Abstract
Lymphatic spread is an important clinical determinant in the prognosis of many human cancers. The lymphangiogenic factor vascular endothelial growth factor‐D (VEGF‐D) is implicated in the promotion of lymphatic metastasis through the development of lymphatic vessels in some human cancers. In this study, we developed an anti‐VEGF‐D monoclonal antibody, cVE199, and investigated its in vitro properties, in vivo effects against tumors and possible target indications to evaluate its potential as a therapeutic antibody. The cVE199 molecule was revealed to have a specific binding reactivity against human VEGF‐D, as well as a specific inhibitory activity against the binding of human VEGF‐D to VEGFR‐3. In addition, cVE199 was found to inhibit the biological activity of VEGF‐D against lymphatic cells in vitro. Because we determined that a neuroblastoma cell line, SK‐N‐DZ, abundantly expressed VEGF‐D, an in vivo efficacy study was performed using a xenograft model of SK‐N‐DZ. We found that cVE199 significantly decreased lymphatic metastasis of SK‐N‐DZ as well as lymphangiogenesis in primary lesions. Finally, we investigated VEGF‐D expression in human neuroblastoma, finding that the molecule was expressed in 11 of 29 human neuroblastoma specimens (37.9%). In conclusion, we found that a novel anti‐VEGF‐D monoclonal antibody, cVE199, with specific reactivity against human VEGF‐D, prevents lymphatic metastasis of neuroblastoma through the inhibition of lymphangiogenesis in an animal model. In addition, our results show that VEGF‐D is expressed in some cases of human neuroblastomas, which suggests that cVE199 is a potential anti‐metastasis therapeutic antibody in neuroblastoma treatment.
Lethality in cancer is primarily associated with metastasis.1 In one type of this phenomenon, known as lymphatic metastasis, cancer cells spread to the lymph nodes. This type of metastasis serves as an important prognostic indicator for disease state and, indeed, lymphatic metastasis is correlated with poor prognosis in many tumor types.2, 3, 4, 5, 6, 7
Multiple steps are required for lymphatic metastasis to occur, including: detachment from the primary tumor mass; invasion into lymphatic vessels; transport through draining lymphatic vessels; and, finally, arrest, survival, and growth in the lymph nodes.8, 9 The first step is lymphangiogenesis, the formation in tumors of new lymphatic vasculature from preexisting vessels. Reliable markers for lymph vessels (e.g. CD44‐related hyaluronan receptor LYVE‐1) have revealed that tumor‐associated upregulation of lymphangiogenesis is linked to increased lymphatic spread and poor prognosis in human cancers.10 Hence, lymphangiogenesis has become a focus of interest in lymphatic metastasis.
A novel member of the vascular endothelial growth factor (VEGF) family, VEGF‐D, has been implicated in the promotion of lymphangiogenesis. This molecule is synthesized as a proprotein. Following synthesis, the VEGF homology domain region is proteolytically cleaved and secreted into the extracellular environment. This processing progressively increases the affinity of the molecule for its receptors.11 In general, VEGF‐D binds to VEGF receptor (VEGFR)‐2, found on both blood and lymphatic vessels, and VEGFR‐3, found predominantly on lymphatic vessels and in some angiogenic tumor blood vessels.12, 13 VEGFR‐3 signaling is the primary factor responsible for the lymphangiogenic response to VEGF‐D stimulation and leads to lymphangiogenesis in mouse models.14 Several experimental and clinical studies have shown positive correlations among VEGF‐D expression, tumor lymphatic vessel density and lymphatic metastasis.15 Moreover, a number of reports have shown that higher VEGF‐D expression correlates with poor prognosis in human tumors.16, 17, 18, 19, 20, 21 Therefore, the blockade of VEGF‐D seems to be a promising method of preventing the lymphatic metastasis of tumors.
To explore this possibility, we generated a novel anti‐VEGF‐D monoclonal antibody, cVE199, and investigated its in vitro binding and neutralizing activity against human VEGF‐D, its in vivo anti‐lymph node metastasis efficacy against VEGF‐D‐expressing tumors and its possible indications in human cancer treatment.
Materials and Methods
Anti‐vascular endothelial growth factor‐D antibody generation
Female BALB/c mice were immunized with recombinant VEGF‐DΔNΔC, a polypeptide that contains amino acid residues 93–201 of human VEGF‐D. After an increase in the serum titer was observed, splenocytes were prepared from the sacrificed mice and fused with myeloma cells. The resulting hybridoma cells were selected using a conventional HAT medium.
Chimeric antibody genes were then constructed by linking the cDNA of the variable regions in the antibody's heavy and light chains with the cDNA of the constant regions from human IgG1 and kappa chains. The resultant chimeric antibodies were then heterologously expressed in HEK293 suspension culture and purified with protein A affinity chromatography and size‐exclusion chromatography.
Binding assay for cVE199
For the binding assay, 96‐well plates were coated with human VEGF‐D (R&D Systems, Minneapolis, MN, USA) at 4°C overnight. After the plates were washed in 0.05% Tween20/PBS, they were incubated with Blocking One (Nacalai Tesque, Kyoto, Japan) for 1 h. The plates were then incubated with monoclonal antibody at the required dilutions. Bound monoclonal antibody was detected with an anti‐human Ig‐HRP (Life Technologies Japan, Tokyo, Japan). The assay was quantified by reading absorbance at 450 nm with an EnVision spectrophotometer (PerkinElmer, Waltham, MA, USA).
The inhibitory activity of cVE199 against the binding of vascular endothelial growth factor‐D to vascular endothelial growth factor receptor‐3
Stable human VEGFR‐3‐expressing CHO cells were aliquoted in a collagen type‐I 96‐well plate (Becton Dickinson, Franklin Lakes, NJ, USA) and incubated for 2 days at 37°C. Following this, 2% FBS/HBSS containing 2.5 μg/mL of His‐tagged recombinant protein (VEGF‐D or VEGF‐C) and/or monoclonal antibody was added, and the cells were then incubated for 1 h. After three washes in 0.05% Tween20/PBS, the cells were incubated with polyHistidine‐HRP MAb (R&D Systems) for 1 h. The assay was quantified by reading absorbance at 405/620 nm with a multi‐well plate reader (Bio‐Rad Laboratories, Hercules, CA, USA).
Immunoprecipitation
His‐tagged human/mouse VEGF‐D and His‐tagged human VEGF‐C were immunoprecipitated by cVE199 and Protein A agarose at 4°C overnight, followed by three washes in PBS. The samples were subsequently analyzed by western blotting.
Neutralization assay with human lung lymphatic microvascular endothelial cells
Human lung lymphatic microvascular endothelial cells (HMVEC‐LLy) were obtained from Takara Bio (Shiga, Japan), and maintained according to the suppliers' instructions. MAZ‐51 was purchased from Sigma‐Aldrich Japan (Tokyo, Japan). For the assay, cells were aliquoted at 5 × 103 cells per well in a 96‐well plate. After a 16‐h incubation period at 37°C, the cells were cultured in RPMI 1640 medium containing human VEGF‐D and 0.1% FBS. Three days later, Cell Counting Kit‐8 solution (Dojindo Laboratories, Kumamoto, Japan) was added. After the cells were incubated for several hours, absorbance at 450 nm was measured with an EnVision spectrophotometer (PerkinElmer).
Cell culture
Cell lines were obtained from the American Type Culture Collection (Manassas, VA, USA), the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany) and the Health Science Research Resources Bank (Sennan, Japan). All cell lines were cultured according to the suppliers' instructions.
Vascular endothelial growth factor‐D quantification by sandwich ELISA
All VEGF‐D quantification by ELISA in this study was performed using the Quantikine Human VEGF‐D ELISA kit (R&D Systems).
For the screening of VEGF‐D‐expressing tumor cells, 100 μg of protein from each cell line was analyzed. For the detection of secreted VEGF‐D, cells (5 × 105/well) were cultured in six‐well plates for 72 h in the condition medium. Following this, the supernatants were analyzed.
Gene expression analysis by Taqman
RNA was isolated from cells using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and cDNA were synthesized using the High Capacity RNA‐to‐cDNA Kit (Applied Biosystems, Foster City, CA, USA). For real‐time PCR analysis, 40 ng of cDNA was used for each reaction. Detection of the human VEGF‐D gene was performed using Taqman probe (Hs01128661_m1; Applied Biosystems). The expression level of VEGF‐D was quantified against the housekeeping gene Gapdh (402869; Applied Biosystems). Reactions were run on the 7900HT Fast Real‐Time PCR system (Applied Biosystems), and the calculation of the delta C t value was performed using Sequence Detection System (SDS) software v2.3.
Western blotting
Western blotting was performed as described previously.22 The following primary antibodies were used: His‐tag (MBL, Nagoya, Japan), VEGF‐D (Santa Cruz Biotechnology, Santa Cruz, CA, USA), VEGFR‐2, VEGFR‐3, pY1175‐VEGFR‐2 (Cell Signaling, Beverly, MA, USA), pY1063/1068‐VEGFR‐3 (Cell Applications, San Diego, CA, USA) and tubulin‐alpha (AbD Serotec, Oxford, UK). Signals were detected using ECL Plus (GE Healthcare, Waukesha, WI, USA), followed by LAS‐4000 (Fujifilm, Tokyo, Japan). Images were edited with MultiGauge (Fujifilm).
Animal care
All in vivo studies described here were conducted following the protocol approved by the Chugai Institutional Animal Care and Use Committee. All animal experiments were performed in accordance with the “Guidelines for the Accommodation and Care of Laboratory Animals” of Chugai Pharmaceutical. All animals were housed in a pathogen‐free environment under controlled conditions (temperature, 20–26°C; humidity, 40–70%; light–dark cycle, 12–12 h). Chlorinated water and irradiated food were provided ad libitum. The animals were allowed to acclimatize and recover from shipping‐related stress for 1 week prior to the study. The health of the mice was monitored daily.
Mouse xenograft study
SK‐N‐DZ_luc cells (5 × 106 in HBSS with BD Matrigel matrix; BD Biosciences, Billerica, MA, USA) were subcutaneously inoculated in the right flanks of 6‐week‐old female CB‐17 mice that had SCID (C.B‐17/Icr‐scid Jcl; Japan CLEA, Tokyo, Japan). After interperitoneal administration of luciferase substrate (65 mg/kg; VivoGloTM Luciferin; Promega, Madison, WI, USA), the anaesthetized mice were imaged using an in vivo imaging system (NightOWL L983; Berthold Technologies GmbH & Co KG, Bad Wildbad, Germany). Luciferase signals were visualized and quantified using WinLight32 (Berthold Technologies GmbH & Co KG) imaging software for NightOWL.
For the detection of lymph node metastasis, resected lymph nodes were sonicated and lysed with 100 μL of Cell Lysis Buffer (Cell Signaling) containing a complete protease inhibitor cocktail (Roche Diagnostics, Penzberg, Germany). The same amount of Bright‐Glo luciferase assay system (Promega) was added. Assays were quantified by reading luminescence with an EnVision spectrophotometer (PerkinElmer).
For lymph vessel staining, three tumors from each treatment group were excised on day 14, weighed, and embedded in O.C.T compound (Tissuetek; Sakura Finetek Japan, Tokyo, Japan), prior to being frozen in liquid nitrogen. Cryosections (5 μm) were fixed with ethanol and immunohistochemically stained for Lyve‐1 using the Ventana automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA). The following staining antibodies were used: polyclonal anti‐mouse Lyve‐1 antibody (RELIATech GmbH, Wolfenbüttel, Germany) and biotinized anti‐rabbit‐HRP antibody (Vector Laboratories, Burlingame, CA, USA). The slides were counterstained with hematoxylin.
Immunohistochemical staining for vascular endothelial growth factor‐D
Using a commercially available tumor microarray, 29 tissue samples were obtained from neuroblastoma patients (US Biomax, Rockville, MD, USA) and tumor sections (TriStar Technology Group, Rockville, MD, USA). Following this, 10% formalin‐fixed paraffin‐embedded (FFPE) slides were stained with anti‐VEGF‐D antibody (sc‐101584; Santa Cruz Biotechnology), using a Ventana automated immunostainer (Ventana Medical Systems), according to the manufacturer's protocol. In brief, slides were pretreated with heat for 90 min by cell‐conditioning solution 1 (Ventana Medical Systems). The first antibody was incubated for 32 min at 37°C. Signals were visualized with an ultraView Universal DAB detection kit (Ventana Medical Systems).
To evaluate VEGF‐D expression levels, the number of positively stained cells per 1000 tumor cells was counted. The expression was scored based on the percentage of positively stained cells. All cases were judged to be positive for VEGF‐D when the proportion of immunoreactive tumor cells was <30%.
Statistical analyses
All statistical tests in this study were performed using the Mann–Whitney U‐test. P < 0.05 was considered to be significant. Statistical analyses were performed using the sas preclinical package (version 8.2; SAS Institute, Cary, NC, USA).
Results
cVE199, a human vascular endothelial growth factor‐D‐specific monoclonal antibody
First, we generated a monoclonal human mouse chimeric antibody against human VEGF‐D, which was designated cVE199, as described in the Materials and Methods.
The binding activity of cVE199 to human VEGF‐D was determined by an antigen‐coated ELISA (Fig. 1a). The binding specificity against human VEGF‐D was evaluated by immunoprecipitation assay using His‐tagged human VEGF‐D, His‐tagged mouse VEGF‐D and His‐tagged human VEGF‐C, the most closely related protein to VEGF‐D. As shown in Figure 1(b), cVE199 specifically bound to human VEGF‐D.
Figure 1.
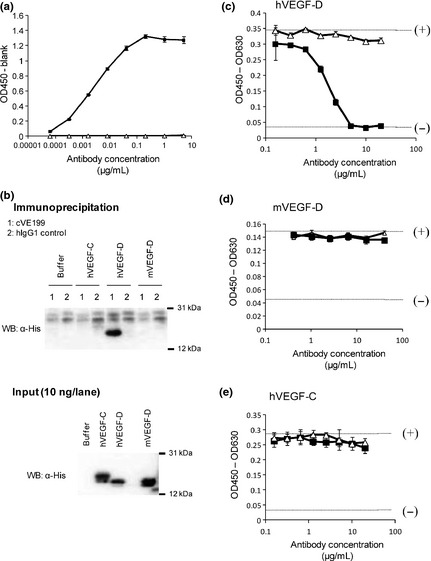
Reaction specificity of cVE199 against rh VEGF‐D. (a) The binding activity of cVE199 to rhVEGF‐D was measured by an ELISA. The various concentrations of cVE199 (black square) and hIgG1 control (white triangle) were reacted to plated hVEGF‐D. Binding was detected by HRP‐labeled anti‐human IgG. (b) The binding specificity of cVE199 to hVEGF‐D was evaluated by immunoprecipitation. (c–e) The inhibitory activity of cVE199 against the binding of (c) hVEGF‐D, (d) mVEGF‐D and (e) hVEGF‐C to VEGFR3 was measured using a cell‐based assay. His‐tagged protein was incubated with VEGFR3 stably expressing CHO cells, in the presence or absence of various concentrations of cVE199 (black square) and hIgG1 control (white triangle). Binding was detected by polyHistidine‐HRP MAb. The absorbance when 1.25 or 0 μg/mL of recombinant protein was added with no antibody is indicated by the (+) or (−) dotted lines, respectively. Points indicate the mean ± SD (n = 3). VEGF, vascular endothelial growth factor.
Following this, we investigated the inhibitory activity of cVE199 against the binding of human VEGF‐D to VEGFR‐3. We found that cVE199 dose‐dependently inhibited the binding of human VEGF‐D to VEGFR‐3 (Fig. 1c). Consistent with the binding specificity shown in Figure 1(b), we found that cVE199 did not inhibit the binding of mouse VEGF‐D or human VEGF‐C to VEGFR‐3 (Fig. 1d,e).
cVE199 neutralizes the biological activity of human vascular endothelial growth factor‐D
To evaluate the neutralizing activity of cVE199 against the biological activity of human VEGF‐D, we used human lymph endothelial cells derived from human lung tissue (HMVEC‐Lly). The HMVEC‐Lly cells were found to express VEGFR‐3 (data not shown), and their proliferation was enhanced by VEGF‐D. When HMVEC‐Lly cells were incubated with cVE199, the VEGF‐D‐dependent growth of HMVEC‐Lly was inhibited in a dose‐dependent manner (Fig. 2a).
Figure 2.
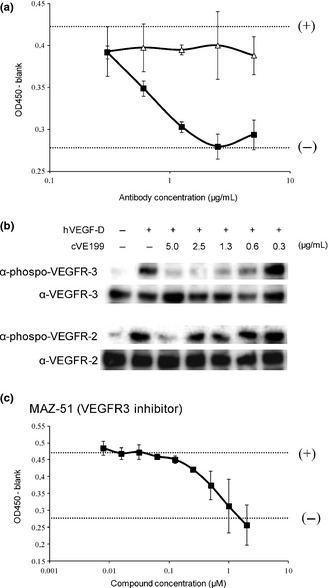
Neutralization activity of cVE199 against rhVEGF‐D. The neutralization activity of cVE199 for VEGF‐D was measured using human lung lymphatic microvascular endothelial cells (HMVEC‐LLy) cells. (a) A total of 500 ng/mL of VEGF‐D was pre‐incubated with serially diluted cVE199 (black square) and hIgG1 control (white triangle) for 15 min at 37°C, and added to HMVEC‐Lly cells for 72 h. The number of living cells was then measured using a Cell Counting Kit‐8 solution. The cell number when 500 or 0 ng/mL of VEGF‐D was added with no antibody is indicated by the (+) or (−) dotted line, respectively. (b) HMVEC‐Lly cells were incubated with cVE199 and VEGF‐D. After 10 min, the cells were harvested and analyzed by western blotting with phospho‐VEGFR‐2 and phospho‐VEGFR‐3 antibodies. (c) MAZ‐51 was used instead of cVE199. Assay was conducted by the same method as (a). All points indicate the mean ± SD (n = 3). VEGF, vascular endothelial growth factor.
Following this, we investigated how cVE199 would alter VEGF‐D‐derived signal transduction in HMVEC‐Lly cells. VEGF‐D induced phosphorylation of VEGFR‐2 and VEGFR‐3, and cVE199 inhibited both phosphorylations at the highest concentration (5 μg/mL). At lower concentrations, cVE199 inhibited VEGFR‐3 but not VEGFR‐2 phosphorylation by VEGF‐D (Fig. 2b). These data suggested that cVE199 preferentially inhibited the VEGF‐D/VEGFR‐3 signal, and so cVE199 inhibited VEGF‐D‐dependent proliferation. To confirm this conclusion, we used MAZ‐51, a commercially available VEGFR‐3 kinase inhibitor.23 We found that MAZ‐51 completely inhibited VEGF‐D‐dependent proliferation of HMVEC‐Lly (Fig. 2c).
SK‐N‐DZ is a vascular endothelial growth factor‐D‐expressing tumor cell line
Many reports have suggested that the VEGF‐D in a tumor has originated in the tumor cell itself.16, 17, 18, 19, 20, 21, 24 Therefore, to evaluate cVE199 activity against tumor progression, we investigated VEGF‐D‐expressing tumor cell lines. VEGF‐D ELISA using cell lysate was performed against 175 tumor cell lines. We found that a neuroblastoma cell line, SK‐N‐DZ, expressed VEGF‐D (Table 1).
Table 1.
Screening of VEGF‐D expressing tumor cells
| Tissue | Cell line | VEGF‐D (pg/mg protein) |
|---|---|---|
| Blood | CMK‐11‐5 | a |
| THP‐1 | a | |
| L‐363 | a | |
| SR | a | |
| ARH‐77 | a | |
| F‐36P | a | |
| Kasumi‐1 | a | |
| MOLM‐13 | a | |
| MV4‐11 | a | |
| SKM‐1 | a | |
| Bone marrow | KHM‐1B | a |
| KMM‐1 | a | |
| KMS‐26 | a | |
| KMS‐11 | a | |
| KMS‐12‐BM | a | |
| KMS‐12‐PE | a | |
| KMS‐20 | a | |
| KMS‐21 BM | a | |
| KMS‐26 | a | |
| KMS‐28 BM | a | |
| KMS‐34 | a | |
| LP‐1 | a | |
| OPM‐2 | a | |
| Breast | MCF7 | a |
| MDA‐MB‐231 | a | |
| MDA‐MB‐468 | a | |
| COLO‐824 | a | |
| MDA‐MB‐453 | a | |
| BT‐474 | a | |
| BT‐483 | a | |
| HCC1395 | a | |
| HCC1599 | a | |
| HCC‐1937 | a | |
| HCC38 | a | |
| JIMT‐1 | a | |
| MFM‐223 | a | |
| T‐47D | a | |
| ZR‐75‐1 | a | |
| HCC‐1806 | a | |
| Colon | COLO 201 | a |
| COLO205 | a | |
| COLO320DM | a | |
| DLD‐1 | a | |
| HCT15 | a | |
| HCT‐8 | a | |
| HT‐29 | a | |
| LoVo | a | |
| LS174T | a | |
| SW1417 | a | |
| SW1116 | a | |
| SW403 | a | |
| SW480 | a | |
| SW620 | a | |
| SW948 | a | |
| WiDr | a | |
| HCT116 | a | |
| RKO | a | |
| T84 | a | |
| Neuron | SH‐SY5Y | a |
| SK‐N‐DZ | 853 | |
| SK‐N‐SH | a | |
| Lung | A549 | a |
| ABC‐1 | a | |
| Calu‐3 | a | |
| HCC‐827 | a | |
| NCI‐H1437 | a | |
| NCI‐H1650 | a | |
| NCI‐H1666 | a | |
| NCI‐H1703 | a | |
| NCI‐H1755 | a | |
| NCI‐H1781 | a | |
| NCI‐H1793 | a | |
| NCI‐H1838 | a | |
| NCI‐H1975 | a | |
| NCI‐H1993 | a | |
| NCI‐H2009 | a | |
| NCI‐H2023 | a | |
| NCI‐H2029 | a | |
| NCI‐H2030 | a | |
| NCI‐H2122 | a | |
| NCI‐H2228 | a | |
| NCI‐H23 | a | |
| NCI‐H2347 | a | |
| NCI‐H441 | a | |
| NCI‐H508 | a | |
| NCI‐H522 | a | |
| NCI‐H716 | a | |
| NCI‐H838 | a | |
| Calu‐6 | a | |
| NCI‐H596 | a | |
| NCI‐H1299 | a | |
| NCI‐H460 | a | |
| NCI‐H661 | a | |
| PC‐13 | a | |
| NCI‐H1048 | a | |
| NCI‐H146 | a | |
| NCI‐H187 | a | |
| NCI‐H2081 | a | |
| NCI‐H209 | a | |
| NCI‐H345 | a | |
| NCI‐H446 | a | |
| NCI‐H526 | a | |
| NCI‐H69 | a | |
| NCI‐H82 | a | |
| SCLC‐21H | a | |
| Calu‐1 | a | |
| NCI‐H2170 | a | |
| NCI‐H226 | a | |
| NCI‐H520 | a | |
| SK‐MES‐1 | a | |
| NCI‐H929 | a | |
| Kidney | 786‐O | a |
| 769‐P | a | |
| Caki‐1 | a | |
| Caki‐2 | a | |
| ACHN | a | |
| Pancreas | Hs 38.T | a |
| HPAF‐II | a | |
| AsPC‐1 | a | |
| BxPC‐3 | a | |
| Capan‐1 | a | |
| Capan‐2 | a | |
| CFPAC‐1 | a | |
| SU.86.86 | a | |
| Hs766T | a | |
| HUP‐T4 | a | |
| MIA PaCa‐2 | a | |
| PANC‐1 | a | |
| YAPC | a | |
| Prostate | PC3 | a |
| 22Rv1 | a | |
| DU145 | a | |
| WERI‐Rb‐1 | a | |
| Skin | HMCB | a |
| MDA‐MB‐435S | a | |
| SK‐MEL‐5 | a | |
| SK‐MEL‐1 | a | |
| SK‐MEL‐2 | a | |
| SK‐MEL‐28 | a | |
| SK‐MEL‐30 | a | |
| C32 | a | |
| AGS | a | |
| Stomach | KATO III | a |
| MKN‐1 | a | |
| MKN‐28 | a | |
| MKN‐45 | a | |
| MKN‐74 | a | |
| NCI‐N87 | a | |
| NUGC‐3 | a | |
| NUGC‐4 | a | |
| SCH | a | |
| SNU‐16 | a | |
| SNU‐5 | a | |
| Bladder | 5637 | a |
| BFTC‐905 | a | |
| HT‐1197 | a | |
| HT‐1376 | a | |
| RT4 | a | |
| SW780 | a | |
| T24 | a | |
| TCCSUP | a | |
| UM‐UC‐3 | a | |
| Liver | SK‐HEP‐1 | a |
| Hep 3B | a | |
| Hep G2 | a | |
| HuH‐7 | a | |
| PLC/PRF/5 | a | |
| Ovary | Caov‐4 | a |
| COLO‐704 | a | |
| ES‐2 | a | |
| OVCAR‐3 | a | |
| SK‐OV‐3 | a | |
| PA‐1 | a |
Indicates under the detection in ELISA assay. The VEGF‐D ELISA assay was performed with various cell lysates, as described in the Materials and Methods. VEGF, vascular endothelial growth factor.
To confirm VEGF‐D expression in SK‐N‐DZ and to investigate the relationship between VEGF‐D expression and neuroblastoma, 10 neuroblastoma cell lines, including SK‐N‐DZ, were analyzed in detail. The expression of VEGF‐D mRNA was evaluated by real‐time PCR. We found that SK‐N‐DZ showed the highest mRNA expression for VEGF‐D among all 10 neuroblastoma cell lines. KELLY and IMR‐32 expressed the next highest levels of VEGF‐D mRNA, after SK‐N‐DZ (Fig. 3a). Consistent with the mRNA expression patterns found, the protein expression of the VEGF‐D precursor (approximately 50 kDa) was strongly detected in SK‐N‐DZ and more weakly detected in KELLY and IMR‐32 using western blot (Fig. 3b). The detection specificity in this assay was confirmed by VEGF‐D siRNA (data not shown). Although these results were obtained by lysate of tumor cells, VEGF‐D is a secreted protein and, thus, we additionally checked for the presence of the secreted form of VEGF‐D in the supernatant of the neuroblastoma cell lines using VEGF‐D ELISA. We found that VEGF‐D was detectable only in the supernatant of SK‐N‐DZ (Fig. 3c). These data indicate that SK‐N‐DZ is a definite VEGF‐D‐expressing line of tumor cells.
Figure 3.
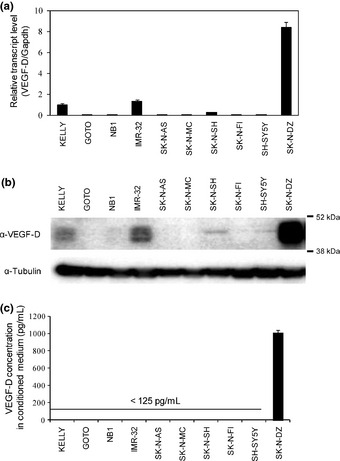
Analysis of vascular endothelial growth factor (VEGF)‐D expression in neuroblastoma cell lines. (a) VEGF‐D mRNA expression in 10 neuroblastoma cell lines was analyzed by the Taqman assay (n = 3 for each cell line). Data were normalized to Gapdh. (b) Expression of VEGF‐D protein in 10 neuroblastoma cell lines was analyzed by western blot using anti‐VEGF‐D antibody. (c) Each cell line was cultured for 72 h in the condition medium. The VEGF‐D concentration in the medium was quantified using the VEGF‐D ELISA kit (n = 3 for each cell line). All values are expressed as the mean ± SD.
We next further confirmed VEGF‐D expression in SK‐N‐DZ xenograft tumors with a VEGF‐D immunohistochemistry (IHC) assay. The specificity of the VEGF‐D staining was confirmed by comparing the staining of human VEGF‐D‐expressing HEK293 transfectant (293‐VEGF‐D) with parental HEK293 cells, which preferentially stained against 293‐VEGF‐D transfectant (Fig. S1). In addition, xenografts of SH‐SY5Y tumors were also used as a negative control for VEGF‐D staining, because SH‐SY5Y did not express VEGF‐D in our in vitro analysis (Fig. 3b,c). As shown in Figure 4(a), SK‐N‐DZ xenografts revealed an immunohistochemical signal for VEGF‐D, and SH‐SY5Y xenografts showed immunonegativity to VEGF‐D. Furthermore, we evaluated the status of lymph vessels in these xenograft tumors with a mLyve‐1 IHC assay. This was done because VEGF‐D is a lymphangiogenic factor. Consistent with the VEGF‐D staining pattern, the SK‐N‐DZ xenografts showed an immunohistochemical signal for mLyve‐1, and SH‐SY5Y xenografts showed immunonegativity to mLyve‐1 (Fig. 4b).
Figure 4.
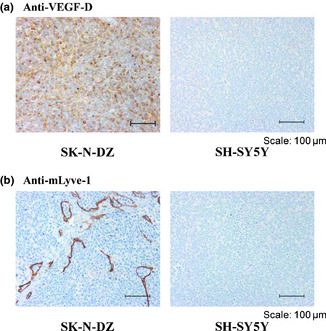
Analysis of vascular endothelial growth factor (VEGF)‐D expression in neuroblastoma xenograft. Expression of VEGF‐D in neuroblastoma xenograft was evaluated by immunohistochemistry with anti‐VEGF‐D antibody. Formalin‐fixed paraffin‐embedded slides were stained by a Ventana automated immunostainer. (a) SK‐N‐DZ showed an immunohistochemical signal for VEGF‐D (b) and SH‐SY5Y showed immunonegativity to VEGF‐D.
cVE199 inhibited in vivo lymphatic metastasis of SK‐N‐DZ
We next investigated the in vivo effect of cVE199 against the lymphatic metastasis of SK‐N‐DZ in mice. To do so, we generated a SK‐N‐DZ cell line stably expressing luciferase (SK‐N‐DZ‐luc) in order to conduct bioimaging in the mice (Fig. S2a). Compared to the SK‐N‐DZ parental cells, the SK‐N‐DZ‐luc cells exhibited similar levels of VEGF‐D expression in vitro (Fig. S2b). Furthermore, the in vitro proliferation of SK‐N‐DZ‐luc cells did not differ from that of SK‐N‐DZ (Fig. S2c). In addition, we evaluated VEGFR‐2 and VEGFR‐3 expression in SK‐N‐DZ‐luc cells to consider the autocrine effect of VEGF‐D. We found that SK‐N‐DZ‐luc cells did not express either VEGFR‐2 or VEGFR‐3 (Fig. S3).
Following this, an in vivo efficacy study was performed by inoculating SK‐N‐DZ‐luc cells into SCID mice and by 10 mg/kg of twice‐weekly intravenous administration of cVE199 (n = 10). On the 14th day after the first administration, there was little difference in the tumor size of SK‐N‐DZ‐luc in the primary lesion between the cVE199‐injected group and the vehicle‐injected group (Fig. 5a,b). In contrast, metastasis of SK‐N‐DZ‐luc into the ipsilateral lymph nodes (lymphatic metastasis) was significantly decreased by cVE199 injection on the 14th day after the first administration (P < 0.05, Fig. 5c). The individual data shown in Figure 5(c) (the amount of photons and the number of metastasized SK‐N‐DZ‐luc) are included in Figure S4. To evaluate the association of lymph vessels with the reduction of metastasis into the lymph nodes, we then analyzed lymph vessels in primary tumors using a mLyve‐1 IHC assay. As shown in Figure 5(d), the immunohistochemical signal for mLyve‐1 in cVE199‐injected xenograft tumors was much weaker than that in control xenograft samples. In contrast, the immunohistochemical signal for mCD31 was not different between the two groups. This indicated that cVE199 did not affect blood vessels within the primary tumors of SK‐N‐DZ‐luc (Fig. S5). These data further indicate that cVE199 decreased the lymphatic metastasis of SK‐N‐DZ‐luc by inhibiting lymph vessel formation.
Figure 5.
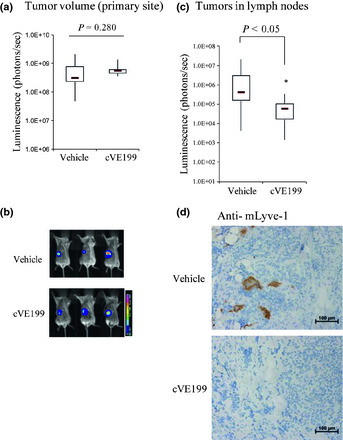
In vivo effect of cVE199 against vascular endothelial growth factor (VEGF)‐D expressing neuroblastoma model. (a and b) Antitumor effect of cVE199 against SK‐N‐DZ primary lesion was using a bioimaging technique. Mice bearing SK‐N‐DZ were give 10 mg/kg of cVE199 twice a week for 2 weeks. Tumor size was measured by administration of luciferase substrate and detection of the photons. Data are shown by a box‐and‐whisker plot. Statistical tests were performed using the Wilcoxon test. (c) Anti‐metastasis activity of cVE199 against SK‐N‐DZ was evaluated by detection of photons from lysed lymph nodes. Data are shown by a box‐and‐whisker plot. Statistical tests were performed using the Wilcoxon test. (d) The presence of lymph vessels in the primary tumors of SK‐N‐DZ was analyzed by mLyve‐1 immunostaining. Formalin‐fixed paraffin‐embedded slides were stained with an anti‐mouse Lyve‐1 antibody using a Ventana automated immunostainer.
Neuroblastoma is a potential indication for cVE199
Because only a neuroblastoma cell line, SK‐N‐DZ, abundantly expressed VEGF‐D in our in vitro culture analysis, we investigated its expression in tumor tissues from neuroblastoma patients. A total of 29 commercially available neuroblastoma specimens were analyzed by IHC, as described in the Materials and Methods. Using the criteria for VEGF‐D immunohistochemical evaluations, VEGF‐D‐positive staining was detected in 11 cases (37.9%). Typical examples of VEGF‐D‐positive and VEGF‐D‐negative staining are given in Figure 6(a,b), respectively. These results indicate that some neuroblastoma patients expressed VEGF‐D.
Figure 6.
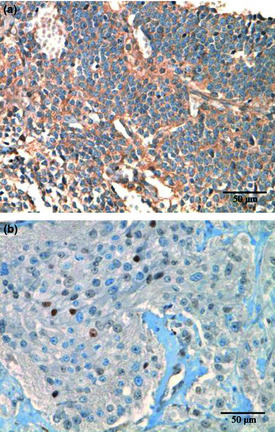
Immunohistochemical analysis of vascular endothelial growth factor (VEGF)‐D in neuroblastomas. Expression of VEGF‐D in neuroblastoma specimens was evaluated by immunohistochemistry with anti‐VEGF‐D antibody. Formalin‐fixed paraffin‐embedded slides were stained by a Ventana automated immunostainer. According to the criteria for VEGF‐D immunohistochemical evaluations, as described in the Materials and Methods, (a) tumor cells showed an immunohistochemical signal for VEGF‐D and (b) the tumor cells showed immunonegativity to VEGF‐D.
Discussion
Lymphatic spread is an important clinical determinant in the prognosis of many tumors. Therefore, therapeutic modalities capable of inhibiting lymphatic spread would be predicted to convey a clinical benefit in the treatment of many tumors. VEGF‐D is implicated in lymphatic spread through the promotion of lymphangiogenesis in human cancers. In addition, high VEGF‐D expression has been shown to correlate well with poor prognosis in many cancers. These findings suggest that anti‐VEGF‐D would be a promising agent in cancer treatment.
In this study, we generated a novel anti‐VEGF‐D chimeric monoclonal antibody, cVE199, which has a specific binding reactivity and a neutralizing activity against human VEGF‐D. The result of an assay using HMVEC‐Lly cells suggested that cVE199 preferentially inhibited VEGF‐D/VEGFR‐3 as compared to the VEGF‐D/VEGFR‐2 signal (Fig. 2b). A recent study showed that N‐terminal residues of VEGF‐D are important for VEGF‐D binding and activation of VEGFR‐3, but not of VEGFR‐2.25 This finding suggests that cVE199 binds to N‐terminal residues of VEGF‐D, and so cVE199 preferentially inhibits the VEGF‐D/VEGFR‐3 signal.
In a xenograft study to evaluate the in vivo effect of cVE199, we used SK‐N‐DZ that natively expresses VEGF‐D, although previous studies have used VEGF‐D‐transfected cell lines. This approach is important because it has a higher clinical relevance compared to overexpression experiments. Our result showing that cVE199 significantly decreased lymph node metastasis of SK‐N‐DZ with reduction of lymphangiogenesis strongly supports previously published conclusions drawn from VEGF‐D overexpressing in vivo studies. These reports largely attribute the metastatic action of VEGF‐D to an induction of lymphangiogenesis. For instance, VEGF‐D stimulated lymphangiogenesis when the molecule was overexpressed in skin keratinocytes;14 it promoted the development of lymphatics and lymph node metastasis when overexpressed in tumors.24, 26
In this study, cVE199 did not alter blood vessels in the primary tumors of SK‐N‐DZ‐luc xenografts. Although the VEGF‐D/VEGFR‐3 pathway is known to play a role not only in tumor lymphangiogenesis but also in pathological angiogenesis the VEGF‐A/VEGFR‐2 signal is known as the predominant regulator of physiological and tumor angiogenesis.27 We found that SK‐N‐DZ‐luc cells expressed VEGF‐A (Fig. S6). In addition, VEGFR‐3 kinase inhibitor or VEGFR3‐Ig, a potent inhibitor of VEGF‐C/VEGF‐D signaling, did not always affect tumor angiogenesis.28, 29 These data suggest that the contribution of VEGF‐D/VEGFR‐3 signaling to tumor angiogenesis is affected by in vivo circumstances, such as VEGF‐A expression. Furthermore, these findings might explain the reason for the ineffectiveness of cVE199 on tumor angiogenesis in the SK‐N‐DZ‐luc xenograft.
Because cVE199 did not affect tumor growth (Fig. 5a,b) or angiogenesis (Fig. S5), it seems that cVE199 inhibits lymph node metastasis of SK‐N‐DZ‐luc by inhibiting lymphangiogenesis. Thus, our finding also supports previously published data showing that formation of a dense lymphatic network facilitates metastatic tumor spread by increasing the likelihood that tumor cells will leave the primary tumor site and invade the lymphatics.30
One of the most important findings of the present study was that cVE199 inhibits the lymphatic metastasis in an experimental neuroblastoma model (Fig. 5) and that VEGF‐D was expressed in some human neuroblastomas (Fig. 6), implicating VEGF‐D in the lymphatic metastasis of neuroblastoma; these findings further suggest cVE199 as a possible therapeutic antibody for neuroblastoma. Although many lower‐stage neuroblastomas regress completely or differentiate into benign ganglioneuroblastoma without treatment, metastatic neuroblastoma (stage IV) in children over 1 year of age is lethal for most patients despite aggressive multimodality therapy.31, 32, 33, 34 Therefore, new therapeutic agents for neuroblastoma are still necessary, and our finding that cVE199 has potential as a therapeutic antibody for neuroblastoma is of significance.
In conclusion, the results of the present study implicate VEGF‐D in the promotion of lymphatic metastasis in neuroblastoma and identify cVE199 as a potential therapeutic antibody against neuroblastoma in the prevention of this process.
Disclosure Statement
All of the authors are employees of Chugai Pharmaceutical Co. Ltd.
Supporting information
Fig. S1. Evaluation of the specificity of vascular endothelial growth factor (VEGF)‐D immunohistochemical analysis.
Fig. S2. Comparison of profile between SK‐N‐DZ and SK‐N‐DZ‐luc cells.
Fig. S3. Expression of VEGFR‐2 and VEGFR‐3 in SK‐N‐DZ‐luc cells.
Fig. S4. Plot of photons and SK‐N‐DZ‐luc number in each mouse shown in Figure 5(c).
Fig. S5. Immunohistochemical analysis of mCD31 in SK‐N‐DZ‐luc xenograft.
Fig. S6. Expression of vascular endothelial growth factor.
Acknowledgments
The authors thank Taeko Masuda and Asuka Motoda for their technical support; Mina Takahashi, Tsukasa Suzuki, Takeshi Baba, Atsushi Narita and Tetsuya Wakabayashi for antibody generation; and Junichi Nezu, Keiko Esaki, Akihisa Sakamoto, Manabu Wada, Masakazu Hasegawa, Naohiro Yabuta and Kunihiro Hattori for helpful discussions.
(Cancer Sci 2012; 103: 2144–2152)
References
- 1. Liotta LA. Cancer cell invasion and metastasis. Sci Am 1992; 266: 54–9, 62–3. [DOI] [PubMed] [Google Scholar]
- 2. Qin LX, Tang ZY. The prognostic significance of clinical and pathological features in hepatocellular carcinoma. World J Gastroenterol 2002; 8: 193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghaferi AA, Wong SL, Johnson TM et al Prognostic significance of a positive nonsentinel lymph node in cutaneous melanoma. Ann Surg Oncol 2009; 16: 2978–84. [DOI] [PubMed] [Google Scholar]
- 4. Grandi C, Alloisio M, Moglia D et al Prognostic significance of lymphatic spread in head and neck carcinomas: therapeutic implications. Head Neck Surg 1985; 8: 67–73. [DOI] [PubMed] [Google Scholar]
- 5. de Manzoni G, Verlato G, Guglielmi A, Laterza E, Genna M, Cordiano C. Prognostic significance of lymph node dissection in gastric cancer. Br J Surg 1996; 83: 1604–7. [DOI] [PubMed] [Google Scholar]
- 6. Lughezzani G, Capitanio U, Jeldres C et al Prognostic significance of lymph node invasion in patients with metastatic renal cell carcinoma: a population‐based perspective. Cancer 2009; 115: 5680–7. [DOI] [PubMed] [Google Scholar]
- 7. Tuna S, Dalkilic Calis M, Sakar B, Aykan F, Camlica H, Topuz E. Prognostic significance of the metastatic lymph node ratio for survival in colon cancer. J BUON 2011; 16: 478–85. [PubMed] [Google Scholar]
- 8. Nguyen TH. Mechanisms of metastasis. Clin Dermatol 2004; 22: 209–16. [DOI] [PubMed] [Google Scholar]
- 9. Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004; 4: 448–56. [DOI] [PubMed] [Google Scholar]
- 10. Raica M, Ribatti D. Targeting tumor lymphangiogenesis: an update. Curr Med Chem 2010; 17: 698–708. [DOI] [PubMed] [Google Scholar]
- 11. Stacker SA, Stenvers K, Caesar C et al Biosynthesis of vascular endothelial growth factor‐D involves proteolytic processing which generates non‐covalent homodimers. J Biol Chem 1999; 274: 32127–36. [DOI] [PubMed] [Google Scholar]
- 12. Achen MG, Jeltsch M, Kukk E et al Vascular endothelial growth factor D (VEGF‐D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA 1998; 95: 548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol 2004; 25: 387–95. [DOI] [PubMed] [Google Scholar]
- 14. Veikkola T, Jussila L, Makinen T et al Signalling via vascular endothelial growth factor receptor‐3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J 2001; 20: 1223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann N Y Acad Sci 2008; 1131: 225–34. [DOI] [PubMed] [Google Scholar]
- 16. White JD, Hewett PW, Kosuge D et al Vascular endothelial growth factor‐D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res 2002; 62: 1669–75. [PubMed] [Google Scholar]
- 17. Nakamura Y, Yasuoka H, Tsujimoto M et al Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long‐term follow‐up. Clin Cancer Res 2003; 9: 716–21. [PubMed] [Google Scholar]
- 18. Yokoyama Y, Charnock‐Jones DS, Licence D et al Expression of vascular endothelial growth factor (VEGF)‐D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin Cancer Res 2003; 9: 1361–9. [PubMed] [Google Scholar]
- 19. Yokoyama Y, Charnock‐Jones DS, Licence D et al Vascular endothelial growth factor‐D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer 2003; 88: 237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juttner S, Wissmann C, Jons T et al Vascular endothelial growth factor‐D and its receptor VEGFR‐3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol 2006; 24: 228–40. [DOI] [PubMed] [Google Scholar]
- 21. Liu B, Ma J, Wang X et al Lymphangiogenesis and its relationship with lymphatic metastasis and prognosis in malignant melanoma. Anat Rec (Hoboken) 2008; 291: 1227–35. [DOI] [PubMed] [Google Scholar]
- 22. Ono N, Yamazaki T, Nakanishi Y et al Preclinical antitumor activity of the novel heat shock protein 90 inhibitor CH5164840 against human epidermal growth factor receptor 2 (HER2)‐overexpressing cancers. Cancer Sci 2012; 103: 342–9. [DOI] [PubMed] [Google Scholar]
- 23. Kirkin V, Mazitschek R, Krishnan J et al Characterization of indolinones which preferentially inhibit VEGF‐C‐ and VEGF‐D‐induced activation of VEGFR‐3 rather than VEGFR‐2. Eur J Biochem 2001; 268: 5530–40. [DOI] [PubMed] [Google Scholar]
- 24. Thelen A, Scholz A, Benckert C et al VEGF‐D promotes tumor growth and lymphatic spread in a mouse model of hepatocellular carcinoma. Int J Cancer 2008; 122: 2471–81. [DOI] [PubMed] [Google Scholar]
- 25. Leppanen VM, Jeltsch M, Anisimov A et al Structural determinants of vascular endothelial growth factor‐D receptor binding and specificity. Blood 2011; 117: 1507–15. [DOI] [PubMed] [Google Scholar]
- 26. Stacker SA, Caesar C, Baldwin ME et al VEGF‐D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001; 7: 186–91. [DOI] [PubMed] [Google Scholar]
- 27. Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 2003; 3: 401–10. [DOI] [PubMed] [Google Scholar]
- 28. Yashiro M, Shinto O, Nakamura K et al Effects of VEGFR‐3 phosphorylation inhibitor on lymph node metastasis in an orthotopic diffuse‐type gastric carcinoma model. Br J Cancer 2009; 101: 1100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor‐secreted vascular endothelial growth factor‐C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res 2005; 65: 9789–98. [DOI] [PubMed] [Google Scholar]
- 30. Detmar M, Hirakawa S. The formation of lymphatic vessels and its importance in the setting of malignancy. J Exp Med 2002; 196: 713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet 2007; 369: 2106–20. [DOI] [PubMed] [Google Scholar]
- 32. Maris JM. Recent advances in neuroblastoma. N Engl J Med 2010; 362: 2202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer 2003; 3: 203–16. [DOI] [PubMed] [Google Scholar]
- 34. Modak S, Cheung NK. Neuroblastoma: therapeutic strategies for a clinical enigma. Cancer Treat Rev 2010; 36: 307–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Evaluation of the specificity of vascular endothelial growth factor (VEGF)‐D immunohistochemical analysis.
Fig. S2. Comparison of profile between SK‐N‐DZ and SK‐N‐DZ‐luc cells.
Fig. S3. Expression of VEGFR‐2 and VEGFR‐3 in SK‐N‐DZ‐luc cells.
Fig. S4. Plot of photons and SK‐N‐DZ‐luc number in each mouse shown in Figure 5(c).
Fig. S5. Immunohistochemical analysis of mCD31 in SK‐N‐DZ‐luc xenograft.
Fig. S6. Expression of vascular endothelial growth factor.


