Abstract
Cancer cells can metastasize throughout the body by various mechanisms, including the lymphatic system, resulting in tumor‐induced lymphangiogenesis that can profoundly affect patient survival. The aim of the present study was to examine the role of lymphangiogenesis in the metastasis of pancreatic cancer to the peripheral nerve plexus. Immunohistochemistry was performed to analyze specimens obtained from 70 ductal adenocarcinoma patients. The markers used included lymphangiogenic factor vascular endothelial growth factor (VEGF)‐C, the lymphatic‐specific marker D2‐40, and cytokeratin 19, an independent prognostic factor for pancreatic tumors. The relationship between survival rate and invasion of both the lymphatic vessels and peripancreatic nerve plexus (PNP) was evaluated, with clearly elevated lymphatic vessel density (LVD) in tissues adjacent to the cancer tissues. In fact, LVD levels were higher in adjacent tissues than in localized cancer tissues, and lymphatic vessel invasion into tissues adjacent to the tumor was significantly correlated with both PNP invasion (P = 0.005) and lymph node metastasis (P = 0.010). Correspondingly, LVD in tissues adjacent to the tumor was correlated with both invasion of lymphatic vessels surrounding the tumor (P = 0.024) and VEGF‐C expression (P = 0.031); in addition, VEGF‐C expression was correlated with invasion of lymphatic vessels around the tumor (P = 0.004). Survival rates were significantly lower in patients in whom there was peritumor lymphatic vessel invasion (P < 0.001), extrapancreatic nerve plexus invasion (P = 0.001), and/or lymph node metastasis (P < 0.001). Based on these results, lymphatic invasion associated with adjacent tumor growth likely contributes to the development of metastatic tumors that invade the PNP.
Tumor cells can spread throughout the body by direct invasion of local tissues or via the circulatory system, either through the cardiovascular system (hematogenous metastasis) or through the lymphatic system (lymphatic metastasis).1, 2 Given its clinical importance, metastasis via the cardiovascular system has been investigated extensively in an attempt to gain a better understanding the mechanisms involved.3 Because vascular endothelial growth factors (VEGFs), previously referred to as VEGF, are the most potent regulators of angiogenesis, they have emerged recently as critical therapeutic targets.4, 5, 6 It has been found that VEGF‐C is an important regulator of lymph vessel growth (lymphangiogenesis) and lymphatic metastasis.7, 8 Despite numerous studies indicating that the lymphatic vessels are the primary pathway for the metastasis of tumor cells,9, 10, 11 and therefore the potential clinical significance of this pathway, relatively little is known about metastasis via the lymphatic system.
The lack of detailed information regarding the mechanisms underlying lymphatic metastasis may be attributed to a lack of viable markers for the identification of lymphatic formations. However, the emergence of VEGF‐C as a dominant regulator of lymphangiogenesis has provided a new and more effective method for distinguishing between lymphatic and blood vessels related to tumor cell movement.12, 13 Increased VEGF‐C expression in the primary tumor has been shown to be closely correlated with increased dissemination of the tumor to regional lymph nodes.14 Coupled with the recent emergence of additional markers of lymphangiogenesis, including the D2‐40 protein specifically expressed in the lymphatic endothelium,15 lymphatic vessels may now be effectively marked without directly staining the vascular endothelium. Markers that specifically denote lymphangiogenesis are crucial to determine the clinical usefulness of the extent of lymph vessel involvement in tumor development as a prognostic factor.
Neurotropic growth has often been used as an indicator of poor prognosis in pancreatic cancer.16 Although invasion of the nervous system is relatively common in patients with pancreatic cancer, the extent of cancer metastasis to the nerves via the lymphatic vessels remains unclear. In a previous study investigating the mechanism of peripancreatic nerve plexus (PNP) invasion, the distribution of lymphatic vessels in the nerve plexus surrounding the superior mesenteric artery was analyzed using D2‐40. The results showed that pancreatic cancer induced lymphangiogenesis, and that lymphatic vessel density (LVD) was highly correlated with tumor differentiation, lymph node metastasis, and PNP invasion.16, 17, 18 Together, these studies indicate that newly formed lymphatic vessels are likely to be the major pathway for the spread of tumors to the PNP.
In the present study, we used cytokeratin 19 (CK19),19 a commonly used prognostic marker, to evaluate lymphatic invasion. It has been reported that CK19 is highly expressed in lymph nodes exhibiting disseminated tumor cells,20 and significant levels of CK19 have been found in the peripheral blood of patients with other malignancies, including liver, breast, and pancreatic cancers.21 Thus, using CK19 as a potential marker for lymphatic invasion of the PNP may both clarify the significance of lymphangiogenesis as a major route for the spread of tumor cells to the nervous system and provide mechanistic information regarding this method of tumor metastasis for further research and clinical application.
Materials and Methods
Patients and specimens
Tissue samples were collected from 70 patients who underwent surgery for pancreatic cancer between September 2003 and December 2004. The patient group consisted of 45 men and 25 women ranging in age from 37 to 82 years (mean age 59 years). For inclusion in the study, patients must not have received chemotherapy, radiotherapy, or any other treatment and were required to be free of any metastases, as determined using imaging or intraoperative examination prior to surgery. Patients with distant organ metastases, as determined by imaging and surgical exploration, were excluded from the study. All patients underwent superior mesenteric artery nerve plexus resection and regional lymph node dissection. Clinical classifications were based on the Classification of Pancreatic Carcinoma.23
The present study was approved by the Ethics Committee of the Second Military Medical University of Shanghai, China. Specimens obtained from the patients included both tumor and tumor‐adjacent tissues (≤2.0 mm). Samples were collected from tumors of the nerve plexus in the pancreatic head and the nerve plexus‐containing tissue around the hepatic artery and hepatoduodenal ligament. Samples of cancerous tissues from the nerve plexus surrounding the celiac artery in the pancreatic body and tail, superior mesenteric arterial plexus tissue (left), and splenic plexus tissue were also obtained. Furthermore, samples of peripancreatic lymph node and adipose tissue were collected.
Tissue samples were approximately 2.0 × 1.5 × 0.3 cm in size. Specimens were fixed in 10% paraformaldehyde for 24 h, embedded in paraffin wax and then sliced (3 μm). Sections were dried at 37°C and stained using H&E, D2‐40, and CK19 dual immunohistochemistry (IHC) or VEGF‐C IHC stains.
Immunohistochemistry
The procedures for D2‐40 and CK19 dual IHC and VEGF‐C IHC were as follows. The paraffin wax was removed from sections using xylene, followed by gradual hydration with ethanol (from 100% to 70%). After tissues had been processed in 3% H2O2 methanol buffer, they were incubated at 4°C overnight in the presence of primary antibodies, namely D2‐40 mouse mAb (1:100 dilution; Dako, Carpinteria, CA, USA), CK19 rabbit mAb (1 : 100 dilution; Epitomics, Burlingame, CA, USA), and VEGF‐C mAb (1:100 dilution; Invitrogen, Camarillo, CA, USA). The following day, tissues were incubated at room temperature for 30 min in the presence of secondary antibodies, namely horseradish peroxidase‐conjugated IgG (Polylink DS kit; Zhong Shan Goldbridge Biotechnology) and alkaline phosphatase (AP)‐conjugated IgG (or streptavidin peroxidase [SP] secondary antibody and peroxidase‐labeled streptavidin in the case of VEGF‐C). Samples were stained with diaminobenzidine and AP‐Red, followed by hematoxylin staining for 1 min. Slides were subsequently examined under a microscope. As a negative control, the primary antibodies were replaced with PBS.
Histopathological analysis
Lymphatic vessels were observed in areas where D2‐40‐positive staining was present as brown‐yellow granules in the lymphatic endothelial membrane and cytoplasm. In addition, D2‐40 staining was used to observe lymphatic vessel morphology and distribution. According to the method of Ohno et al.,24 D2‐40 staining was used to determine LVD in intratumor tissues, tumor‐adjacent tissues, and PNP tissues. Briefly, D2‐40 positivity was determined by light microscopy at a magnification of ×40, with the number of positive lymphatic capillaries counted at a magnification of ×200 (0.739 mm2/field for three fields). Mean values were then calculated to obtain LVD. Scoring of CK19‐positive cells was as described previously.25
The VEGF‐C‐positive cells appeared as brown‐yellow granules in the cytoplasm of pancreatic cancer cells. Each section was examined a minimum of 10 times at a magnification of ×400 to count both the number of positive cells and the total number of cells. Samples in which >30% cells (be area) were stained were considered to be VEGF‐C positive.
Data analysis
Data were analyzed using spss 13.0 (SPSS, Chicago, IL, USA), with differences between groups evaluated by the Chi‐squared test. Differences in the LVD of tumors, tumor‐adjacent tissues, and peripancreatic plexus tissues were compared using either t‐test or one‐way anova with post hoc Dunnett's test. Survival rates were evaluated using the Kaplan–Meier estimator and Cox's proportional hazards model. Survival rates were compared using the log‐rank test. P < 0.05 was considered significant.
Results
Clinical data
Analysis of samples tissues revealed that most tumors were located in the head of the pancreas or in the lesser pancreas (71.4%). Of the tumors sampled, 68.6% demonstrated high levels of differentiation. A large proportion of tumors was observed to be associated with positive peripancreatic nerve invasion (67.1%) or negative large vascular invasion (72.9%). Finally, high levels of VEGF‐C expression were detected in 61.4% of samples. These data support a relationship between pancreatic cancer progression and peripancreatic nerve invasion, with lymphangiogenesis as a primary means for the spread of tumor cells to the nervous system.
Lymphatic vessel and peripancreatic plexus invasion in non‐cancerous tissues associated with lymph node metastasis
To explore the role of lymphangiogenesis in the metastatic process, 47 patients (67.1%) with peripancreatic plexus invasion were analyzed (Fig. 1). Using IHC analysis revealed that both lymphatic endothelial cells and pancreatic cells were positive for D2‐40, as evidenced by brown‐yellow staining, with approximately half of all sampled tissues exhibiting positive staining (Table 1). In contrast, CK19 staining (red cytoplasmic staining on microscopic slides) was detected in pancreatic cancer cells only. Red areas corresponding to CK19 staining indicate high levels of tumor invasion by lymphatic vessels into non‐cancerous tissues, including adjacent tissues and the peripancreatic nerves (Fig. 2). The VEGF‐C‐positive cells were detected primarily in the cytoplasm of cancerous cells, although there was some limited staining for VEGF‐C in the stroma (data not shown). In all, tissue samples from 41 patients (58.6%) were found to be positive for peripancreatic lymphatic vessel invasion by tumor cells. Of these patients, 13 (31.7%) showed evidence of tumor cell invasion into the peripancreatic nerve adjacent to the lymphatic structures. The lymphatic vessels in the remaining 29 patients (41.4%) were unaffected, wherein the peripancreatic nerve surrounding the lymphatic vessel exhibited no signs of tumor cell invasion. Based on Chi‐squared analysis, invasion of tumor cells from lymphatic vessels into adjacent tissues was significantly associated with PNP metastasis (P = 0.005), lymph node metastasis (P = 0.010), and VEGF‐C expression (P = 0.004; Table 2). Notably, there was no correlation between the invasion of tumor cells from lymphatic vessels and gender (P = 0.492), tumor size (P = 0.441), tumor differentiation (P = 0.104), or tumor location (P = 0.701). There was no significant correlation between invasion of the PNP and lymph node metastasis (P = 0.108).
Figure 1.
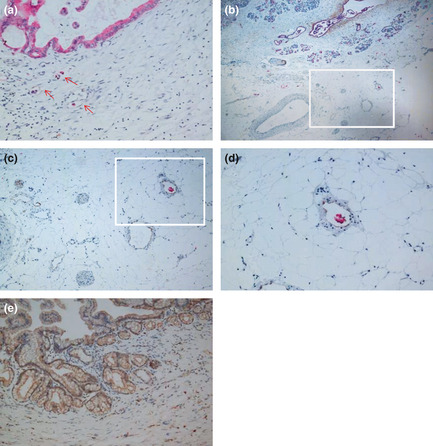
Cytokeratin 19 (CK19) and D2‐40 dual immunohistochemistry revealing lymphatic vessel (LV) invasion, peripancreatic plexus invasion, and lymph node metastasis. (A) High‐density area of LV in peripancreatic tissue. Most lymphatic vessels exhibited lumen‐like structures with tumor cells inside. Red arrows indicate lymphatic endothelial cells stained by D2‐40 and pancreatic cancer cells stained by CK19 (original magnification ×200). (B) Lymphatic vessels, blood vessels and nerve bundles showing CK19‐stained tumor cells inside the LV (original magnification ×40). (C) Staining of pancreatic tumor cells for CK19 (red) and peripancreatic LV for D2‐40 (brown‐yellow; original magnification ×100). Panel C shows the boxed area in (b) and panel D shows the boxed area in (c). (D) Tumor cells staining positive for CK19 (red) were found inside the peripancreatic LV (brown‐yellow; original magnification ×200). (E) Vascular endothelial growth factor‐C‐stained regions found in the plasma of tumor cells (brown‐yellow yellow; original magnification ×200).
Table 1.
Patient characteristics
| Characteristics | |
|---|---|
| Total no. patients | 70 |
| Age (years) | |
| Mean | 59.0 |
| Range | 37–82 |
| Gender | |
| No. men | 45 (64.3) |
| No. women | 25 (35.7) |
| Tumor size | |
| Diameter ≥4.0 cm | 40 (57.1) |
| Diameter <4.0 cm | 30 (42.9) |
| Tumor location | |
| Head of pancreas/lesser pancreas | 50 (71.4) |
| Body and tail of pancreas | 20 (28.6) |
| Tumor differentiation | |
| High | 48 (68.6) |
| Low | 22 (31.4) |
| Large vascular invasion | |
| Positive | 19 (27.1) |
| Negative | 51 (72.9) |
| Lymph node metastasis | |
| Positive | 37 (52.9) |
| Negative | 33 (47.1) |
| Peripancreatic nerve invasion | |
| Positive | 47 (67.1) |
| Negative | 23 (32.9) |
| Peritumoral LVI | |
| Positive | 41 (58.6) |
| Negative | 29 (41.4) |
| VEGF‐C expression | |
| Positive | 43 (61.4) |
| Negative | 27 (38.6) |
Unless indicated otherwise, data show the number of patients in each group, with percentages given in parentheses. LVI, lymphatic vessel invasion; VEGF‐C, vascular endothelial growth factor C.
Figure 2.
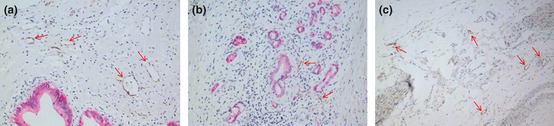
Cytokeratin 19 (CK19) and D2‐40 dual immunohistochemical analysis of lymphatic vessel density (LVD). (a) Peripancreatic tissue showing areas of high LVD. Most lymphatic vessels maintained lumen‐like structures (original magnification ×200). (b) Within pancreatic cancer tissues, low LVD was observed. The lymphatic vessels lost their structure due to the pressure created by the tumor (CK19 [red]‐stained pancreatic cancer cells in D2‐40 [brown‐yellow]‐stained lymphatic endothelial cells; original magnification ×200). (c) The peripancreatic nerve plexus region showing areas of high LVD. Most lymphatic vessels presented with enlarged lumen‐like structures. Arrows indicate lymphatic endothelial cells stained with D2‐40. (Original magnification ×100.)
Table 2.
Invasion of lymphatic vessels into adjacent tissues and clinicopathological features
| Clinicopathological features | n | Invasion of the lymphatic vessels into adjacent tissues | P‐value | |
|---|---|---|---|---|
| Positive (n) | Negative (n) | |||
| Gender | ||||
| Male | 45 | 25 | 20 | 0.492 |
| Female | 25 | 16 | 9 | |
| Tumor location | ||||
| Head of pancreas/lesser pancreas | 50 | 30 | 20 | 0.701 |
| Body and tail of pancreas | 20 | 11 | 9 | |
| Tumor size | ||||
| Diameter ≥4.0 cm | 40 | 25 | 15 | 0.441 |
| Diameter <4.0 cm | 30 | 16 | 14 | |
| Tumor differentiation | ||||
| High | 48 | 25 | 23 | 0.104 |
| Low | 22 | 16 | 6 | |
| Lymph node metastasis | ||||
| Positive | 37 | 27 | 10 | 0.010 |
| Negative | 33 | 14 | 19 | |
| PNP invasion | ||||
| Positive | 47 | 33 | 14 | 0.005 |
| Negative | 23 | 8 | 15 | |
| VEGF‐C expression | ||||
| Positive | 43 | 31 | 12 | 0.004 |
| Negative | 27 | 10 | 17 | |
PNP, peripancreatic nerve plexus; VEGF‐C, vascular endothelial growth factor C.
Peripancreatic nerve plexus invasion and analysis of lymphatic vessel distribution in the pancreas, peripancreatic region, and adjacent lymph nodes
Lymphatic vessels, blood vessels, and nerve bundles were all located in both tumor‐adjacent tissues and peripancreatic tissue. Lymphatic vessels specifically demonstrated non‐uniform distribution by staining, indicating a collapse of the lymphatic vessels around the tumor that resulted in partial or complete loss of their whole lumen‐like structure (Fig. 2b). Conversely, lymphatic vessels of the peripancreatic tissue, particularly those of the plexus, maintained their lumen structure (data not shown). In all, 15 patients (21.4%) exhibited peripancreatic lymphatic vessels that remained in close contact with, or even penetrated, the nerve bundle (Fig. 3).
Figure 3.
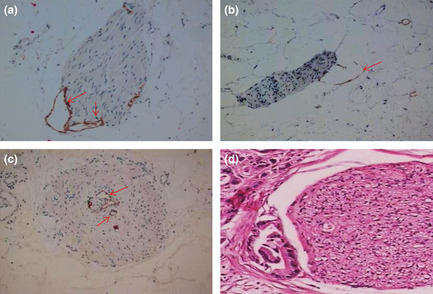
(a–c) Cytokeratin 19 (CK19) and D2‐40 dual immunohistochemical analysis of the distribution of lymphatic vessels (LV) in the peripancreatic plexus. (a) Peripancreatic LV accompanied by the peripancreatic plexus, showing close contact or invasion into the epineurium (original magnification ×200). (b) Peripancreatic LV in close contact or invading the epineurium. The LV are seen as semi‐open structures (original magnification ×200). (c) Peripancreatic LV directly entering the peripancreatic nerve bundle (original magnification ×200). (d) Invasion of the peripancreatic plexus showing infiltration of the epineurium and partial perineurium (H&E staining; original magnification ×100). Arrows indicate lymphatic endothelial cells stained by D2‐40.
Patients were grouped according to tumor location and classification, and the relationship between LVD and peripancreatic neural invasion analyzed. Patients were first divided into two groups, one consisting of 50 patients with tumors located in the head of the pancreas/lesser pancreas and the second consisting of 20 patients with tumors in the body and tail of the pancreas. The LVD was determined for each tissue individually (Table 3), with the results indicating that the average LVD was higher in tumor‐adjacent tissues than in tumor tissues (11.1 ± 4.8 vs 3.7 ± 2.1/field, respectively; P < 0.001; Fig. 2). The LVD in the nerve plexus region near the head of the pancreas was significantly higher than that in tissues adjacent to the hepatic artery and the hepatoduodenal ligament plexus (P = 0.026). In addition, LVD in both the celiac plexus tissues and splenic plexus tissues was significantly higher than that in the nerve plexus tissue surrounding the superior mesenteric artery (P < 0.001). The rate of PNP invasion in tissues from the pancreatic head plexus and celiac plexus (left) was 60.0% and 65.0%, respectively. The rate of PNP invasion was higher in both these tissues compared with that in other tissues of the pancreatic plexus (Table 3), indicating the likelihood of a relationship between tumor location and PNP invasion.
Table 3.
Comparison between lymphatic vessel density and peripancreatic neural invasion
| Tumor location | Specimen of pancreatic plexus | n | LVD | Positive pancreatic plexus invasion (%) |
|---|---|---|---|---|
| Head of pancreas | Pancreatic head plexus tissue | 30 | 6.5 ± 3.2* | 60.0 |
| Common hepatic artery tissue and hepatoduodenal ligament plexus tissue | 22 | 5.2 ± 2.5 | 44.0 | |
| Body and tail of pancreas | Celiac plexus tissue (left) | 13 | 8.0 ± 3.1† | 65.0 |
| Splenic plexus tissue | 7 | 6.9 ± 2.9† | 25.0 | |
| Superior mesenteric arterial plexus tissue (left) | 5 | 3.5 ± 1.9 | 35.0 |
*P < 0.05 compared with common hepatic artery tissue and hepatoduodenal ligament plexus tissue. †P < 0.001 compared with superior mesenteric arterial plexus tissue (left). Lymphatic vessel density (LVD) data are given as the mean ± SD.
The relationship between adjacent lymphatic vessels and pancreatic tumors was, in turn, related to clinicopathological factors. As indicated in Table 4, there was no significant difference in LVD in tumor‐adjacent tissues positive for lymph node invasion (P = 0.549). However, there was evidence of significantly greater PNP invasion in groups positive for lymphatic vessel invasion in adjacent tissue compared with the group negative group for lymphatic vessel invasions (P = 0.042). Similarly, significantly higher PNP invasion was seen in tumor‐adjacent tissues that were VEGF‐C positive compared with those that were VEGF‐C negative (P = 0.031). Finally, LVD in tumor‐adjacent tissues was higher in tissues positive for lymphatic vessel invasion (P = 0.024). Together, these results suggest that invasion of lymphatic vessels into tumor‐adjacent tissue is likely to be related to pancreatic cancer prognosis. Furthermore, there may be a relationship between prognosis and lymphatic vessel invasion, tumor differentiation, PNP invasion, and lymph node metastasis.
Table 4.
Lymphatic vessel density distribution in adjacent tissues and clinicopathological features
| Clinicopathological features | n | LVD | P‐value |
|---|---|---|---|
| Gender | |||
| Male | 45 | 11.3 ± 4.7 | 0.686 |
| Female | 25 | 10.8 ± 5.0 | |
| Tumor location | |||
| Head of pancreas/lesser pancreas | 50 | 10.9 ± 4.7 | 0.601 |
| Body and tail of pancreas | 20 | 11.6 ± 5.1 | |
| Tumor size | |||
| Diameter ≥4.0 cm | 40 | 11.0 ± 4.4 | 0.821 |
| Diameter <4.0 cm | 30 | 11.2 ± 5.3 | |
| Tumor differentiation | |||
| High or middle | 48 | 11.0 ± 5.0 | 0.800 |
| Low | 22 | 11.3 ± 4.3 | |
| Lymphatic vessel invasion in adjacent tissue | |||
| Positive | 41 | 12.2 ± 4.8 | 0.024 |
| Negative | 29 | 9.6 ± 4.4 | |
| Lymph node metastasis | |||
| Positive | 37 | 11.4 ± 4.4 | 0.549 |
| Negative | 33 | 10.8 ± 5.2 | |
| PNP invasion | |||
| Positive | 47 | 11.9 ± 4.6 | 0.042 |
| Negative | 23 | 9.4 ± 4.9 | |
| VEGF‐C expression | |||
| Positive | 43 | 12.1 ± 4.7 | 0.031 |
| Negative | 27 | 9.6 ± 4.6 | |
Lymphatic vessel density (LVD) data are given as the mean ± SD. PNP, peripancreatic nerve plexus; VEGF‐C, vascular endothelial growth factor C.
Survival rate analysis
Univariate analysis
Follow‐up was initiated after radical surgery for pancreatic cancer for the 60 patients (85.7%) who underwent this procedure, resulting in a median survival of 21 months. The survival rate by the end of 1st, 2nd, 3rd, and 5th years after surgery was 84.2%, 38.7%, 18.5%, and 7.7%, respectively (Fig. 4). Kaplan–Meier estimation was used to analyze the potential effects of individual factors on survival rates, including gender, tumor location, tumor size, tumor differentiation, large vasculature (e.g. portal vein and superior mesenteric vein) invasion, invasion of lymphatic vessels into tumor‐adjacent tissue, PNP invasion, and lymph node metastasis.
Figure 4.
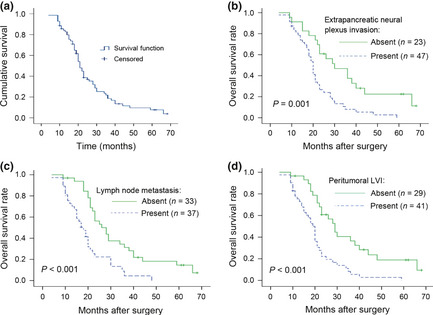
Survival analysis (Kaplan–Meier method). (a) Follow‐up was initiated after surgery for pancreatic cancer. A total of 60 patients (85.7%) completed follow‐up interviews. The median survival time was 21 months. At the end of 1, 2, 3, and 5 years after surgery, survival rates were 84.2%, 38.7%, 18.5%, and 7.7%, respectively. (b) Peripancreatic nerve plexus (PNP) invasion was closely correlated with survival (P = 0.001). Survival at 5 years was significantly greater in the patient group without PNP invasion. (c) Lymphatic vessel (LV) metastasis was closely correlated with survival (P = 0.001). Patients with no LV metastasis had significantly greater survival at 5 years. (d) Peritumor LV invasion (LVI) was correlated with survival (P < 0.001). Patients with no LVI had significantly better survival at 5 years.
Survival rates between groups were compared using the log‐rank test. The results indicate that the survival rate of pancreatic cancer patients is significantly correlated with vascular invasion (P = 0.003), lymph node metastasis into adjacent tissues (P < 0.001), the extent of tumor differentiation (P = 0.030), and PNP invasion (P = 0.001). These factors strongly influenced long‐term survival, as indicated in Table 5.
Table 5.
Factors contributing to prognosis, as determined using univariate analysis
| No. patients | Medium survival rate (months) | 2‐year survival rate (%) | 5‐year survival rate (%) | P‐value for 5‐year survival rate | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 45 | 23 | 43.7 | 11.0 | 0.074 |
| Female | 25 | 18 | 29.3 | 0.0 | |
| Tumor location | |||||
| Head of pancreas/lesser pancreas | 50 | 21 | 38.3 | 6.2 | 0.907 |
| Body and tail of pancreas | 20 | 21 | 40.0 | 10.0 | |
| Tumor size | |||||
| Diameter ≥4.0 cm | 40 | 21 | 33.6 | 3.1 | 0.459 |
| Diameter <4.0 cm | 30 | 23 | 45.1 | 15.2 | |
| Tumor differentiation | |||||
| Low | 22 | 16 | 24.1 | 0.0 | 0.030 |
| High | 48 | 23 | 45.1 | 11.7 | |
| Major vascular invasion | |||||
| Yes | 19 | 15 | 17.4 | 0 | 0.003 |
| No | 51 | 23 | 46.4 | 10.1 | |
| Lymphatic vessel invasion in adjacent tissue | |||||
| Yes | 41 | 19 | 22.2 | 0 | <0.001 |
| No | 29 | 29 | 60.8 | 18.9 | |
| Lymph node metastasis | |||||
| Positive | 37 | 18 | 22.3 | 0 | <0.001 |
| Negative | 33 | 28 | 56.3 | 14.6 | |
| PNP invasion | |||||
| Positive | 47 | 20 | 26.7 | 0 | 0.001 |
| Negative | 23 | 30 | 60.9 | 22.5 | |
P‐values were calculated by the log‐rank test. PNP, peripancreatic nerve plexus.
Multivariate analysis
The factors identified by univariate analysis were subsequently examined by Cox's proportional hazard models. This analysis revealed that survival rates were independently affected by major vascular invasion (P = 0.009), invasion of lymphatic vessel into tumor‐adjacent tissues (P = 0.011), PNP invasion (P = 0.046), and lymph node metastasis (P = 0.002). However, tumor differentiation had no significant effect on prognosis (P = 0.221). Table 6 lists the P‐values, associated risk factors, and 95% confidential intervals for each of these factors.
Table 6.
Cox's proportional hazards model of factors influencing survival rate
| Factors that may influence prognosis | P‐value | Risk (95% CI) |
|---|---|---|
| Adjacent lymphatic vessel invaded | 0.011 | 2.099 (1.90–3.705) |
| Peripancreatic neural invasion | 0.046 | 1.822 (1.010–3.289) |
| Regional lymphatic metastasis | 0.002 | 2.428 (1.385–4.256) |
| Major vascular invasion | 0.009 | 2.189 (1.212–3.953) |
| Extent of tumor differentiation | 0.221 | 1.424 (0.809–2.506) |
CI, confidence interval.
Discussion
Pancreatic cancer is a devastating neoplasm commonly associated with poor prognosis, particularly in cases of metastasis through the lymphatic system. The 5‐year survival rate for pancreatic adenocarcinoma26 is <5% and the only currently available method to treat the disease is complete tumor removal. Even in the case of successful tumor removal, long‐term survival may only be improved by as little as 18–24%.27 In 64–100% of patients, pancreatic cancer is complicated by neural invasion.28 Neural invasion is a cause of distant metastasis and retroperitoneal recurrence. Understanding the mechanisms and pathways governing nerve invasion will provide valuable insight for the development of novel therapeutic strategies.29
In the present study, 46 patients (59.7%) with peripancreatic neural invasion and 47 patients (61%) with regional lymph node invasion were evaluated, including dual staining for CK19 and D2‐40 or standard IHC staining for VEGF‐C in samples from 41 patients. The results revealed that the lymphatic vessels present in tumor‐adjacent tissues were often invaded by cancerous cells. Perineural lymphatic invasion was also found in 13 patients (18.6%). Most commonly, when lymphatic vessels surrounding the tumor were unaffected, the peripancreatic nerve was not invaded.
Analysis of lymphatic vessels within the PNP showed differential LVD distribution according to region, with the highest levels observed in pancreatic head plexus tissues and progressively lower levels observed in tissues from the celiac plexus, splenic plexus, common hepatic artery, hepatoduodenal ligament plexus, and superior mesenteric arterial plexus (Table 3). Consequently, metastasis can be considered to occur more frequently in certain regions, such as the pancreatic head plexus.30 The consistency between elevated LVD surrounding tissues and PNP invasion implies that the distribution of lymphatic vessels strongly influences the invasion of PNP. A reasonable explanation for this observation is that the lymphatic system represents the predominant route through which tumor cells spread to the peripancreatic nerve. A previous study has shown that tumors of the pancreatic head tend to grow towards the celiac plexus and posterior hepatic plexus, a finding that differs considerably from that of tumors of the pancreatic body and tail, which tend to spread towards the splenic plexus and celiac plexus.31 The findings of the present study support those of previous studies, indicating the potential prognostic importance of varying levels of LVD.
Major vascular invasion, lymphatic vessel invasion in tumor‐adjacent tissue, PNP invasion, and lymph node metastasis were shown to be the most dominant factors affecting survival rate, as determined using Kaplan–Meier analysis. Thus, both lymphatic metastasis and PNP invasion are critical factors impacting survival rates. Recent studies have shown that lymphangiogenesis may be tumor induced, thus providing a means for invasion and metastasis.32, 33 Both VEGF‐C and VEGF‐D have been shown to be capable of stimulating lymphatic vessel formation by activating VEGF receptor 3 (VEGFR‐3). Signaling via this pathway assists lymph node metastasis of various tumors, including pancreatic cancer, whereas blocking the VEGF‐C/D–VEGFR‐3 signaling pathway inhibits lymphangiogenesis and lymph node metastasis.34, 35, 36 In contrast, binding of VEGF‐C to VEGFR‐3 induces lymphangiogenesis in tumor tissues and causes lymphatic vessel dilation in surrounding tissues.36, 37 Although binding of VEGF‐C to VEGFR‐2 enhances microvascular formation, the binding of VEGF‐C to VEGFR‐3 promotes lymphangiogenesis.39 Clinically, high VEGFR‐3 expression has been associated with lymphatic vessel metastasis, ultimately impacting long‐term survival.40
The formation of new lymphatic vessels associated with tumor growth may be an important prognostic factor. Bjorndahl et al.41 reported that insulin‐like growth factor (IGF)‐1 and IGF‐2 were capable of inducing and promoting lymphangiogenesis and lymph node metastasis. The results of the present study demonstrate that VEGF‐C/D–VEGFR‐3 signaling pathways may interact with IGF to form new lymphatic vessels, a potential route for metastasis. In addition, VEGF‐C has been found to play a further role in lymphatic endothelial cell proliferation as a specific factor involved in chemotaxis. The entry of cancerous cells from the pancreas into lymphatic vessels is likely facilitated by endothelial junctions with the assistance of proteinase, an inhibitor of apoptosis.42 These cells may also invade into nerves through neural adhesion molecules.43 The process of metastasis through the lymphatic system involves several pathways, each of which is affected by the growth of cancerous tissue in the pancreas.
The close association between pancreatic cancer and peripancreatic nerve invasion has prompted extensive research into the mechanism of pancreatic cancer cell affinity for this particular neural structure. Studies have consistently suggested that nerve growth factor is a crucial mediator of neural invasion.44, 45 The determination of other growth factors that are involved in neural invasion, such as transforming growth factor‐α,46 glial cell line‐derived neurotrophic factor,47 artemin,48 and fractalkine,49 has allowed for the emergence of the molecular model of neurotrophism. Furthermore, neural cells and pancreatic cancer cells share a number of similar biological properties, including similarities in growth factor receptors and cell surface adhesion molecules, which may promote their interaction.30 The relationship between perineural invasion and the lymphatic system surrounding those nerves suggests that perineural invasion occurs not only as a result of direct infiltration, but also by way of lymphatic vessels, most commonly through newly formed lymph vessels, possibly due to cancerous growth.
Together, the results of the present study indicate that tumor cells are able to release several factors, including VEGF‐C, that serve to stimulate the formation of lymphatic vessels in tissues adjacent to the cancerous growth. Furthermore, some of these secreted factors may play a role in both the creation of new lymph vessels and the promotion of tumor cell entry into these newly formed passages, thus providing a route for metastatic invasion of surrounding neural tissues. The involvement of neurotropic adhesion facilitates further spread of tumor cells, accounting for common metastasis into the nerve plexus.
The present study provides strong evidence that tumor‐induced lymphatic vessel formation and PNP invasion are particularly important factors influencing patient survival. Regulation of lymphangiogenesis by VEGF‐C and regulation of neurotrophism using nerve growth factors is a potential goal that may result in the development of more effective clinical treatments, allowing for medical intervention in cases of pancreatic cancer than may result in an improved prognosis for these patients. The results of the present study are the first step in a process that will require further investigations, using both animal models and advanced molecular biology techniques, before the findings can be applied to a clinical setting.
Disclosure Statement
The authors declare that they have no conflict of interest.
Acknowledgment
This work was supported by a grant from the National Natural Science Foundation of China (30772139).
(Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02364.x, 2012)
References
- 1. Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002; 2: 573–83. [DOI] [PubMed] [Google Scholar]
- 2. Stacker SA, Baldwin ME, Achen MG. The role of tumor lymphangiogenesis in metastatic spread. FASEB J 2002; 16: 922–34. [DOI] [PubMed] [Google Scholar]
- 3. Risau W. Mechanisms of angiogenesis. Nature 1997; 386: 671–4. [DOI] [PubMed] [Google Scholar]
- 4. Ranieri G, Patruno R, Ruggieri E, Montemurro S, Valerio P, Ribatti D. Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr Med Chem 2006; 13: 1845–57. [DOI] [PubMed] [Google Scholar]
- 5. Rykala J, Przybylowska K, Majsterek I et al Angiogenesis markers quantification in breast cancer and their correlation with clinicopathological prognostic variables. Pathol Oncol Res 2011; 17: 809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Baumgarten L, Brucker D, Tirniceru A et al Bevacizumab has differential and dose‐dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res 2011; 17: 6192–205. [DOI] [PubMed] [Google Scholar]
- 7. Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF‐C‐induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007; 109: 1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Veikkola T, Enholm B, Hytönen M et al Adenoviral VEGF‐C overexpression induces blood vessel enlargement, tortuosity, and leakiness but no sprouting angiogenesis in the skin or mucous membranes. FASEB J 2002; 16: 1041–9. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Rawi MA, Jiang WG. Lymphangiogenesis and cancer metastasis. Front Biosci 2011; 16: 723–39. [DOI] [PubMed] [Google Scholar]
- 10. Rasmussen JC, Tan IC, Marshall MV et al Human lymphatic architecture and dynamic transport imaged using near‐infrared fluorescence. Transl Oncol 2010; 3: 362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roma AA, Magi‐Galluzzi C, Kral MA, Jin TT, Klein EA, Zhou M. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod Pathol 2006; 19: 392–8. [DOI] [PubMed] [Google Scholar]
- 12. Tang RF, Itakura J, Aikawa T et al Overexpression of lymphangiogenic growth factor VEGF‐C in human pancreatic cancer. Pancreas 2001; 22: 285–92. [DOI] [PubMed] [Google Scholar]
- 13. Zampell J, Avraham T, Yoder N et al Lymphatic function is regulated by a coordinated expression of lymphangiogenic and anti‐lymphangiogenic cytokines. Am J Physiol Cell Physiol 2012; 302: C392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skobe M, Hawighorst T, Jackson DG et al Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 15. Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2‐40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 2002; 15: 434–40. [DOI] [PubMed] [Google Scholar]
- 16. Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int 2002; 1: 469–76. [PubMed] [Google Scholar]
- 17. Saad RS, Kordunsky L, Liu YL, Denning KL, Kandil HA, Silverman JF. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol 2006; 19: 1317–23. [DOI] [PubMed] [Google Scholar]
- 18. Kurahara H, Takao S, Shinchi H et al Significance of lymphangiogenesis in primary tumor and draining lymph nodes during lymphatic metastasis of pancreatic head cancer. J Surg Oncol 2010; 102: 809–15. [DOI] [PubMed] [Google Scholar]
- 19. Schmitt AM, Anlauf M, Rousson V et al WHO 2004 criteria and CK19 are reliable prognostic markers in pancreatic endocrine tumors. Am J Surg Pathol 2007; 31: 1677–82. [DOI] [PubMed] [Google Scholar]
- 20. Chang CC, Yang SH, Chien CC et al Clinical meaning of age‐related expression of fecal cytokeratin 19 in colorectal malignancy. BMC Cancer 2009; 9: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stathopoulos EN, Sanidas E, Kafousi M et al Detection of CK‐19 mRNA‐positive cells in the peripheral blood of breast cancer patients with histologically and immunohistochemically negative axillary lymph nodes. Ann Oncol 2005; 16: 240–6. [DOI] [PubMed] [Google Scholar]
- 22. Gradilone A, Gazzaniga P, Silvestri I et al Detection of CK19, CK20 and EGFR mRNAs in peripheral blood of carcinoma patients: Correlation with clinical stage of disease. Oncol Rep 2003; 10: 217–22. [PubMed] [Google Scholar]
- 23. Japan Pancreas Society . Classification of Pancreatic Carcinoma, 2nd English edn Tokyo: Kanehara Shuppan, 2003. [Google Scholar]
- 24. Ohno M, Nakamura T, Kunimoto Y, Nishimura K, Chung‐Kang C, Kuroda Y. Lymphagenesis correlates with expression of vascular endothelial growth factor‐C in colorectal cancer. Oncol Rep 2003; 10: 939–43. [PubMed] [Google Scholar]
- 25. El Demellawy D, Nasr A, Alowami S. Application of CD56, P63 and CK19 immunohistochemistry in the diagnosis of papillary carcinoma of the thyroid. Diagn Pathol 2008; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klapman J, Malafa MP. Early detection of pancreatic cancer: Why, who, and how to screen. Cancer Control 2008; 15: 280–7. [DOI] [PubMed] [Google Scholar]
- 27. Yeo CJ, Abrams RA, Grochow LB et al Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single‐institution experience. Ann Surg 1997; 225: 621–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirai I, Kimura W, Ozawa K et al Perineural invasion in pancreatic cancer. Pancreas 2002; 24: 15–25. [DOI] [PubMed] [Google Scholar]
- 29. McColl BK, Loughran SJ, Davydova N, Stacker SA, Achen MG. Mechanisms of lymphangiogenesis: targets for blocking the metastatic spread of cancer. Curr Cancer Drug Targets 2005; 5: 561–71. [DOI] [PubMed] [Google Scholar]
- 30. Pour PM, Bell RH, Batra SK. Neural invasion in the staging of pancreatic cancer. Pancreas 2003; 26: 322–5. [DOI] [PubMed] [Google Scholar]
- 31. Makino I, Kitagawa H, Ohta T et al Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas 2008; 37: 358–65. [DOI] [PubMed] [Google Scholar]
- 32. Harrell MI, Iritani BM, Ruddell A. Tumor‐induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol 2007; 170: 774–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilting J, Hawighorst T, Hecht M, Christ B, Papoutsi M. Development of lymphatic vessels: tumour lymphangiogenesis and lymphatic invasion. Curr Med Chem 2005; 12: 3043–53. [DOI] [PubMed] [Google Scholar]
- 34. Kodera Y, Katanasaka Y, Kitamura Y et al Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res 2011; 13: R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kowanetz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res 2006; 12: 5018–22. [DOI] [PubMed] [Google Scholar]
- 36. Yang H, Kim C, Kim MJ et al Soluble vascular endothelial growth factor receptor‐3 suppresses lymphangiogenesis and lymphatic metastasis in bladder cancer. Mol Cancer 2011; 10: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aoki Y, Tosato G. Lymphatic regeneration: New insights from VEGFR‐3. J Natl Cancer Inst 2005; 97: 2–3. [DOI] [PubMed] [Google Scholar]
- 38. Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002; 2: 573–83. [DOI] [PubMed] [Google Scholar]
- 39. Goldman J, Rutkowski JM, Shields JD et al Cooperative and redundant roles of VEGFR‐2 and VEGFR‐3 signaling in adult lymphangiogenesis. FASEB J 2007; 21: 1003–12. [DOI] [PubMed] [Google Scholar]
- 40. Li R, Younes M, Wheeler TM et al Expression of vascular endothelial growth factor receptor‐3 (VEGFR‐3) in human prostate. Prostate 2004; 58: 193–9. [DOI] [PubMed] [Google Scholar]
- 41. Bjorndahl M, Cao R, Nissen LJ et al Insulin‐like growth factors 1 and 2 induce lymphangiogenesis in vivo . Proc Natl Acad Sci USA 2005; 102: 15593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Takahashi H, Funahashi H, Sawai H et al Synthetic serine protease inhibitor, gabexate mesilate, prevents nuclear factor‐κB activation and increases TNF‐α‐mediated apoptosis in human pancreatic cancer cells. Dig Dis Sci 2007; 52: 2646–52. [DOI] [PubMed] [Google Scholar]
- 43. Li R, Wheeler T, Dai H, Ayala G. Neural cell adhesion molecule is upregulated in nerves with prostate cancer invasion. Hum Pathol 2003; 34: 457–61. [DOI] [PubMed] [Google Scholar]
- 44. Demir IE, Ceyhan GO, Liebl F, D'Haese JG, Maak M, Friess H. Neural invasion in pancreatic cancer: the past, present and future. Cancers 2010; 2: 1513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhu Z, Friess H, diMola FF et al Nerve growth factor expression correlates with perineural invasion and pain in human pancreatic cancer. J Clin Oncol 1999; 17: 2419. [DOI] [PubMed] [Google Scholar]
- 46. Bockman DE, Büchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 1994; 107: 219–30. [DOI] [PubMed] [Google Scholar]
- 47. Ito Y, Okada Y, Sato M et al Expression of glial cell line‐derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery 2005; 138: 788–94. [DOI] [PubMed] [Google Scholar]
- 48. Ceyhan GO, Giese NA, Erkan M et al The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg 2006; 244: 274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marchesi F, Piemonti L, Fedele G et al The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res 2008; 68: 9060–9. [DOI] [PubMed] [Google Scholar]


