scholar]
- 8. Strimpakos AS, Karapanagiotou EM, Saif MW, Syrigos KN. The role of mTOR in the management of solid tumors: an overview. Cancer Treat Rev 2009; 35: 148–59. [DOI] [PubMed] [Google Scholar]
- 9. Minguez B, Tovar V, Chiang D, Villanueva A, Llovet JM. Pathogenesis of hepatocellular carcinoma and molecular therapies. Curr Opin Gastroenterol 2009; 25: 186–94. [DOI] [PubMed] [Google Scholar]
- 10. Newman DJ. Natural products as leads to potential drugs: an old process or the new hope for drug discovery? J Med Chem 2008; 51: 2589–99. [DOI] [PubMed] [Google Scholar]
- 11. Price KR, Johnson IT, Fenwick GR. The chemistry and biological significance of saponins in foods and feedingstuffs. Crit Rev Food Sci Nutr 1987; 26: 27–135. [DOI] [PubMed] [Google Scholar]
- 12. Taylor WG, Elder JL, Chang PR, Richards KW. Microdetermination of diosgenin from fenugreek (Trigonella foenum‐graecum) seeds. J Agric Food Chem 2000; 48: 5206–10. [DOI] [PubMed] [Google Scholar]
- 13. Vincken JP, Heng L, de GrootA, Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007; 68: 275–97. [DOI] [PubMed] [Google Scholar]
- 14. Haridas V, Higuchi M, Jayatilake GS et al Avicins: triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc Natl Acad Sci USA 2001; 98: 5821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mujoo K, Haridas V, Hoffmann JJ et al Triterpenoid saponins from Acacia victoriae (Bentham) decrease tumor cell proliferation and induce apoptosis. Cancer Res 2001; 61: 5486–90. [PubMed] [Google Scholar]
- 16. Bang KH. The Medicinal Plant of Korea. Seoul: Kyo‐Hak Press, 1999. [Google Scholar]
- 17. Wang Q, Zheng XL, Yang L et al Reactive oxygen species‐mediated apoptosis contributes to chemosensitization effect of saikosaponins on cisplatin‐induced cytotoxicity in cancer cells. J Exp Clin Cancer Res 2010; 29: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yap TA, Garrett MD, Walton MI, Raynaud F, de BonoJS, Workman P. Targeting the PI3K‐AKT‐mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol 2008; 8: 393–412. [DOI] [PubMed] [Google Scholar]
- 19. Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol 2003; 3: 371–7. [DOI] [PubMed] [Google Scholar]
- 20. Zhao J. Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: a perspective on plant biotechnology application. Recent Pat Biotechnol 2007; 1: 75–97. [DOI] [PubMed] [Google Scholar]
- 21. McKnight JJ, Gray SB, O'Kane HF, Johnston SR, Williamson KE. Apoptosis and chemotherapy for bladder cancer. J Urol 2005; 173: 683–90. [DOI] [PubMed] [Google Scholar]
- 22. Dowsett M, Archer C, Assersohn L et al Clinical studies of apoptosis and proliferation in breast cancer. Endocr Relat Cancer 1999; 6: 25–8. [DOI] [PubMed] [Google Scholar]
- 23. Wang XY, Chen XL, Tang HF, Gao H, Tian XR, Zhang PH. Cytotoxic triterpenoid saponins from the rhizomes of Anemone taipaiensis. Planta Med 2011; 77: 1550–4. [DOI] [PubMed] [Google Scholar]
- 24. Liu H, Chou GX, Wang JM, Ji LL, Wang ZT. Steroidal saponins from the rhizomes of Dioscorea bulbifera and their cytotoxic activity. Planta Med 2011; 77: 845–8. [DOI] [PubMed] [Google Scholar]
- 25. Song G, Guo S, Wang W et al Intestinal metabolite compound K of ginseng saponin potently attenuates metastatic growth of hepatocellular carcinoma by augmenting apoptosis via a Bid‐mediated mitochondrial pathway. J Agric Food Chem 2010; 58: 12753–60. [DOI] [PubMed] [Google Scholar]
- 26. Xiang ZL, Zeng ZC, Fan J, Tang ZY, Zeng HY, Gao DM. Gene expression profiling of fixed tissues identified HIF‐1{alpha}, VEGF, and MMP‐2 as biomarkers of lymph node metastasis in hepatocellular carcinoma. Clin Cancer Res 2011; 17: 5463–72. [DOI] [PubMed] [Google Scholar]
- 27. Chen QJ, Zhang MZ, Wang LX. Gensenoside Rg3 inhibits hypoxia‐induced VEGF expression in human cancer cells. Cell Physiol Biochem 2010; 26: 849–58. [DOI] [PubMed] [Google Scholar]
- 28. Tian F, Zhang X, Tong Y et al PE, a new sulfated saponin from sea cucumber, exhibits anti‐angiogenic and anti‐tumor activities in vitro and in vivo. Cancer Biol Ther 2005; 4: 874–82. [DOI] [PubMed] [Google Scholar]
- 29. Arai M, Hayashi A, Sobou M et al Anti‐angiogenic effect of triterpenoidal saponins from Polygala senega. J Nat Med 2011; 65: 149–56. [DOI] [PubMed] [Google Scholar]
- 30. Chen PS, Shih YW, Huang HC, Cheng HW. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC‐3 cells by reducing matrix metalloproteinases expression. PLoS ONE 2011; 6 (Pt 5): e20164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tommasi S, Pinto R, Pilato B, Paradiso A. Molecular pathways and related target therapies in liver carcinoma. Curr Pharm Des 2007; 13: 3279–87. [DOI] [PubMed] [Google Scholar]
- 32. Sun CH, Chang YH, Pan CC. Activation of the PI3K/Akt/mTOR pathway correlates with tumour progression and reduced survival in patients with urothelial carcinoma of the urinary bladder. Histopathology 2011; 58: 1054–63. [DOI] [PubMed] [Google Scholar]


