Abstract
CD98 is a heterodimeric glycoprotein of 125‐kDa, which consists of a 90‐kDa heavy chain (hc) subunit and 35‐kDa to 55‐kDa light chain (lc) subunits. It is strongly expressed on the surface of proliferating normal cells and almost all tumor cells. To investigate the participation of CD98 in cellular proliferation and malignant transformation, we analyzed cell‐cycle progression of NIH3T3 clones transfected with cDNA of human CD98hc. Although NIH3T3 and control transfectant cells grown to the subconfluent state were arrested in the G0/G1 phase by serum starvation, considerable portions of CD98hc‐transfected cells resided at S and G2/M phases. Under serum‐starved and confluent conditions, significant fractions (20–25%) of NIH3T3 and control transfectant cells contained less than 2n content DNA, indicating occurrence of apoptosis, whereas no apoptotic cells were detected in CD98hc‐transfectant cells. Under serum‐starved conditions, a marked increase in the levels of cyclin D1 and cyclin E and a decrease in p16 were observed in CD98hc‐transfectant cells. The reverse was true for NIH3T3 and control transfectant cells. Our results suggest that resistance to G1 arrest and apoptosis by CD98 overexpression are associated with high G1‐cyclins and low p16 levels. (Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02304.x, 2012)
CD98, which was originally identified as an early lymphocyte activation antigen with a 4F2 monoclonal antibody (mAb),1 B3 mAb2 or RL388 mAb,3 respectively, in human, rat or mouse species, is a disulfide‐linked heterodimeric protein with relative molecular mass of 125 000. This protein consists of a CD98 heavy chain (hc) subunit with molecular mass of 90 000 Da and one of six CD98 light chain (lc) subunits with molecular mass of 35 000 to 55 000 Da. CD98lc are 12‐pass non‐glycosylated transmembrane proteins, and are sorted to the plasma membrane through association with CD98hc single‐pass type II transmembrane glycoprotein.4, 5
In the functional aspect, involvement of CD98hc in the transport of various amino acids has been reported;6, 7 however, CD98hc is now regarded as a molecular chaperone of CD98lc or a regulator of amino‐acid transport activity by CD98lc, because six CD98lc have been identified as the main body of amino‐acid transport (systems L, y+L, xc− and asc).8, 9, 10, 11, 12, 13
In addition to the role in amino‐acid transport, the involvement of CD98 in cellular calcium uptake,14, 15, 16 homotypic aggregation and apoptosis of lymphoid progenitor cells,17 virus‐mediated cell fusion18, 19 and regulation of integrin affinity20 have been reported. Several groups report the higher expression of CD98 in restricted normal tissues, which contain actively dividing cells.1, 2, 21, 22, 23, 24, 25
As to the involvement of CD98 in cancers, we have reported higher expression of CD98 in various cancer cells using specific anti‐CD98hc mAb,2, 26 growth inhibition of cancer cells by anti‐CD98hc mAb27, 28, 29 or by liposomes containing anti‐cancer drug and coated with anti‐CD98hc mAb,30 malignant transformation of mouse fibroblasts by DNA transfection of human and rat cDNA of CD98hc,31, 32, 33 and cooperation between CD98hc and CD98lc in the malignant transformation.32 We have recently demonstrated that the CD98lc responsible for CD98hc‐mediated malignant transformation is L‐type amino‐acid transporter 1 (LAT1), also referred to as solute carrier (SLC) 7A5, among six CD98lc, namely, LAT1, LAT2 (SLC7A8), y+LAT1 (SLC7A7), y+LAT2 (SLC7A6), asc1 (SLC7A10) and xCT (SLC7A11), from experimental genetics using LAT1 gene‐disrupted chicken DT40 cells.34
Although many cellular functions are attributed to the CD98 complex, the precise role(s) of CD98 in cellular proliferation and malignant transformation in mammalian cells remains unclear. As mentioned above, we have found that murine fibroblasts transfected with human or rat CD98hc cDNA show various malignant phenotypes.31, 32, 33 Proliferation of normal mammalian cells is directly related to the cell cycle,35 and deregulation of cell cycle progression results in oncogenesis.36 To understand the function of CD98 with respect to cellular proliferation and malignancy, in the present study, we analyze the cell‐cycle progression and expression of G1 cyclins, CDK, and their inhibitors using NIH3T3 clones transfected with full‐length cDNA of human CD98hc.
Materials and Methods
Cell Culture
NIH3T3 cells (American Type Culture Collection, Bethesda, MD, USA) were grown in DMEM supplemented with 10% heat‐inactivated FBS (ICN Biomedicals, Aurora, OH, USA). Stable NIH3T3 transfectant clones expressing full‐length human CD98hc protein (NIH/hH‐1, NIH/hH‐2 and NIH/hH‐3) and a control NIH3T3 transfectant clone (NIH/neo) were maintained in DMEM containing 10% FBS and 400 μg/mL of Geneticin disulfate (G418; Wako Pure Chemical Industries, Osaka, Japan).31, 33 Multiple aliquots of individual clones (passages 5–10) were frozen, and each aliquot was used for no more than 2 weeks, to avoid potential phenotypic changes. Expression of human CD98hc protein on the cell surface of each clone was confirmed periodically by flow cytometry, as described previously.31, 33
Primary antibodies
HBJ127 (IgG1) mouse mAb recognizes CD98hc of human cells26 and shows species‐specific reactivity.30 Rabbit polyclonal antibodies against cyclin E (M‐20), p16 (M‐156), p27 (M‐19), CDK2 (M‐2) and CDK4 (C‐22), and a mouse mAb against cyclin D1 (72‐13G) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). A rabbit mAb against cyclin D1 (SP4) and integrin β1 (EP1041Y) were obtained from Abcam (Tokyo, Japan).
Cell‐cycle analysis
For synchronization, cells grown to approximately 50–80% confluency were washed twice with FBS‐free DMEM and starved in DMEM containing 0.2% FBS for 48 h. Cells were harvested by a brief trypsinization, and washed twice with PBS by centrifugation. Cellular DNA was stained using a Cycle Test Plus DNA reagent kit (Beckton‐Dickinson, Sunnyvale, CA, USA) according to the manufacturer's protocol. Flow cytometric analysis was performed using the FACScan flow cytometer and Cell Fit‐DNA software (Beckton‐Dickinson).
Analysis of apoptotic cells
Detection of DNA fragmentation by agarose gel electrophoresis was performed, as described previously.37 To detect DNA fragmentation by flow cytometry, separated nuclei were stained using the MEBSTAIN Apoptosis kit (MBL, Nagoya, Japan) based TUNEL and analyzed on a FACScan flow cytometer, as described previously.38
Immunoprecipitation and Immunoblot
Monolayered cells were rinsed twice with ice‐cold PBS, and harvested by a brief trypsinization. Cells were extracted by addition of lysis buffer (50 mM Tris, pH 7.6, 150 mM NaCl, 1 mM EDTA containing 1% SDS, 1% Triton X‐100, 1 mM dithiothreitol and inhibitors [1 mM PMSF, 20 μg/mL aprotinin, 20 μg/mL leupeptin, 10 μg/mL pepstatin, 1 mM NaF, 0.1 mM Na3VO4 and 10 mM glycerophosphate]) three to five times its volume of cell pellet(s). The solution was incubated on ice for 20 min and then ultracentrifuged at 18 000g for 45 min at 4°C. The cleared cell lysate (100 μg protein in each lane) was separated on SDS‐PAGE and transferred to Fluorotrans membranes (Pall BioSupport, Port Washington, NY, USA) using the semidry transfer apparatus. Membranes were treated with Block Ace (Dainihon Seiyaku, Osaka, Japan) 1:2 diluted with PBS, and incubated sequentially with rabbit polyclonal antibodies (2 μg/mL in 1% BSA‐PBS) and HRP‐conjugated protein A (Zymed Laboratories, South San Francisco, CA, USA) diluted 1:10 000 in PBS containing 0.05% Tween 20 (T‐PBS). When mouse mAb were used as primary antibodies, membranes were successively treated with mouse mAb, rabbit anti‐mouse immunoglobulins (Dako Japan, Kyoto) diluted 1:200 in 1% BSA‐PBS, and HRP‐conjugated protein A. Between each step, membranes were washed extensively with T‐PBS. HRP activity was detected using 0.05% 3,3′‐diaminobenzidine (Wako) and 0.01% hydrogen peroxide in 0.1 M Tris buffer, pH 7.5. For the analyses of immunocomplex, monolayer‐cultured cells were treated with ice‐cold NP‐40 lysis buffer, and the lysates sonicated and cleared by the ultracentrifugation at 18 000g for 45 min at 4°C. These cleared lysates were incubated successively with rabbit polyclonal antibodies to CDK4 or cyclin E followed by protein A‐Sepharose (Pharmacia, Uppsala, Sweden). The resultant immune complexes were washed extensively with the lysis buffer. Pellets containing immune complexes were analyzed by SDS‐PAGE.
Immunocytochemistry
Cells grown on Cellgen (collagen type 1, Koken, Tokyo, Japan) or poly‐L‐lysine (Sigma, 10 μg/mL) pretreated eight‐chamber culture slides (Falcon, Franklin Lakes, NJ, USA) with or without HBJ127 anti‐human CD98hc mAb (10 μg/mL) were rinsed once with PBS, fixed with acetone–methanol or 4% paraformaldehyde (2% sucrose) in PBS, and permeated with Triton buffer (0.5% Triton X‐100 in 20 mM HEPES, pH 7.4, 50 mM NaCl, 3 mM MgCl2 and 300 mM sucrose). After a rinse with PBS, cells in each well were overlaid with Block Ace 1:2 diluted with PBS for 1 h at 37°C, and either immediately used or stored at 4°C for up to 10 days until use. For the antibody labeling, cells were treated with 2 μg/mL of primary antibodies in PBS containing 1% BSA for 12 h at 4°C. After washing with PBS, cells were treated with biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA, USA), which were diluted 1:200 in PBS containing 1% BSA for 1 h. After washing with PBS, cells were treated with ABC reagent (Vector) diluted 1:100 in PBS for 1 h. Following extensive washing with PBS, cells were incubated with 0.05% 3,3′‐diaminobenzidine and 0.01% hydrogen peroxide in 0.1 M Tris buffer (pH 7.5), rinsed with water, dehydrated with ethanol, cleared by xylene and mounted by Permount (Fisher Scientific, Fair Lawn, NJ, USA). Localization of antibody‐defined components was observed under a microscope (Zeiss Axiolab, Thornwood, NY, USA) and photographed.
Results
CD98hc‐overexpressing NIH3T3 cells resist G0/G1 arrest under serum‐starved and subconfluent conditions
We have demonstrated that CD98hc‐transfected murine 3T3 clones are able to grow in soft agar and to develop tumors in athymic mice.31, 32, 33 In the study, CD98hc‐transfected NIH3T3 cells were assessed for cell cycle progression under the serum‐starved condition. The distribution patterns in the cell cycle of subconfluent (50–80% confluent) cells cultured in the medium containing 10% FBS were indistinguishable between the control (NIH3T3 and NIH/neo) and CD98hc‐transfected cells (Fig. 1, left). Following the culture of subconfluent cells in the medium containing 0.2% FBS for 48 h, more than 90% of the NIH3T3 and NIH/neo cells were arrested in the G0/G1 phase and less than 5% of the cells were in the S phase. In contrast, <75% of the CD98hc‐transfected clone NIH/hH‐1 and hH‐2 cells were in the G0/G1 phase and more than 10% of the cells were in the S phase (Fig. 1, middle). However, 66 h after culturing subconfluent cells with 0.2% FBS, more than 95% of the NIH3T3, NIH/neo and three CD98hc‐transfected cells were arrest in the G0/G1 phase (Fig. 1, right). In these culture conditions, sub‐G0/G1 peak showing a peak of cells having less than 2n content of DNA was not detected in NIH3T3, NIH/neo and two CD98hc‐transfected cells.
Figure 1.
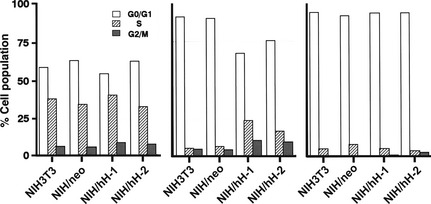
Cell‐cycle analysis of CD98hc‐transfected clones. Asynchronously growing control (NIH3T3 and NIH/neo) and CD98hc‐transfected clones (NIH/hH‐1 and hH‐2) were transferred to various culture conditions; namely, subconfluent cells were cultured in a medium supplemented with 10% FBS for 48 h (left) or in a medium with 0.2% FBS for 48 h (middle) or 66 h (right). Cells were subjected to flow cytometric analysis after propidium iodide staining (20 000 cells were routinely counted for each sample.
CD98hc‐overexpressing NIH3T3 cells resist apoptosis under serum‐starved and confluent conditions
When cells were allowed to reach confluence and then cultured in the medium containing 0.2% FBS for 48 h, NIH3T3 and NIH/neo showed a sub‐G0/G1 peak on the FACS histogram, corresponding to 30 and 26% of the population, respectively. However, the sub‐G0/G1 peak was not detected with any of the two CD98hc‐transfected clones (Fig. 2).
Figure 2.
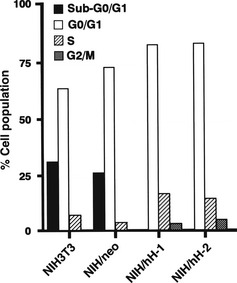
Cell‐cycle analysis of CD98hc‐transfected clones in confluent and serum‐starved conditions. Confluent NIH3T3, NIH/neo and CD98hc‐transfected clones (NIH/hH‐1 and hH‐2) were cultured in the medium containing 0.2% FBS for 48 h and subjected to flow cytometric analysis after propidium iodide staining.
To determine whether the emergence of sub‐G0/G1 peaks of NIH3T3 and NIH/neo cells cultured under confluent and serum‐starved conditions were caused by apoptosis, we performed TUNEL assays followed by flow cytometry (Fig. 3). Relatively large numbers of NIH3T3 and NIH/neo cells showed high fluorescence intensity, indicative of increased free DNA ends, whereas for all three CD98hc cDNA‐transfected clones, the number of cells showing high fluorescence intensity were negligible. Next, we prepared genomic DNA from control cells and CD98hc‐transfected clones cultured for 48 h under various culture conditions, and examined DNA fragmentation by agarose gel electrophoresis (Fig. 4). Under the subconfluent conditions with 10% (Fig. 4, right) or 0.2% FBS (Fig. 4, left), DNA fragmentation was not detected in the control or CD98hc‐transfected cells. DNA fragmentation was evident in confluent NIH3T3 and NIH/neo cells but not in all the two CD98hc‐transfected clones, which were cultured under the confluent and serum‐starved conditions (Fig. 4, middle).
Figure 3.
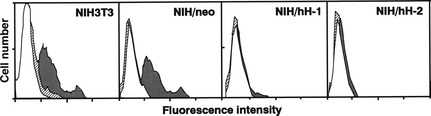
Analysis of resistance to apoptosis by overexpression of CD98hc using TUNEL method. Cells were cultured under the following three conditions: standard culture conditions with 10% FBS (△), or culture for 48 h with 0.2% FBS after subconfluence ( ) or confluence (
) or confluence ( ). Permeabilized cells were subjected to TUNEL. Cells were incubated with fluorescein‐conjugated avidin and subjected to flow cytometry.
). Permeabilized cells were subjected to TUNEL. Cells were incubated with fluorescein‐conjugated avidin and subjected to flow cytometry.
Figure 4.
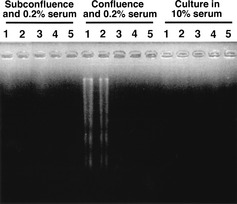
Analysis of resistance to apoptosis by overexpression of CD98hc revealed with DNA fragmentation. Genomic DNA was isolated from cells cultured in 10% FBS medium (right), or 0.2% FBS medium after subconfluence (left) or confluence (middle). Soluble DNA was subjected to agarose gel electrophoresis and stained with ethidium bromide. Lanes 1, NIH3T3; 2, NIH/neo; 3, NIH/hH‐1; 4, NIH/hH‐2; 5, NIH/hH‐3.
CD98hc‐overexpression influences the expression of cell‐cycle regulators under the conditions that induce cell‐cycle arrest or apoptosis in NIH3T3 cells
Serum components (growth factors) induce the expression of cyclin D1 in G0/G1‐arrested cells. Because overexpression of CD98hc in NIH3T3 cells induces resistance to G0/G1 arrest or apoptosis caused by serum starvation, we examined whether overexpression of CD98hc is associated with altered expression and/or activity of cell cycle regulators. Immunocytochemical analysis of subconfluent cells cultured under the serum‐starved conditions for 48 h revealed that p16 was strongly expressed in NIH/neo as compared with NIH/hH‐1, whereas cyclin D1 and cyclin E were strongly expressed in the nuclei of NIH/hH‐1 cells, but not in NIH/neo cells (Fig. 5). Higher expression of cyclin D1 in NIH/hH‐1was remarkably decreased by the addition of anti‐human CD98hc mAb to the culture of NIH/hH‐1 cells (Fig. 6). Next, expression of various cell cycle regulators was examined in the normal (10% FBS) and serum‐starved (0.2% FBS) conditions with subconfluent cells using immunoblot and immunoprecipitation analysis (Figs 7 and 8). Two CD98hc‐overexpressing clones expressed higher levels of cyclin D1 than parental NIH3T3 and control NIH/neo transfectant cells under normal or serum‐starved culture conditions (Fig. 7). As for the expression of cyclin E, CD98hc‐transfected clones, NIH/hH‐1 and hH‐2 expressed higher levels of this cyclin than control cells in the culture with 0.2% FBS. Two CD98hc‐transfected clones expressed higher levels of cyclin E as compared with the control cells in the normal culture condition. We next examined the expression of CDK4 and CDK2 that form a complex with cyclin D1 and cyclin E, respectively. In the culture with 10% FBS, the levels of CDK4 and CDK2 were almost the same between the control and CD98hc‐overexpressing cells. Under the serum‐starved condition, the levels of CDK4 and CDK2 in both two CD98hc‐transfected clones were slightly higher than those of the control cells. Inhibitor proteins such as p16 and p27 regulate the activities of CDK4 and CDK2, and the expression of these proteins in normal cells increases upon serum starvation and decreases after stimulation by growth factors. The levels of p27 in CD98hc‐overexpressing cells were similar to those in the NIH3T3 and NIH/neo cells. Although p16 was highly expressed in NIH3T3 and NIH/neo cells, it was not expressed in NIH/hH‐1 and NIH/hH‐2 cells under either the normal growth or serum‐starved conditions.
Figure 5.
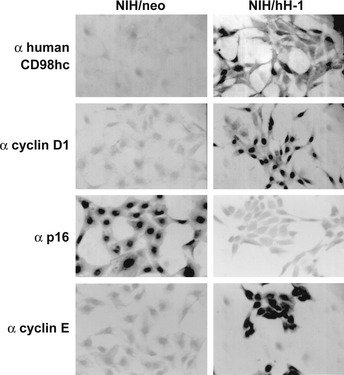
Immunocytochemical analysis of cell‐cycle regulators in control and CD98hc‐transfected cells. Formaldehyde‐fixed, and Triton X100‐permeated control (NIH/neo) as well as CD98hc‐transfected cells (NIH/hH‐1) grown on poly‐L‐lysine‐treated slides were successively treated with indicated antibodies, biotinylated secondary antibodies and ABC reagent.
Figure 6.
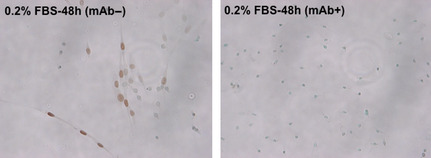
Effect of anti‐human CD98hc mAb on the expression of cyclin D1 in CD98hc‐transfected cells. Acetone‐methanol‐fixed cells grown on collagen‐treated slides cultured with or without anti‐human CD98hc mAb were successively treated with anti‐cyclin D1 rabbit mAb, biotinylated secondary antibodies and ABC reagent.
Figure 7.
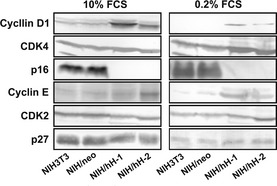
Immunoblot analysis of the expression levels of the G1/S Cyclin‐CDK complex components. Equal amounts of lysate proteins from NIH3T3, NIH/neo and two CD98hc‐transfected clones cultured in the medium with 10% (left) or 0.2% (right) FBS were subjected to SDS‐PAGE, blotted onto membranes and immunostained with antibodies with indicated cell‐cycle regulators.
Figure 8.
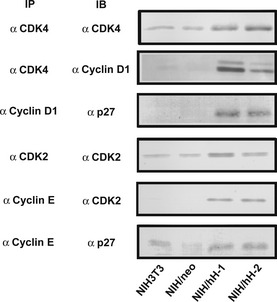
Immunoprecipitation and immunoblot analysis of cyclin D1‐CDK4 and cyclin E‐CDK2 complexes. Equal amounts of lysate proteins from NIH3T3, NIH/neo and two CD98hc‐transfected clones cultured in 0.2% FBS‐medium were treated with indicated antibodies (IP) for immunoprecipitation. Immunoprecipitates were subjected to SDS‐PAGE, blotted onto membranes and stained with indicated antibodies.
We next examined the complex formation between cyclins and CDK or p27 in subconfluent cells cultured in the serum‐starved conditions for 48 h (Fig. 8). Immunoprecipitation with anti‐CDK4 revealed the association of cyclin D1 with CDK4 in CD98hc‐overexpressing cells. The association of p27 with cyclin D1 was also observed in CD98hc‐overexpressing cells. The cyclin E‐CDK2 complex was detected in CD98hc‐overexpressing cells, and the association of p27 with cyclin E was also observed in these cells.
Discussion
Over the past ten years, accumulating reports have demonstrated the multiple functions of CD98hc‐lc molecules, including amino‐acid transport,4, 5, 8, 9, 10, 11, 12, 13 regulation of integrin affinity20 and virus‐induced cell fusion,18, 19 as well as the possible correlation of this molecule with lymphocyte activation1, 2, 3, 21, 23, 24, 25 and cell growth.2, 22, 23 We have demonstrated that murine fibroblasts transfected with human or rat CD98hc acquire the transformed phenotypes to grow in soft agar and to form tumors in nude mice,31, 32, 33 and that CD98hc‐mediated transformation requires cooperation with CD98lc,32 especially with LAT1.34 In this study, we have demonstrated that overexpression of CD98hc allows subconfluent cells to resist G0/G1 arrest and confluent cells to escape from apoptosis upon serum starvation.
In normal cells, growth arrest occurs in response to mitogen deprivation due to the inhibition of CDK kinase activities via specific posttranslational modifications of the CDK subunits39 or association with CDK inhibitors.40 NIH3T3 and NIH/neo cells were arrested in the G0/G1 phase 48 h after serum deprivation, whereas CD98hc‐transfected clones resisted arrest in the G0/G1 phase. However, after 66 h, CD98hc‐transfected cells were also arrested in the G1 phase, indicating that overexpression of CD98hc in NIH3T3 cells modulates the growth inhibitory mechanism but does not cancel the requirement of cells for serum growth factors to grow. The remarkable character of CD98hc‐transfected clones is that they express no or negligible levels of p16INK4a, even under the serum‐deprived condition. The levels of cyclin D1 and cyclin E in NIH3T3 and NIH/neo were very low, whereas those in CD98hc‐overexpressing cells cultured in serum‐starved conditions for 48 h were relatively high. Thus, loss or low levels of p16 and relatively high levels of cyclin D1 and cyclin E might explain the resistance of CD98hc‐transfected clones to G0/G1 arrest. Decreased expression of cyclin D1 by anti‐human CD98hc mAb might also substantiate the functional significance of overexpressed CD98hc in NIH/hH‐1 cells (Fig. 6).
Several lines of evidence suggest that regulation of apoptosis is closely related to the regulation of the cell cycle.41, 42, 43, 44 Although apoptosis can be induced at any point of the cell cycle, inducibility of apoptosis greatly differs depending on the point in the cell cycle.45 Induction of apoptosis by growth factor deprivation appears to be associated with cell cycle regulation, because a failure of the passage through a certain point of G1 phase results in apoptotic cell death in normal cells.46, 47 The progression through the G1 phase depends on the balance between cyclin D1 and p16, according to their positive and negative influence on G1 progression. In this context, it is interesting that mutations or abnormal expression of p16 and cyclin D1 were detected in various cancer cells.48, 49 Thus, the CD98hc‐transfected cells cultured under the serum‐starved and subconfluent conditions, which exhibit loss or low levels of p16 and relatively high levels of cyclin D1, are probably arrested in the late G1 phase that is beyond the apoptosis‐sensitive point.50
At present, why CD98hc‐transfected clones express no or relatively low levels of p16INK4a and relatively high levels of cyclin D1 and cyclin E under serum‐starved conditions as compared to control cells is not well understood. Cell growth is dependent on the stimuli by cell adhesion to extracellular matrix (ECM) proteins as well as serum growth factors. Both stimuli are required for the induction of cyclin D1 mRNA,51 and the ECM is also required for the translation of cyclinD1 mRNA.52 Cell anchorage to substratum reflects the interaction of the ECM with integrin: a family of cell surface receptors comprised of α and β chains that heterodimerize in distinct combinations to confer ligand specificity.53 Furthermore, it has been demonstrated that the physical interaction between the cytoplasmic domains of integrin β chains and CD98hc leads to the activation of integrin affinity through a conformational change in the ligand‐binding site of the integrins.20 Cross‐linking of CD98hc by anti‐CD98hc mAb promotes integrin‐like signaling and anchorage‐independent growth through the stimulation of phosphatidylinositol 3‐kinase, focal adhesion kinase and protein kinase B,54 and mAb‐induced CD98 activation increases surface expression and clustering of β1 integrin.55 CD98hc participates in fibronectin matrix assembly by mediating integrin signaling,56 and the association with integrin β1 is required for CD98hc‐mediated transformation.57
The present study has demonstrated that overexpression of CD98hc allows subconfluent cells to resist G0/G1 arrest and confluent cells to escape from apoptosis upon serum starvation, and might link CD98hc‐mediated activation of integrin β1 to CD98hc‐mediated malignant transformation and tumorigenesis. We are now trying to analyze the mechanism(s) of CD98‐mediated cell‐cycle progression and resistance to apoptosis, by evaluating the link to CD98hc‐mediated activation of integrin β1 in NIH3T3 cells. In this context, CD98hc‐overexpressing NIH/hH‐1 cells showed higher levels of integrin β1 and stronger adhesive activity to collagen (type 1) compared with NIH3T3 cells (data not shown). Studies are currently underway to further address the mechanism of CD98‐mediated malignancy and resistance to cell cycle arrest and apoptosis by evaluating possible roles of CD98lc,32, 34 and by searching for novel CD98‐associated molecules.58, 59, 60
CD98hc and CD98lc (LAT1 and xCT) were overexpressed on the surface of almost all tumor cells irrespective of the tissue of origin,26, 59, 60, 61 unlike typical receptor‐type oncoproteins, such as members of the HER family with restricted tumor distribution; therefore, analysis of CD98hc‐mediated transformation might reveal general mechanisms involved in the oncogenic process and might provide a novel target for cancer therapy. The significance of LAT1 in addition to CD98hc for the emergence of the malignant phenotype has been recently revealed; namely, we have demonstrated the essential role of LAT1 in malignant transformation through experimental genetics using targeted disruption of the LAT1 gene in chicken DT40 cells.34 Furthermore, we characterized human LAT1 as a promising target for the therapy of human cancers using experiments showing in vitro and in vivo growth inhibition of human cancer cells with hLAT1 small interfering RNA and anti‐hLAT1 mAb.34, 59, 61 As for novel CD98‐associated molecules, we now focus on CD44 variant (CD44v) molecules expressed on the surface of cancer stem cells (CSC) in the precancerous region of gastric adenocarcinomas of K19‐Wnt1/C2mE transgenic mice.62 Our recent data provide evidence that the expression of CD44v and its association with an xCT CD98lc block the reactive oxygen species‐induced stress signaling that results in growth arrest, cell differentiation and senescence, and, thereby, promote the proliferation of cancer cells and the formation of lethal gastrointestinal tumors,58 and that anti‐CD44v fully human mAb could inhibit tumor formation or tumor growth of xenografted human cancers.63 Given that CD44v‐expressing CSC play a central role in resistance to cancer therapy, it has been suggested that definitive treatment should target the xCT‐CD44vhigh cell population in cancer.58 We expect that anti‐CD98 therapeutic reagents will be applied to various types of human cancers, and that CD98hc/CD98lc (LAT1 and xCT) will become an excellent molecular target, possibly even superior to existing target proteins.
Disclosure Statement
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by the “Academic Frontier” Project (Kinki University, 2005–2007) and the “Antiaging Center” Project (Kinki University, 2008–2012) for Private Universities, with a matching fund subsidy from the Ministry of Education, Culture, Sports, Science and Technology, and was also supported by the “Adaptable and Seamless Technology Transfer Program through R&D” Project (2009–2011), with a matching fund subsidy from the Japan Science and Technology Agency.
References
- 1. Haynes BF, Hemler ME, Mann DL et al Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol 1981; 126: 1409–14. [PubMed] [Google Scholar]
- 2. Hashimoto Y, Masuko T, Yagita H, Endo N, Kanazawa J, Tazawa J. A proliferation‐associated rat cell surface antigen recognized by a murine monoclonal antibody. Gann 1983; 74: 819–21. [PubMed] [Google Scholar]
- 3. Luscher B, Rousseaux M, Lees R et al Cell surface glycoproteins involved in the stimulation of interleukin 1‐dependent interleukin 2 production by a subline of EL‐4 thymoma cells. II. Structure, Biosynthesis, and Maturation. J Immunol 1985; 135: 3951–7. [PubMed] [Google Scholar]
- 4. Mastroberardino L, Spindler B, Pfeiffer R et al Amino‐acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 1998a; 395: 288–91. [DOI] [PubMed] [Google Scholar]
- 5. Kanai Y, Segawa H, Miyamoto K et al Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem 1998; 273: 23629–32. [DOI] [PubMed] [Google Scholar]
- 6. Bertran J, Magagnin S, Werner A et al Stimulation of system y(+)‐like amino acid transport by the heavy chain of human 4F2 surface antigen in Xenopus laevis oocytes. Proc Natl Acad Sci USA 1992; 89: 5606–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wells RG, Lee WS, Kanai Y, Leiden JM, Hediger MA. The 4F2 antigen heavy chain induces uptake of neutral and dibasic amino acids in Xenopus oocytes. J Biol Chem 1992; 267: 15285–8. [PubMed] [Google Scholar]
- 8. Mastroberardino L, Spindler B, Pfeiffer R et al Amino‐acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 1998b; 395: 288–91. [DOI] [PubMed] [Google Scholar]
- 9. Pfeiffer R, Rossier G, Spindler B, Meier C, Kuhn L, Verrey F. Amino acid transport of y+L‐type by heterodimers of 4F2hc/CD98 and members of the glycoprotein‐associated amino acid transporter family. EMBO J 1999; 18: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 1999; 274: 11455–8. [DOI] [PubMed] [Google Scholar]
- 11. Liu X, Charrier L, Gewirtz A, Sitaraman S, Merlin D. CD98 and intracellular adhesion molecule I regulate the activity of amino acid transporter LAT‐2 in polarized intestinal epithelia. J Biol Chem 2003; 278: 23672–7. [DOI] [PubMed] [Google Scholar]
- 12. Rajan DP, Kekuda R, Huang W et al Cloning and expression of a b(0,+)‐like amino acid transporter functioning as a heterodimer with 4F2hc instead of rBAT. A new candidate gene for cystinuria. J Biol Chem 1999; 274: 29005–10. [DOI] [PubMed] [Google Scholar]
- 13. Fukasawa Y, Segawa H, Kim JY et al Identification and characterization of a Na(+)‐independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral D‐ and L‐amino acids. J Biol Chem 2000; 275: 9690–8. [DOI] [PubMed] [Google Scholar]
- 14. Dong S, Hughes RC. Galectin‐3 stimulates uptake of extracellular Ca2+ in human Jurkat T‐cells. FEBS Lett 1996; 395: 165–9. [DOI] [PubMed] [Google Scholar]
- 15. Posillico JT, Srikanta S, Eisenbarth G, Quaranta V, Kajiji S, Brown EM. Binding of monoclonal antibody (4F2) to its cell surface antigen on dispersed adenomatous parathyroid cells raises cytosolic calcium and inhibits parathyroid hormone secretion. J Clin Endocrinol Metab 1987; 64: 43–50. [DOI] [PubMed] [Google Scholar]
- 16. Michalak M, Quackenbush EJ, Letarte M. Inhibition of Na+/Ca2+ exchanger activity in cardiac and skeletal muscle sarcolemmal vesicles by monoclonal antibody 44D7. J Biol Chem 1986; 261: 92–5. [PubMed] [Google Scholar]
- 17. Warren AP, Patel K, McConkey DJ, Palacios R. CD98: a type II transmembrane glycoprotein expressed from the beginning of primitive and definitive hematopoiesis may play a critical role in the development of hematopoietic cells. Blood 1996; 87: 3676–87. [PubMed] [Google Scholar]
- 18. Ohgimoto S, Tabata N, Suga S et al Molecular characterization of fusion regulatory protein‐1 (FRP‐1) that induces multinucleated giant cell formation of monocytes and HIV gp160‐mediated cell fusion. FRP‐1 and 4F2/CD98 are identical molecules. J Immunol 1995; 155: 3585–92. [PubMed] [Google Scholar]
- 19. Suga S, Tsurudome M, Ohgimoto S et al Identification of fusion regulatory protein (FRP)‐1/4F2 related molecules: Cytoskeletal proteins are associated with FRP‐1 molecules that regulate multinucleated giant cell formation of monocytes and HIV‐induced cell fusion. Cell Struct Funct 1995; 20: 473–83. [DOI] [PubMed] [Google Scholar]
- 20. Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 1997; 390: 81–5. [DOI] [PubMed] [Google Scholar]
- 21. Macdonald HR, Lees RK, Bron C. Cell surface glycoproteins involved in the stimulation of interleukin 1‐dependent interleukin 2 production by a subline of EL‐4 thymoma cells. I. Functional characterization by monoclonal antibodies. J Immunol 1985; 135: 3944–50. [PubMed] [Google Scholar]
- 22. Patterson JA, Eisinger M, Haynes BF, Berger CL, Edelson RL. Monoclonal antibody 4F2 reactive with basal layer keratinocytes: studies in the normal and a hyperproliferative state. J Invest Dermatol 1984; 83: 210–3. [DOI] [PubMed] [Google Scholar]
- 23. Masuko T, Abe J, Yagita H, Hashimoto Y. Human bladder cancer cell‐surface antigens recognized by murine monoclonal antibodies raised against T24 bladder cancer cells. Jpn J Cancer Res 1985a; 76: 386–94. [PubMed] [Google Scholar]
- 24. Yagita H, Masuko T, Takahashi N, Hashimoto Y. Monoclonal antibodies that inhibit activation and proliferation of lymphocytes. I. Expression of the antigen on monocytes and activated lymphocytes. J Immunol 1986a; 136: 2055–61. [PubMed] [Google Scholar]
- 25. Tanaka T, Masuko T, Hashimoto Y. Appearance of a proliferation‐associated antigen, gp125, on rat and human lymphocytes by co‐stimulation with phorbol ester and calcium ionophore. J Biochem 1988; 103: 644–9. [DOI] [PubMed] [Google Scholar]
- 26. Masuko T, Abe J, Yagita H, Hashimoto Y. Human bladder cancer cell‐surface antigens recognized by murine monoclonal antibodies raised against T24 bladder cancer cells. Jpn J Cancer Res 1985b; 76: 386–94. [PubMed] [Google Scholar]
- 27. Yagita H, Masuko T, Hashimoto Y. Inhibition of tumor cell growth in vitro by murine monoclonal antibodies that recognize a proliferation‐associated cell surface antigen system in rats and humans. Cancer Res 1986b; 46: 1478–84. [PubMed] [Google Scholar]
- 28. Itoh K, Inoue K, Hirooka K et al Phage display cloning and characterization of monoclonal antibody genes and recombinant Fab fragment against the CD98 oncoprotein. Jpn J Cancer Res 2001; 92: 1313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Itoh K, Ohshima M, Sonobe M et al Antibody epitope peptides as potential inducers of IgG antibodies against CD98 oncoprotein. Cancer Sci 2009; 100: 126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka T, Suzuki S, Masuko T, Hashimoto Y. In vitro targeting and cytotoxicity of adriamycin in liposomes bearing monoclonal antibody against rat or human gp125 cell proliferation‐associated antigen. Jpn J Cancer Res 1989; 80: 380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hara K, Kudoh H, Enomoto T et al Malignant transformation of NIH3T3 cells by overexpression of early lymphocyte activation antigen CD98. Biochem Biophys Res Commun 1999; 262: 720–5. [DOI] [PubMed] [Google Scholar]
- 32. Shishido T, Uno S, Kamohara M et al Transformation of BALB3T3 cells caused by over‐expression of rat CD98 heavy chain (HC) requires its association with light chain: mis‐sense mutation in a cysteine residue of CD98HC eliminates its transforming activity. Int J Cancer 2000a; 87: 311–6. [DOI] [PubMed] [Google Scholar]
- 33. Hara K, Kudoh H, Enomoto T et al Enhanced tumorigenicity caused by truncation of the extracellular domain of GP125/CD98 heavy chain. Oncogene 2000; 19: 6209–15. [DOI] [PubMed] [Google Scholar]
- 34. Ohkawa M, Ohno Y, Takeuchi A et al Oncogenic character of L‐type amino‐acid transporter 1 (LAT1) revealed by the targeted disruption of LAT1 gene in chicken DT40 cells. Biochem Biophys Res Commun 2011; 406: 649–55. [DOI] [PubMed] [Google Scholar]
- 35. Weinberg RA. The retinoblastoma protein and cell cycle control. Cell 1995; 81: 323–30. [DOI] [PubMed] [Google Scholar]
- 36. Morgan DO. Principles of CDK regulation. Nature 1995; 374: 131–4. [DOI] [PubMed] [Google Scholar]
- 37. Gavrieli Y, Sherman Y, Ben‐Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992; 119: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yagi H, Masuko T. Multi‐color flow cytometric analysis using UV‐excited fluorochromes: study on the expression of CD98 amino‐acid transporter. Cytometry Res 2002; 12: 65–72. [Google Scholar]
- 39. Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin‐dependent kinases. Genes 1995; 9: 1149–63. [DOI] [PubMed] [Google Scholar]
- 40. Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science 1996; 271: 1861–4. [DOI] [PubMed] [Google Scholar]
- 41. Meikrantz W, Schlegel R. Suppression of apoptosis by dominant negative mutants of cyclin‐dependent protein kinases. J Biol Chem 1996; 271: 10205–9. [DOI] [PubMed] [Google Scholar]
- 42. Kranenburg O, van der Eb AJ, Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J 1996; 15: 46–54. [PMC free article] [PubMed] [Google Scholar]
- 43. Gil‐Gomez G, Berns A, Brady HJ. A link between cell cycle and cell death: Bax and Bcl‐2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J 1998; 17: 7209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sugiyama K, Akiyama T, Shimizu M et al Decrease in susceptibility toward induction of apoptosis and alteration in G1 checkpoint function as determinants of resistance of human lung cancer cells against the antisignaling drug UCN‐01 (7‐Hydroxystaurosporine). Cancer Res 1999; 59: 4406–12. [PubMed] [Google Scholar]
- 45. Leach SD, Scatena CD, Keefer CJ et al Negative regulation of Wee1 expression and Cdc2 phosphorylation during p53‐mediated growth arrest and apoptosis. Cancer Res 1998; 58: 3231–6. [PubMed] [Google Scholar]
- 46. Levkau B, Koyama H, Raines EW et al Cleavage of p21Cip1/Waf1 and p27Kip1 mediates apoptosis in endothelial cells through activation of Cdk2: role of a caspase cascade. Mol Cell 1998; 1: 553–63. [DOI] [PubMed] [Google Scholar]
- 47. Han EK, Begemann M, Sgambato A et al Increased expression of cyclin D1 in a murine mammary epithelial cell line induces p27kip1, inhibits growth, and enhances apoptosis. Cell Growth Differ 1996; 7: 699–710. [PubMed] [Google Scholar]
- 48. Hunter T. Oncoprotein networks. Cell 1997; 88: 333–46. [DOI] [PubMed] [Google Scholar]
- 49. Nobori T, Wu DJ, Lois A, Takabayashi K, Carson DA. Deletions of the cyclin‐dependent kinase‐4 inhibitor gene in multiple human cancers. Nature 1994; 368: 753–6. [DOI] [PubMed] [Google Scholar]
- 50. Afrakhte M, Heldin NE, Westermark B. Inhibition of G1 cyclin‐dependent kinase activity in cell density‐dependent growth arrest in human fibroblasts. Cell Growth Differ 1998; 9: 983–8. [PubMed] [Google Scholar]
- 51. Bohmer RM, Scharf E, Assoian RK. Cytoskeletal integrity is required throughout the mitogen stimulation phase of the cell cycle and mediates the anchorage‐dependent expression of cyclin D1. Mol Biol Cell 1996; 7: 101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu X, Ohtsubo M, Bohmer RM, Roberts JM, Assoian RK. Adhesion‐dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E‐cdk2, and phosphorylation of the retinoblastoma protein. J Cell Biol 1996; 133: 391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 1992; 69: 11–25. [DOI] [PubMed] [Google Scholar]
- 54. Rintoul RC, Buttery RC, Mackinnon AC et al Cross‐linking CD98 promotes integrin‐like signaling and anchorage‐independent growth. Mol Biol Cell 2002; 13: 2841–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim SM, Hahn J‐H. CD98 activation increases surface expression and clustering of β1 integrins in MCF‐7 cells through FAK/Src‐ and cytoskeleton‐independent mechanisms. Exp Mol Med 2008; 40: 261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Feral CC, Zijlstra A, Tkachenko E et al CD98hc (SLC3A2) participates in fibronectin matrixassembly by mediating integrin signaling. J Cell Biol 2007; 178: 701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Henderson NC, Collis EA, Mackinnon AC et al CD98hc (SLC3A2) Interaction with β1 Integrins Is Required for Transformation. J Biol Chem 2004; 279: 54731–41. [DOI] [PubMed] [Google Scholar]
- 58. Shishido T, Ohkawa M, Itoh A, Enomoto T, Hashimoto Y, Masuko T. Colocalization of GP125/CD98 with tropomyosin isoforms at the cell–cell adhesion boundary. J Biochem 2000b; 127: 253–61. [DOI] [PubMed] [Google Scholar]
- 59. Masuko T, Ohno Y, Masuko K et al Towards therapeutic monoclonal antibodies to membrane oncoproteins by a robust strategy using rats immunized with transfectants expressing target molecules fused to green fluorescent protein. Cancer Sci 2011; 102: 25–35. [DOI] [PubMed] [Google Scholar]
- 60. Ishimoto T, Nagano O, Yae T et al CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc– and thereby promotes tumor growth. Cancer Cell 2011; 19: 387–400. [DOI] [PubMed] [Google Scholar]
- 61. Ohno Y, Suda K, Masuko K, Yagi H, Hashimoto Y, Masuko T. Production and characterization of highly tumor‐specific rat monoclonal antibodies recognizing the extracellular domain of human LAT1 amino‐acid transporter. Cancer Sci 2008; 99: 1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ishimoto T, Ohshima H, Ohshima M et al CD44+ slow‐cycling tumor cell expansion is triggered by the cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci 2010; 101: 673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Masuko K, Okazaki S, Satoh M et al Anti‐tumor effect against human cancer xenografts by a fully human monoclonal antibody to a variant 8‐epitope of CD44R1 expressed on cancer stem cells. PLoS ONE 2012; 19: 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]


