Abstract
Glioblastoma (GBM) is a highly aggressive brain tumor characterized by increased proliferation and resistance to chemotherapy and radiotherapy. Recently, a growing body of evidence suggests that glioma‐initiating cells (GICs) are responsible for the initiation and recurrence of GBM. However, the factors determining the differential development of GICs remain poorly defined. In the present study, we show that curcumin, a natural compound with low toxicity in normal cells, significantly induced differentiation of GICs in vivo and in vitro by inducing autophagy. Moreover, curcumin also suppressed tumor formation on intracranial GICs implantation into mice. Our results suggest that autophagy plays an essential role in the regulation of GIC self‐renewal, differentiation, and tumorigenic potential, suggesting autophagy could be a promising therapeutic target in a subset of glioblastomas. This is the first evidence that curcumin has differentiating and tumor‐suppressing actions on GICs. (Cancer Sci 2012; 103: 684–690)
Glioblastoma (GBM) is a highly aggressive form of brain cancer associated with complicating therapeutic intervention. Recent evidence has indicated that the initiation and growth of GBM has been linked to glioma‐initiating cells (GICs), which share features with neural stem cells and are poorly differentiated.1, 2 Furthermore, it has been reported that bone‐morphogenetic protein, as well as distinct microRNAs, can suppress the tumorigenicity of GICs by promoting their differentiation.3, 4, 5 Thus, it may be possible to target GICs by developing differentiation‐inducing therapies for GBM tumors. This approach requires an understanding of the mechanisms involved in the maintenance of the poorly differentiated, tumor‐initiating cell state.
Autophagy is essential for maintaining cellular energy homeostasis during nutritional stress, and also appears to play important roles in development and differentiation. A recent study has reported that embryonic stem cells lacking beclin 1 or Atg5 are defective in forming cavitated embryoid bodies in vitro, due to the failure in clearing apoptotic cells.6 Autophagy is also involved in the differentiation, survival, and death of leukocytes, although there are differences between the different cell types. In a human myeloid leukemia cell line (K562), which can be differentiated to mimic erythropoiesis in vitro, it was determined that autophagic activity is high during this process.7 The B cell development was blocked at the pro‐B cell stage in the bone marrow in the absence of Atg5.8 Beclin 1 autophagy gene plays an essential, but selectively, early role during T cell development.9 In our previous report, we provided evidence that autophagy defect in GICs contributes to differentiation block and glioma radioresistance.10 In this study, we analyze the role of autophagy in GIC differentiation in more detail.
Curcumin, a low molecular weight compound, has been consumed by humans for centuries as an anti‐inflammatory and antioxidant in Ayurvedic medicine.11, 12 Curcumin reduces oxidative damage and cognitive deficits in animal models of Alzheimer's disease, ischemic stroke, and traumatic brain injury.13, 14, 15 Recently, curcumin was found to possess anticancer activities in multiple human carcinomas including melanoma, head and neck, breast, colon, pancreatic, prostate, and ovarian cancers.16, 17, 18, 19, 20, 21, 22, 23, 24 The mechanisms by which curcumin exerts its anticancer effects are comprehensive and diverse, targeting many levels of regulation in the processes of cellular growth. In a previous study, we reported that autophagy plays an essential role in the regulation of GIC self‐renewal, differentiation, and tumorigenic potential.10 Here, we investigated the anticancer effect of curcumin on GICs in vitro and in vivo. We found that curcumin induced differentiation of GICs and efficiently inhibited growth of GICs by activating autophagy. These findings support a role for curcumin as an adjunct to traditional chemotherapy in the treatment of GBM.
Materials and Methods
Patients
Tumor samples classified as GBM based on World Health Organization criteria were obtained from patients undergoing surgical treatment. The subjects’ consent was obtained according to the Declaration of Helsinki. The study protocol was approved by the Medical Review Board of Soochow University Medical School (Suzhou, China).
Cell culture
We obtained GICs, named SU‐2 and SU‐3, from surgically resected human GBM samples, as reported previously.10, 25 Cells proliferated in the presence of fibroblast growth factor (FGF) (20 ng/mL; Gibco, Frederick, MD, USA), epidermal growth factor (EGF) (20 ng/mL; Gibco, Camarillo, CA, USA), and N2 supplement (Gibco) in serum‐free media DMEM/F12 (Gibco) and differentiated in the presence of serum or in the absence of basic fibroblast growth factor (bFGF) and EGF.
Antibodies and reagents
The following antibodies were used: Nestin (clone 10C2); Musashi (EP1302); O4 (clone 81); GFAP (clone GFA‐02); MAP‐2 (HM‐2); βIII‐tubulin (clone 2G10); and β‐actin (clone EP1123Y). Curcumin (Sigma, St Louis, MO, USA) was dissolved in DMSO to produce a 100 mM stock solution.
Clonogenic survival assay
Cells were untreated, treated with 2 μM curcumin (curcumin group), or 2 μM curcumin + 3‐methyladenine (curcumin + 3‐MA group). To determine whether the effect on GICs resulted from autophagy induction, we used 3‐MA (Cat: M9281; Sigma) to inhibit autophagy induction. Cells in the curcumin + 3‐MA group were treated with 3‐MA (10 mM) 10 min before curcumin. After treatment in various groups, GIC neurospheres were dissociated in single‐cell suspension prior to plating. Cells were seeded in DMEM/F12 without EGF/bFGF for 24 h, then cultured in DMEM/F12 with 10% FBS in six‐well plates until visible colony formation. Each treatment for each cell type from each source was carried out in triplicate. After 15 days in culture, cell colonies were fixed with ethanol and stained with 0.5% crystal violet (Sigma). Colonies that contained ≥50 cells were counted.
Neurosphere formation assay
Cells treated as described above were dissociated into single cells and plated in 24‐well plates at 10 cells per well. After 7 days, the percentage of wells containing neurospheres and the total number of cells were quantified.
Autophagy assay
Cells were untreated, or treated with curcumin, 3‐MA + curcumin, or E64d + curcumin. Glioma‐initiating cells were untreated, or treated with curcumin (2 μM) for 24 h. To detect the effects of the autophagy inhibitor 3‐MA or the lysosomal inhibitor E64 on LC3‐II, cells were treated with 3‐MA (10 mM) or E64 (10 μg/mL), respectively, 10 min before curcumin (2 μM). Cells were then subjected to immunofluorescence analysis or Western blot analysis.
Intracranial tumor cell injection into immunocompromised mice and post‐treatment experiments
All mouse experiments were approved by and carried out according to Chinese laws governing animal care. As previously reported, an intracranial orthotropic model was used for evaluation of tumorigenicity of GICs.10, 25 The GICs were dissociated and resuspended in 2 μL PBS. Cells (n = 200 000) were injected stereotactically into the right caudate nucleus (1.0 mm anterior and 2.5 mm lateral to the bregma) of 6‐week‐old athymic nude mice. The rate was 0.2 μL/min for a total of 10 min. On day 5 post‐tumor implantation, mice were randomized into two groups (14 mice per group): untreated (control) and curcumin. Curcumin was given as an i.p. injection (300 mg/kg) and repeated every 3 days. This dose was tolerated well in pilot experiments. Mice in each group were placed in either the tumor analysis group (n = 6) or the survival group (n = 8). Mice were observed daily to monitor external appearance, feeding behavior, and locomotion for a total of 120 days. Five weeks after tumor implantation, mice in the tumor analysis group were killed and their brains removed for H&E staining and transmission electron microscope examination. Mice in survival groups were observed daily until they became moribund. The survival time after GICs implantation until onset of a moribund state was recorded.
Results
Treatment with curcumin induced GIC differentiation
We obtained GICs, named SU‐2 and SU‐3, from surgically resected human GBM samples, as reported previously.10, 25, 26 We assessed the effect of various treatments on differentiation following the two mentioned differentiation protocols. Neurospheres were incubated with FGF2 and EGF under serum‐free conditions. A time‐course experiment showed that cells treated with curcumin acquired the markers of differentiation Tuj1, GFAP, and Olig2 (Fig. 1A) earlier than untreated cells and co‐treated cells. We also observed an increase in the expression of differentiation markers (GFAP and βIII‐tubulin), together with a decrease in the expression of neural stem/progenitor markers (CD133 and Nestin) as detected by immunocytochemical assays (Fig. 1B). Moreover, neurospheres were induced to differentiate in the absence of EGF and FGF for 3 days. Cells treated with curcumin expressed high levels of differentiation markers (Tuj1, GFAP, Olig2, and βIII‐tubulin) and low levels of neural stem/progenitor markers (CD133 and Nestin), whereas cells in the other groups expressed low levels of differentiation markers and high levels of neural stem/progenitor markers, as detected by immunocytochemical assays and as quantified by real‐time quantitative reverse transcription PCR (Fig. 2). Overall, our results indicated that curcumin induced the differentiation of GICs.
Figure 1.
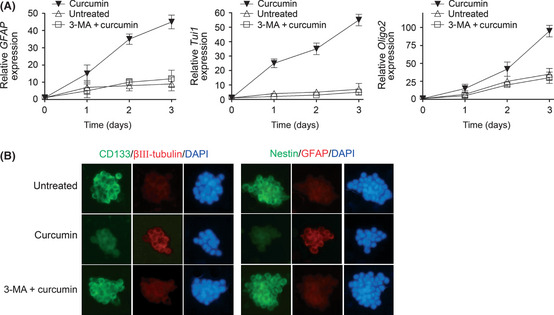
Curcumin promotes differentiation of glioblastoma neurospheres. (A) Quantitative real‐time PCR analysis was carried out to determine the mRNA levels of the differentiation markers GFAP, Tuj1, and Olig2 in SU‐2 neurospheres after 3 days of the indicated treatments. The GAPDH mRNA levels were used as an internal normalization control. Error bars represent the mean ± SD. (B) Immunocytochemistry for the indicated proteins was carried out in SU‐2 neurospheres after 3 days of the indicated treatments on poly‐l‐lysine‐coated coverslips. Nuclei were counterstained with DAPI. Similar results were observed in SU‐3 neurospheres (data not shown). 3‐MA, 3‐methyladenine.
Figure 2.
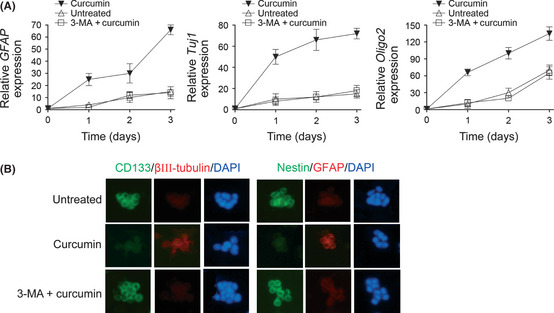
(A) Quantitative real‐time PCR analysis was used to determine the mRNA levels of the differentiation markers GFAP, Tuj1, and Olig2 in SU‐3 single glioma‐initiating cells after 3 days of the indicated treatments. The GAPDH mRNA levels were used as an internal normalization control. Error bars represent the mean ± SD. (B) Immunocytochemistry for the indicated proteins was carried out in SU‐3 neurospheres after 3 days of the indicated treatments on poly‐l‐lysine‐coated coverslips. Nuclei were counterstained with DAPI. Similar results were observed in SU‐2 cells (data not shown). 3‐MA, 3‐methyladenine.
Curcumin decreases GIC self‐renewal
We decided to evaluate the effect of curcumin on GIC self‐renewal following a well‐described protocol based on the ability of GICs to generate neurospheres.27 Cells treated as described in “Materials and Methods” were dissociated into single cells and cultured in serum‐free media with FGF2 and EGF. Relative to cells in the untreated group and co‐treated group, cells in the curcumin‐treated group became more attached to the culture plate, losing their spherical shape (Fig. 3B) after 7 days of the indicated treatments. The newly formed neurospheres and the total number of cells were counted. Treatment with curcumin decreased the amount and size of the newly formed neurospheres as well as the total number of cells, indicating that the curcumin represses GIC self‐renewal (Fig. 3A,B).
Figure 3.
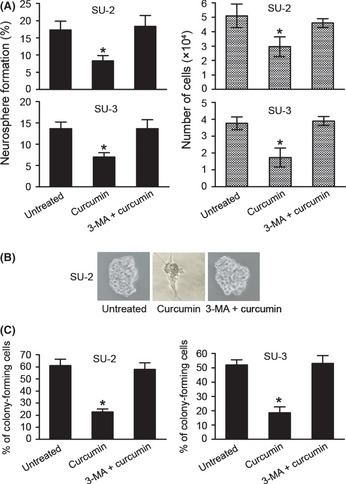
Effect of curcumin on glioma‐initiating cell self‐renewal. (A) Curcumin attenuated the efficiency of glioma‐initiating cells to form neurospheres. The percentage of neurosphere‐forming cells and the total number of cells were determined. *P < 0.05. (B) Representative images of SU‐2 neurospheres. Similar results were observed in SU‐3 neurospheres (data not shown). (C) The number of colonies was determined after staining with 0.01% crystal violet. *P < 0.05. Error bars represent the mean ± SD. 3‐MA, 3‐methyladenine.
To further validate the effect of curcumin on GIC self‐renewal, we carried out a clonogenic survival assay. Cells previously treated with curcumin generated a decreased number of colonies as compared to untreated cells or co‐treated cells (Fig. 3C). The decrease in self‐renewal and colony formation capacity was in line with the significant decrease in Nestin and Sox2 immunopositivity with curcumin treatment, whereas GFAP and βIII‐tubulin staining concomitantly increased.
Overall, our results indicated that curcumin not only induced the differentiation of GICs but also deregulated GIC self‐renewal.
Increased autophagy induction in GICs and xenograft tumors following treatment with curcumin
We investigated whether treating GICs with curcumin would result in autophagy. Although microtubule‐associated protein light chain 3 (LC3) has several homologs in mammals, LC3‐II is most commonly used for autophagy assays. LC3‐II, or the protein tagged at its N‐terminus with a fluorescent protein has been used to monitor autophagy through indirect immunofluorescence. Another approach is to detect LC3 conversion (LC3‐I to LC3‐II) by immunoblot analysis because the amount of LC3‐II is clearly correlated with the number of autophagosomes.28 After GIC exposure to untreated, curcumin, 3MA + curcumin, or E64d + curcumin, changes in cellular localization of punctate LC3 expression were observed (Fig. 4A). Quantification of the percentage of punctate LC3 expression in each treatment (Fig. 4B) showed that untreated and treatment with 3MA + curcumin resulted in approximately 4% and 5% of punctuate LC3 expression, respectively, compared to 70% of punctuate LC3 expression in single treatment with curcumin in GICs. To determine whether elevation in LC3 expression resulted from autophagy induction or sluggish autophagy flux, we used 3‐MA to inhibit autophagy induction and used E64d, a lysosomal protease inhibitor to inhibit the autophagy flux. As expected, the expression of LC3 was blocked by 3‐MA, which inhibits type III PI3K, initiator of autophagy. LC3 expression were elevated by E64d, which blocked the flux of autophagy. The changes in protein levels of LC3‐I (18 kDa) and LC3‐II (16 kDa) in GICs with the same treatments as mentioned above were further confirmed by immunoblotting analysis (Fig. 4C).
Figure 4.
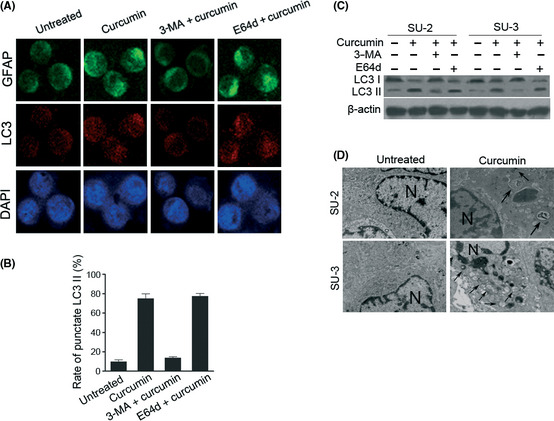
Autophagy detection in glioma‐initiating cells treated with curcumin. (A) Changes in LC3 and GFAP in SU‐2 after the indicated treatments assessed by immunocytochemistry. After specific treatment, the cells were subjected to immunofluorescence with anti‐LC3‐II antibody and anti‐GFAP antibody. (B) Quantification of cells with LC3 dots in SU‐2 treated by indicated treatments. The percentage of cells with punctate LC3 fluorescence was calculated relative to all GFP‐positive cells. Similar results were observed in SU‐3 (data not shown). (C) Cells were harvested in the indicated time for Western blot analyses for LC3‐II. (D) Representative electron micrograph images showing increased formation of autophagosomes following curcumin treatment in an in vivo SU‐3 xenograft model. Transmission electron microscope images showed intact nuclear membrane and normal structure of chromatin and mitochondria in untreated and co‐treated groups. The images show a remarkable increase of double membrane autophagosomes (arrows) in the curcumin‐treated group. Four mice in each group and 10 fields for each mouse were examined and displayed similar morphological changes. 3‐MA, 3‐methyladenine; N, nucleus. Scale bar = 1 μm.
We next carried out xenograft experiments to investigate the effect of treatment with curcumin in animal models. Electron microscope showed a remarkable increase in the number of autophagosomes in tumor sections obtained from our in vivo tumor study on curcumin treatment (Fig. 4D). As such, the effects of curcumin on GIC differentiation were specifically due to autophagy induction. Taken together, these findings indicate that curcumin promoted differentiation of GICs by activating autophagy.
Increased survival of mice bearing GIC intracranial xenograft tumor treated with curcumin
We next sought to establish if delivery of curcumin in vivo might prevent intracerebral glioma tumor establishment and growth. Animals with untreated therapy developed large tumor masses that showed characteristic GBM features, including nuclear atypia, expression of aberrant glia, and extensive neovascularization, and invaded the lateral, third, and fourth ventricles (Fig. 5A).29 Mice receiving curcumin therapy displayed small, confined lesions (Fig. 5A). Lesions in curcumin‐treated animals included few neoplastic cells, conversely, tumors in the untreated groups contained pleiomorphic, neoplastic elements, with malignant, infiltrating cells (Fig. 5A). Untreated intracranial glioma‐bearing mice developed cachexia and poor feeding that did not improve, whereas curcumin therapy resulted in less toxicity. Survival in animals bearing GIC intracranial xenografts was evaluated using log–rank analysis from a Kaplan–Meier survival curve (Fig. 5B). Nearly all mice receiving curcumin therapy survived the study period of 120 days, whereas three months post‐injection all animals in the untreated groups died. The data indicate that treatment of nude mice bearing intracranial GIC glioma xenografts with curcumin results in a significant increase in animal survival when compared with untreated controls. In summary, these findings indicate that curcumin suppresses the tumorigenicity of GICs and are consistent with our results in vitro.
Figure 5.
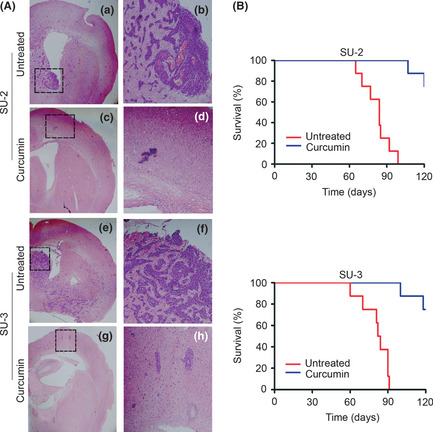
Treatment with curcumin inhibits tumorigenicity of glioma‐initiating cells. (A) Sections stained with H&E 5 weeks after tumor implantation in mice reveal the antitumor efficacy of curcumin. Tumors in untreated control mice contained pleiomorphic, neoplastic, and malignant, infiltrating cells that had invaded the ventricular system (a,b,e,f). Curcumin‐treated animals generated very small non‐invasive tumors (c,d,g,h). Magnification, ×2 (a,c,e,g) and ×10 (b,d,f,h). (B) Kaplan–Meier curves indicate that treatment with curcumin increased the survival of mice bearing glioma‐initiating cell intracranial xenografts. P < 0.05, untreated control versus curcumin.
Treatment with curcumin decreases tumorigenicity of GICs through induction of differentiation
Given the phenotypic changes we observed in curcumin‐treated GICs in vitro, we next tested whether treatment with curcumin affects in vivo differentiation potential of GICs (Fig. 6). The majority of cells within the untreated groups were Nestin positive, whereas only a few GFAP‐positive cells and a few βIII‐tubulin‐positive cells could be detected per field. In contrast, the xenograft lesions in the curcumin‐treated group showed decreased Nestin expression and a concomitant overall increase of GFAP and βIII‐tubulin positivity. These in vivo effects parallel the effects of curcumin on neurosphere cells observed in vitro.
Figure 6.
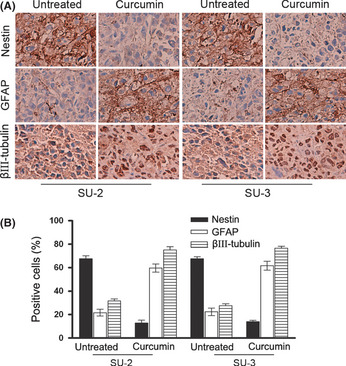
(A) Representative images of immunohistochemical staining of sections of tumors in pre‐transplantation treatment experiments with antibodies against GFAP, βIII‐tubulin, and Nestin at 35 days. Scale bar = 200 μm. (B) Quantitation of Nestin‐positive cells in tumors derived from SU‐2 glioma‐initiating cells.
Discussion
Glioblastomas are common and aggressive brain tumors in adults. The current standard treatments for malignant gliomas include surgical resection, radiation therapy, and chemotherapy. Despite progress in understanding the molecular mechanisms involved in the genesis and progression of glioma, the prognosis and treatment of this tumor type continue to be dismal. The median survival of patients with gliomas is only 9–12 months.30 Glioblastomas are highly heterogeneous both phenotypically and genotypically and are composed of several types of differentiated and undifferentiated cells. Like neural stem cells, poorly differentiated GICs have been shown to possess the capacity of self‐renewal and multilineage differentiation and are thus able to fuel tumor growth. One therapeutic strategy to specifically target the stem/progenitor‐like cell pool in a tumor involves forcing these cells to undergo differentiation. In our previous study, we provided several lines of evidence supporting a critical role for autophagy in maintaining a tumorigenic, stem cell‐like state.10
Autophagy is an evolutionarily highly conserved catabolic pathway that degrades cellular macromolecules and organelles.31, 32 Recent studies suggest that autophagy might be important in the regulation of cancer development and progression and in determining the response of tumor cells to anticancer therapy. However, the role of autophagy in these processes is complicated and may, depending on the circumstances, have diametrically opposite consequences for the tumor. In addition to its established roles in regulating nutrient supply and cell death, autophagy also functions in cellular development and differentiation. Accumulating evidence indicates that alterations in autophagy could influence cell fate during mammalian development and differentiation. Embryonic stem cells lacking beclin 1 or Atg5 are defective in forming cavitated embryoid bodies in vitro, due to the failure in clearing apoptotic cells.6 Autophagy is also a key cellular catabolic pathway that is also used for multiple other functions carried out by cells of the blood. This also includes their differentiation. Lack of autophagic activity (as a consequence of defective Atg5 expression) results in a differentiation block in the B cell lineage.8 These findings have given rise to the concept that autophagy might regulate development by performing critical cellular remodeling functions required for differentiation. Rather, the identification of a subset of GBMs that has this particular differentiation block will be of great utility for further understanding glioma pathophysiology, glioma stem cell biology, and ultimately design rationale for therapeutic strategies. Our previous results suggest that autophagy plays an essential role in the regulation of GIC self‐renewal, differentiation, tumorigenic potential, and radiosensitization, suggesting autophagy could be a promising therapeutic target in a subset of GBMs.10 Here, we report that curcumin induced differentiation of GICs and inhibited glioma growth. This is associated with the induction of autophagy. Our data showed that curcumin induced autophagy in vitro and in vivo. The basal activity of autophagy in the untreated GICs was very low, which was consistent with our previous study.26, 33 These results are interesting in the context of our recent findings,10 showing that autophagy can alter the differentiation and tumor‐initiating capacity of GICs.
Accumulating evidence shows that curcumin, a natural product, has a potent anticancer effect on a variety of cancer cell types, such as leukemia, breast cancer, prostate cancer, and pancreatic cancer.34, 35 However, the efficacy of curcumin for GICs is not yet fully determined. Here, we show that curcumin activated autophagy and triggered the differentiation cascade in GICs isolated from human GBMs. This was followed by a reduction in self‐renewal and clonogenic ability, and increased expression of neural differentiation markers. Analysis of the expression of differentiation markers by quantitative real‐time PCR revealed that GICs treated with curcumin expressed higher (greater than fourfold) levels of Tuj1, GFAP, and Olig2 relative to untreated cells including EGF and FGF free‐cultured GICs. Moreover, addition of curcumin induced a pro‐differentiation effect of GICs as reflected by a threefold increase of GFAP‐positive cells and a concurrent reduction of Nestin immunoreactivity in GIC‐derived xenografts. Importantly, curcumin suppressed the tumorigenicity of GICs as indicated by a strongly reduced tumor burden and increased survival of GIC‐carrying animals by inducing autophagy. Therefore, both the maintenance of stem cell‐like characteristics in vitro and tumorigenic potential in vivo were dependent on autophagy activity, further strengthening the notion that “forced” differentiation and concomitant depletion of the GIC pool can suppress brain tumor formation.1, 3, 4 In the light of these findings, autophagy‐inducer proteins such as curcumin might be promising targets for the treatment of GBM. The overall function of curcumin probably depends on its cellular context. Future investigations will provide further insights into the cell type‐specific role of autophagy and its implication in stem cell maintenance and brain tumorigenesis.
Disclosure Statement
The authors have no conflicts of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 30873052, 81072656, 81102466), the Natural Science Foundation of Jiangsu Province, China (Grant No. BK2010226), the Applied Basic Research Programs of Suzhou City (Grant No. SYS201105), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- 1. Lee J, Kotliarova S, Kotliarov Y et al Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum‐cultured cell lines. Cancer Cell 2006; 9: 391–403. [DOI] [PubMed] [Google Scholar]
- 2. Bao S, Wu Q, McLendon RE et al Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–60. [DOI] [PubMed] [Google Scholar]
- 3. Lee J, Son MJ, Woolard K et al Epigenetic‐mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma‐initiating cells. Cancer Cell 2008; 13: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piccirillo SG, Reynolds BA, Zanetti N et al Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour‐initiating cells. Nature 2006; 444: 761–5. [DOI] [PubMed] [Google Scholar]
- 5. Silber J, Lim DA, Petritsch C et al miR‐124 and miR‐137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med 2008; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qu X, Zou Z, Sun Q et al Autophagy gene‐dependent clearance of apoptotic cells during embryonic development. Cell 2007; 128: 931–46. [DOI] [PubMed] [Google Scholar]
- 7. Fader CM, Colombo MI. Multivesicular bodies and autophagy in erythrocyte maturation. Autophagy 2006; 2: 122–5. [DOI] [PubMed] [Google Scholar]
- 8. Miller BC, Zhao Z, Stephenson LM et al The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy 2008; 4: 309–14. [DOI] [PubMed] [Google Scholar]
- 9. Arsov I, Adebayo A, Kucerova‐Levisohn M et al A role for autophagic protein beclin 1 early in lymphocyte development. J Immunol 2011; 186: 2201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhuang W, Li B, Long L, Chen L, Huang Q, Liang Z. Induction of autophagy promotes differentiation of glioma‐initiating cells and their radiosensitivity. Int J Cancer 2011; 11: 2720–31. [DOI] [PubMed] [Google Scholar]
- 11. Singh S, Aggarwal BB. Activation of transcription factor NF‐kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem 1995; 270: 24995–5000. [DOI] [PubMed] [Google Scholar]
- 12. Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol 2007; 5: 25–37. [DOI] [PubMed] [Google Scholar]
- 13. Cole GM, Teter B, Frautschy SA. Neuroprotective effects of curcumin. Adv Exp Med Biol 2007; 595: 197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 2001; 21: 8370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci 2004; 74: 969–85. [DOI] [PubMed] [Google Scholar]
- 16. Aggarwal S, Takada Y, Singh S, Myers JN, Aggarwal BB. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor‐kappaB signaling. Int J Cancer 2004; 111: 679–92. [DOI] [PubMed] [Google Scholar]
- 17. LoTempio MM, Veena MS, Steele HL et al Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res 2005; 11: 6994–7002. [DOI] [PubMed] [Google Scholar]
- 18. Wang D, Veena MS, Stevenson K et al Liposome‐encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kappaB by an AKT‐independent pathway. Clin Cancer Res 2008; 14: 6228–36. [DOI] [PubMed] [Google Scholar]
- 19. Mukhopadhyay A, Bueso‐Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 2001; 20: 7597–609. [DOI] [PubMed] [Google Scholar]
- 20. Mehta K, Pantazis P, McQueen T, Aggarwal BB. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer Drugs 1997; 8: 470–81. [DOI] [PubMed] [Google Scholar]
- 21. Hanif R, Qiao L, Shiff SJ, Rigas B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin‐independent pathway. J Lab Clin Med 1997; 130: 576–84. [DOI] [PubMed] [Google Scholar]
- 22. Elattar TM, Virji AS. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res 2000; 20: 1733–8. [PubMed] [Google Scholar]
- 23. Lin YG, Kunnumakkara AB, Nair A et al Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor‐kappaB pathway. Clin Cancer Res 2007; 13: 3423–30. [DOI] [PubMed] [Google Scholar]
- 24. Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R. Curcumin‐induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IkappaB kinase and nuclear factor kappaB activity and are independent of the B‐Raf/mitogen‐activated/extracellular signal‐regulated protein kinase pathway and the Akt pathway. Cancer 2005; 104: 879–90. [DOI] [PubMed] [Google Scholar]
- 25. Huang Q, Zhang QB, Dong J et al Glioma stem cells are more aggressive in recurrent tumors with malignant progression than in the primary tumor, and both can be maintained long‐term in vitro. BMC Cancer 2008; 8: 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhuang W, Li B, Long L, Chen L, Huang Q, Liang ZQ. Knockdown of the DNA‐dependent protein kinase catalytic subunit radiosensitizes glioma‐initiating cells by inducing autophagy. Brain Res 2011; 1371: 7–15. [DOI] [PubMed] [Google Scholar]
- 27. Seaberg RM, van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J Neurosci 2002; 22: 1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kabeya Y, Mizushima N, Ueno T et al LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19: 5720–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galli R, Binda E, Orfanelli U et al Isolation and characterization of tumorigenic, stem‐like neural precursors from human glioblastoma. Cancer Res 2004; 64: 7011–21. [DOI] [PubMed] [Google Scholar]
- 30. Furnari FB, Fenton T, Bachoo RM et al Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 2007; 21: 2683–710. [DOI] [PubMed] [Google Scholar]
- 31. Levine B, Klionsky DJ. Development by self‐digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6: 463–77. [DOI] [PubMed] [Google Scholar]
- 32. Hoyer‐Hansen M, Jaattela M. AMP‐activated protein kinase: a universal regulator of autophagy? Autophagy 2007; 3: 381–3. [DOI] [PubMed] [Google Scholar]
- 33. Zhao Y, Huang Q, Yang J et al Autophagy impairment inhibits differentiation of glioma stem/progenitor cells. Brain Res 2010; 1313: 250–8. [DOI] [PubMed] [Google Scholar]
- 34. Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 2003; 23: 363–98. [PubMed] [Google Scholar]
- 35. Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci 2005; 1056: 206–17. [DOI] [PubMed] [Google Scholar]


