Abstract
The escape of preneoplastic cells from the immune system, which is caused by immune tolerance, occurs during the development of several types of tumors. Indoleamine 2,3‐dioxygenase (IDO) plays a critical role in the induction of immune tolerance. In the present study we investigated the effects of 1‐methyltryptophan (1‐MT), an IDO inhibitor, and (−1;)‐epigallocatechin gallate (EGCG), the major catechin in green tea, on the development of azoxymethane (AOM)‐induced colonic preneoplastic lesions by focusing on the inhibition of IDO. To induce colonic premalignant lesions, male F344 rats were injected with AOM (20 mg/kg body weight, s.c.) once a week for 2 weeks. They also received 0.2% 1‐MT or 0.1% EGCG in their drinking water for 4 weeks, starting 1 week before the first dose of AOM. Both 1‐MT and EGCG significantly decreased the total number of aberrant crypt foci and β‐catenin‐accumulated crypts, which overexpressed IDO protein. Treatment with EGCG decreased IDO mRNA expression in both the colonic epithelium and stroma of rats induced by AOM. The AOM‐induced increase in cyclooxygenase‐2 mRNA expression in the colonic stroma was significantly decreased by EGCG. Furthermore, AOM‐induced increases in IDO activity in the serum and stroma were significantly inhibited by 1‐MT and EGCG. Inhibition of IDO activity by 1‐MT and EGCG was also observed in cell‐free assays. These findings suggest that upregulation of IDO activity is observed in the early stages of colon carcinogenesis and that the use of IDO inhibitors, such as 1‐MT and EGCG, which suppress the occurrence of colonic preneoplastic lesions, could be a novel strategy for the chemoprevention of colon cancer. (Cancer Sci 2012; 103: 951–958)
The immune system recognizes preneoplastic cells and, in most cases, eliminates these cells before they expand into clinically detectable tumors. Therefore, the escape of precancerous cells from the immune system, which is closely associated with immune tolerance, is involved in the development of several types of tumors.1 Recent studies have suggested that indoleamine 2,3‐dioxygenase (IDO) plays a crucial role in the induction of immune tolerance.2 Indoleamine 2,3‐dioxygenase is an intracellular enzyme that catalyses the first and rate‐limiting steps in the catabolism of the essential amino acid tryptophan along the kynurenine pathway.3 In the tumor microenvironment, increased IDO activity inhibits the proliferation of T and natural killer cells and induces apoptosis through tryptophan depletion and the production of toxic tryptophan catabolites.4 Overexpression of IDO has been shown to be correlated with poor clinical outcome in patients with ovarian carcinoma, endometrial carcinoma, and colorectal carcinoma.5, 6, 7 We have recently reported that, in diffuse large B‐cell lymphoma, IDO expression in tumor cells and serum concentrations of l‐kynurenine, which reflect IDO activity, are useful indicators of a poor prognosis.8, 9 Several preclinical studies using rodent cancer models have demonstrated that IDO inhibitors, such as 1‐methyltryptophan (1‐MT), are therapeutically beneficial, especially when combined with different types of cytotoxic chemotherapeutic agents.10, 11 These reports suggest that targeting IDO, and therefore regulating tryptophan catabolism, may be an effective strategy for the treatment of certain types of human malignancies.12 However, the possibility of cancer chemoprevention by inhibiting IDO expression and/or activity has not been considered.
(−)‐Epigallocatechin gallate (EGCG), one of the major catechins in green tea, is the most biologically active component of green tea. It has been shown to exert its cancer chemopreventive and anti‐carcinogenic effects in various organs, including the colon.13, 14 Previously, we demonstrated that EGCG can inhibit the growth of and induce apoptosis in human colorectal cancer cells.15, 16, 17 The inhibitory effects of EGCG on both inflammation‐ and obesity‐related colon carcinogenesis have also been demonstrated.18, 19 In addition, green tea polyphenols in the drinking water have been shown to inhibit the development of putative preneoplastic lesions called aberrant crypt foci (ACF) in rats treated with azoxymethane (AOM), which induces ACF.20, 21, 22
Recently, it was been reported that EGCG administration suppresses the expression of IDO in interferon (IFN)‐γ‐stimulated murine dendritic cells23 and human oral cancer cell lines.24 Although the mechanisms underlying the role of IDO in carcinogenesis have not yet been clarified, we hypothesized that the inhibitory effect of EGCG on IDO expression may contribute to the anti‐carcinogenic properties of EGCG. To confirm our hypothesis, we examined the effects of 1‐MT and EGCG on the development in the colon of AOM‐induced preneoplastic lesions, namely ACF21 and β‐catenin‐accumulated crypts (BCAC),25in male F344 rats by focusing on the inhibition of IDO expression and activity.
Materials and Methods
Animals, chemicals, and diets
Male F344 rats, aged 4 weeks (Charles River Japan, Tokyo, Japan), were maintained at the Gifu University Animal Facility according to Institutional Animal Care Guidelines. All rats were housed in plastic cages with free access to drinking water and a pelleted basal diet (CRF‐1; Oriental Yeast, Tokyo, Japan). Both 1‐MT and AOM were purchased from Sigma (St Louis, MO, USA), whereas EGCG was obtained from Mitsui Norin (Tokyo, Japan). For 1‐MT and EGCG treatment of rats, 1‐MT (0.2%) and EGCG (0.1%) solutions were prepared in tap water and administered to the rats in their drinking water ad libitum. Fresh test solutions were prepared three times a week. The concentration of EGCG (0.1%) used in the present study was chosen on the basis of the results of previous chemopreventive studies19, 26 and was within the physiologic range of the daily intake of green tea catechins in humans on a per unit body weight basis.27
Experimental procedure
As shown in Figure 1, 60 male F344 rats were quarantined for the first 7 days and then randomized into one of three groups to receive either 0.2% 1‐MT, 0.1% EGCG, or no test compounds. One week later, the rats in each group were further grouped to receive subcutaneous injections of AOM (20 mg/kg body weight) or saline (200 μL) once a week for 2 weeks. Rats were given control and test drinking water for 4 weeks, starting 1 week before the first AOM injection. All measurements, including the large bowel excision and the collection of blood samples from the inferior vena cava, were performed from rats that had been killed by CO2 asphyxiation at Week 4 (9 weeks of age). One‐quarter of the excised colons (cecum side) was used for crypt isolation, whereas the remainder was used to determine the number of colonic ACF and BCAC (see below). After the number of ACF had been counted, the colon was rolled like a “Swiss roll”28 and paraffin‐embedded sections were prepared using routine procedures for subsequent histopathologic and immunohistochemical examinations.
Figure 1.
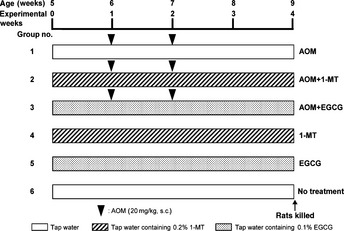
Experimental protocol. Rats (5 weeks old) were allocated to one of six groups and treated over a period of 4 weeks, as indicated. AOM, azoxymethane; 1‐MT, 1‐methyltryptophan; EGCG, (−)‐epigallocatechin gallate.
Counting colonic ACF and BCAC
The number of ACF and BCAC was determined as described previously.25, 29, 30 Briefly, buffered formalin‐fixed colons were stained with 0.5% methylene blue solution for 20 s and then placed on microscope slides to count the number of ACF and to determine their size. The number of ACF in the colon was recorded along with the number of crypts in each focus and the data are expressed as the total number of ACF per colon, total number of aberrant crypts (ACs) per colon, number of ACs per focus, and total number of large ACF (i.e. ACF with four or more aberrant crypts) per colon.29 After the number of ACF had been counted, the rectal mucosa (2.0 cm from the anus) was cut and embedded in paraffin to identify BCAC intramucosal lesions, with 4‐μm sections obtained from an en face preparation. The number of BCAC on histological sections stained with β‐catenin was counted and is expressed as the number of BCAC per cm2 mucosa.
Immunohistochemical analysis
After endogenous peroxidase activity had been blocked with H2O2, sections were incubated overnight at 4°C with primary antibodies: anti‐β‐catenin (1 : 1000; BD Biosciences PharMingen, San Diego, CA, USA), anti‐IDO (1 : 1000; LYFESPAN, Seattle, WA, USA), and anti‐l‐kynurenine (1 : 1000; Abnova, Taipei City, Taiwan). Subsequently, sections for the immunohistochemistry of β‐catenin and IDO were incubated with biotinylated secondary antibodies against the primary antibodies (DAKO, Carpinteria, CA, USA), followed by incubation with avidin‐coupled peroxidase. The sections for l‐kynurenine immunohistochemistry were incubated with peroxidase‐labeled polymer‐conjugated secondary antibodies against the primary antibodies. They were then developed with 3,3′‐diaminobenzidine using DAKO Liquid DAB Substrate‐Chromogen System (DAKO) and counterstained with hematoxylin.
Crypt isolation
Colonic tissue was washed twice with 1 × Hank's balanced salt solution (HBSS; Sigma) and then incubated with 1 × HBSS containing 30 mM EDTA at 37°C for 15 min. The tissue was dispersed in 1 × HBSS solution by vortexing and separated into epithelial crypts and stromal tissues as described previously.31
Quantitative real‐time RT‐PCR
Total RNA was extracted from isolated epithelial crypts and stromal tissues using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Total RNA (1 μg) was used for the synthesis of first‐strand cDNA. Quantitative real‐time RT‐PCR was performed using specific primer/probe sets that amplified the IDO, tryptophan 2,3‐dioxygenase (TDO), cyclooxygenase (COX)‐2, IFN‐γ, and GAPDH genes (TaqMan Gene Expression Assays; Applied Biosystems, Foster City, CA, USA) and TOYOBO Real‐time PCR Master Mix (TOYOBO, Osaka, Japan). Each sample was analyzed on a LightCycler 1.0 (Roche Diagnostics, Mannheim, Germany), as described previously.32 The expression of each gene was normalized against that of GAPDH using the standard curve method.
Determination of IDO activity
Indoleamine 2,3‐dioxygenase activity was determined by calculating the ratio of l‐kynurenine/l‐tryptophan in serum and colonic tissues.33 Serum samples were deproteinized with 3% perchloric acid. Isolated epithelial crypt and stromal samples were homogenized in 2 μL of 3% perchloric acid per mg tissue. After centrifugation at 4°C and 20 000 g for 10 min, aliquots of the supernatant were collected for HPLC determination of l‐tryptophan and l‐kynurenine concentrations, as described previously.34
The enzymatic activity of IDO was also measured using cell‐free assays. An aliquot of recombinant human IDO (R&D Systems, Minneapolis, MN, USA) was diluted in 50 mM 2‐(N‐morpholino)ethanesulfonic buffer (pH 6.5). The reaction mixture contained 50 μL enzyme preparation and 50 μL substrate solution, which consisted of 100 mM potassium phosphate buffer (pH 6.5), 50 μM methylene blue, 20 μg catalase, 50 mM ascorbate, 0.4 mM l‐tryptophan, and 2000 μM 1‐MT or 200 μM EGCG. After incubation of the reaction mixture at 37°C for 1 h, the concentrations of the enzymatic products were measured by HPLC.35 Enzymatic activity is expressed as the product content per hour.
Statistical analysis
All data are expressed as the mean ± SD. Differences between groups were analyzed by two‐way anova and, when statistical significance was found, individual differences were evaluated using the Tukey–Kramer multiple comparison test. P < 0.05 was considered significant.
Results
General observations
All rats remained healthy and none died during the experimental period. There were no significant differences in the consumption of food (data not shown) and drinking water (Table 1) between the different groups. Body, liver, and relative liver weights, as well as the length of the large bowel, at the end of the study are given in Table 1. The mean body weight of the AOM + 1‐MT group was only significantly less than that of the 1‐MT group (P < 0.05). This decrease may have been due to AOM toxicity, as observed in previous studies,19, 36, 37 because 1‐MT alone did not reduce body weight in the absence of AOM. Other measurements did not differ significantly among the groups. Histopathologically, there were no findings suggesting toxicity of 1‐MT or EGCG in the liver, kidney, or spleen of rats (data not shown).
Table 1.
General parameters
| Treatment | No. rats examined | Drinking water intake (g/day) | Body weight (g) | Liver weight (g) | Relative liver weight (g/100 g body weight) | Length of the large bowel (cm) |
|---|---|---|---|---|---|---|
| AOM alone | 14 | 27.3 ± 1.3 | 203 ± 11 | 10.2 ± 1.0 | 5.0 ± 0.5 | 19.0 ± 1.8 |
| AOM + 0.2% 1‐MT | 14 | 25.2 ± 2.3 | 198 ± 13* | 10.1 ± 0.7 | 5.1 ± 0.4 | 19.6 ± 1.1 |
| AOM + 0.1% EGCG | 14 | 26.1 ± 2.9 | 202 ± 8 | 9.8 ± 0.8 | 4.9 ± 0.5 | 19.0 ± 0.9 |
| 0.2% 1‐MT | 6 | 25.3 ± 4.3 | 217 ± 12 | 10.6 ± 0.8 | 4.9 ± 0.2 | 20.3 ± 1.6 |
| 0.1% EGCG | 6 | 26.5 ± 1.4 | 212 ± 16 | 10.2 ± 0.9 | 4.8 ± 0.6 | 19.5 ± 1.1 |
| No treatment | 6 | 26.8 ± 0.8 | 208 ± 9 | 9.6 ± 0.8 | 4.6 ± 0.4 | 19.0 ± 2.1 |
Data are given as the mean ± SD. *P < 0.05 compared with 0.2% 1‐methyltryptophan (1‐MT) alone. AOM, azoxymethane; EGCG, (−)‐epigallocatechin gallate.
Effects of 1‐MT and EGCG on AOM‐induced ACF and BCAC in F344 rats
All rats in the AOM, AOM + 1‐MT, and AOM + EGCG groups (i.e. all those treated with AOM) developed ACF and BCAC. In the 1‐MT, EGCG, and untreated groups, there were no microscopically observable changes, including ACF or BCAC, in the colon. Compared with the group treated with AOM alone, daily oral administration of 1‐MT and EGCG in the drinking water significantly reduced the frequency of ACF (P < 0.001 for each comparison). The reduction in the frequency of ACF was significantly greater following EGCG administration than after 1‐MT administration (P < 0.05). We also noticed a significant reduction in the percentage of large ACF, consisting of four or more aberrant crypts, in the AOM + 1‐MT and AOM + EGCG groups compared with the AOM group (P < 0.001 for each comparison; Fig. 2a). In addition, the number of BCAC per cm2 in the AOM + 1‐MT and AOM + EGCG groups was significantly less than that in the AOM group (P < 0.001 for each comparison; Fig. 2b).
Figure 2.
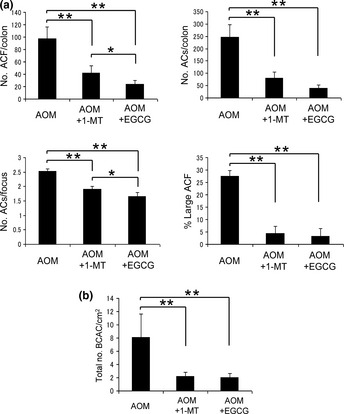
Effects of 1‐methyltryptophan (1‐MT) and (−)‐epigallocatechin gallate (EGCG) on azoxymethane (AOM)‐induced formation of aberrant crypt foci (ACF) and β‐catenin‐accumulated crypts (BCAC). (a) Number of ACF per colon, total number of aberrant crypts (ACs) per colon, the number of ACs in each focus, and the percentage of large ACF (i.e. those with four or more ACs). (b) Number of BCAC per cm2. Data are the mean ± SD (n = 6). *P < 0.05, **P < 0.001.
Immunohistochemical analysis of IDO and l‐kynurenine in the colonic mucosa
The expression of IDO and l‐kynurenine was determined in colonic crypts and preneoplastic lesions (i.e. ACF and BCAC) using immunohistochemical analysis. Compared with colonic crypt cells in untreated control rats, which exhibited only weak positive cytoplasmic staining to IDO, there was a significant increase in IDO staining in the atypical cell cytoplasm of the ACF and BCAC that had developed in AOM‐treated rats. Furthermore, l‐kynurenine expression, which was very weak in normal crypts of untreated control rats, was slightly increased in the ACF and BCAC of AOM‐treated rats (Fig. 3a). Neither EGCG nor 1‐MT treatment significantly altered the AOM‐induced increases in IDO and l‐kynurenine staining (Fig. 3b,c).
Figure 3.
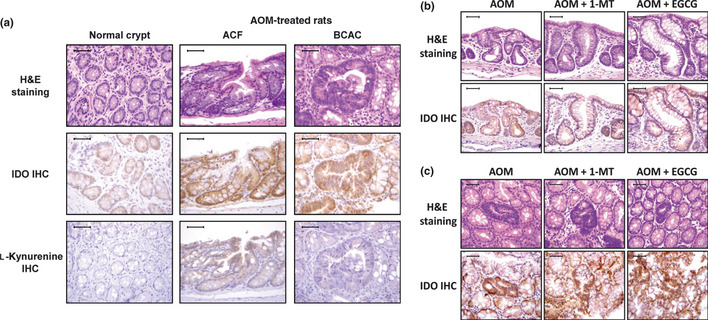
Immunohistochemical evaluation of the expression of indoleamine 2,3‐dioxygenase (IDO) and l‐kynurenine in normal crypts from untreated rats and aberrant crypt foci (ACF), and β‐catenin‐accumulated crypts (BCAC) in the colonic mucosa of rats treated with azoxymethane (AOM). (a) Expression of IDO and l‐kynurenine in representative samples of colonic mucosa, as evidenced by H&E staining and immunohistochemistry (IHC). (b,c) Effects of 1‐methyltryptophan (1‐MT) and (−)‐epigallocatechin gallate (EGCG) on the expression of IDO in ACF (b) and BCAC (c), as determined by IHC. Scale lines, 50 μm.
Effects of 1‐MT and EGCG on IDO and TDO expression in isolated epithelial crypts and stromal cells
In cancer tissues, IDO is overexpressed in both tumor epithelial cells and antigen‐presenting cells in the stroma.38 Tryptophan 2,3‐dioxygenase, a hepatic enzyme that catalyses the first step of tryptophan degradation, is also expressed in many tumors.39 Therefore, after crypt isolation, we determined whether there was increased expression of IDO and TDO in both epithelial crypts and stromal tissues in the colon of AOM‐treated rats. As indicated in Figure 4(a,b), quantitative RT‐PCR analysis revealed a significant increase in IDO expression in both the crypts and stromal cells of the AOM‐treated group compared with the untreated control group (P < 0.05 for each comparison). Furthermore, these increases were significantly inhibited by EGCG treatment (P < 0.05 for each comparison). Although 1‐MT treatment tended to decrease IDO expression in crypts and stromal tissues, the difference failed to reach statistical significance. In the absence of AOM treatment, IDO mRNA expression was not affected by the administration of either 1‐MT or EGCG. In contrast with IDO, AOM did not induce an increase in TDO mRNA expression and neither EGCG nor 1‐MT had any effect on TDO expression in crypts and stromal tissues (Fig. 4c,d).
Figure 4.
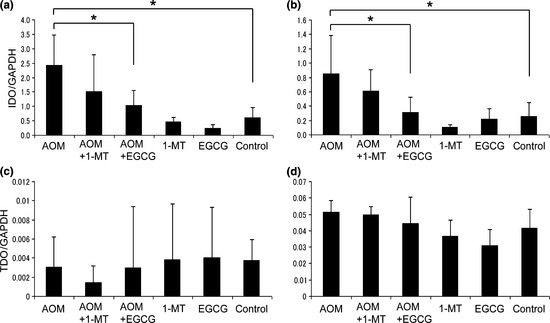
Effects of 1‐methyltryptophan (1‐MT) and (−)‐epigallocatechin gallate (EGCG) on the expression of (a,b) indoleamine 2,3‐dioxygenase (IDO) and (c,d) tryptophan 2,3‐dioxygenase (TDO) in the epithelium (a,c) and stroma (b,d). Total RNA was extracted from epithelial crypts and stromal tissues and IDO and TDO mRNA expression evaluated by quantitative RT‐PCR. Expression is normalized against that of GAPDH. AOM, azoxymethane. Data are the mean ± SD (n = 6). *P < 0.05.
Effects of 1‐MT and EGCG on IDO activity
We next examined the enzymatic activity of IDO in serum and colon tissues of AOM‐treated rats by measuring the concentrations of l‐kynurenine and l‐tryptophan. The l‐kynurenine/l‐tryptophan ratios in the serum (Fig. 5a) and stromal cells (Fig. 5c) of the AOM‐treated group were significantly higher than in the untreated control group (P < 0.05 for each comparison). Treatment of rats with 1‐MT and EGCG resulted in a significant decrease in these ratios in AOM‐treated rats (P < 0.05 for each comparison), suggesting that 1‐MT and EGCG significantly inhibit both the systemic (serum) and focal (colonic stromal) AOM‐induced increases in IDO activity. In epithelial cells, there were no significant differences in the l‐kynurenine/l‐tryptophan ratios between the different groups (Fig. 5b). In the absence of AOM treatment, neither 1‐MT nor EGCG alone had any effect on the l‐kynurenine/l‐tryptophan ratios (Fig. 5a–c).
Figure 5.
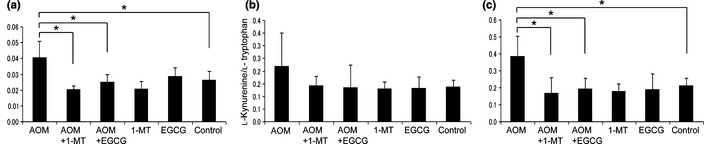
Effects of 1‐methyltryptophan (1‐MT) and (−)‐epigallocatechin gallate (EGCG) on indoleamine 2,3‐dioxygenase (IDO) activity in the (a) serum and colonic (b) epithelium and (c) stroma. Functional IDO activity was determined by measuring the concentrations of l‐kynurenine and tryptophan using HPLC. The l‐kynurenine/l‐tryptophan ratio indicates IDO activity. AOM, azoxymethane. Data are the mean ± SD (n = 6). *P < 0.05.
To further investigate whether 1‐MT and EGCG directly influence IDO activity, we measured IDO enzyme kinetics (kynurenine production) using recombinant human IDO in a cell‐free system. As shown in Figure 6, levels of l‐kynurenine produced by IDO were significantly inhibited by 1‐MT and EGCG treatment (P < 0.001 for each comparison). These findings suggest that both 1‐MT and EGCG act directly to inhibit IDO activity.
Figure 6.
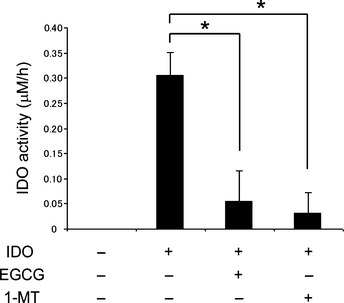
Effects of 1‐methyltryptophan (1‐MT) and (−)‐epigallocatechin gallate (EGCG) on indoleamine 2,3‐dioxygenase (IDO) activity in cell‐free assays. Functional activity of recombinant human IDO enzyme in response to 1‐MT and EGCG was determined by measuring the concentrations of enzymatic products (l‐kynurenine) using HPLC. Enzymatic activity is expressed as the product content per hour (μM/h). Data are the mean ± SD. *P < 0.001.
Effects of 1‐MT and EGCG on COX‐2 and IFN‐γ expression in stromal cells
We next assessed the inhibitory effects of 1‐MT and EGCG on COX‐2 and IFN‐γ expression, because both are regulated by inflammatory cells in the stroma and are implicated in the induction of IDO.40, 41, 42, 43 Using quantitative RT‐PCR, we found that the expression of COX‐2 mRNA in stromal tissues was markedly upregulated in the AOM‐treated group, but this upregulation was significantly inhibited by EGCG treatment (Fig. 7a). In addition, although AOM increased IFN‐γ mRNA expression in stromal cells, this increase was not inhibited by EGCG (Fig. 7b). Treatment of rats with 1‐MT did not have any significant effect on AOM‐induced increases in the expression of COX‐2 or IFN‐γ mRNA. In the absence of AOM treatment, neither 1‐MT nor EGCG alone had any effect on COX‐2 or IFN‐γ mRNA levels (Fig. 7a,b).
Figure 7.
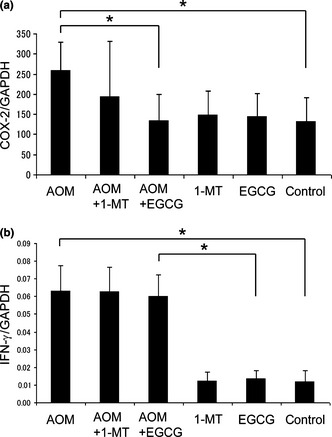
Effects of 1‐methyltryptophan (1‐MT) and (−)‐epigallocatechin gallate (EGCG) on the expression of (a) cyclooxygenase (COX)‐2 and (b) interferon (IFN)‐γ in stromal cells, as determined by quantitative RT‐PCR. Expression is normalized against that of GAPDH. AOM, azoxymethane. Data are the mean ± SD (n = 6). *P < 0.05.
Discussion
The results of the present study suggest that upregulation of IDO is possibly involved in colon carcinogenesis, as evidenced by higher IDO expression (Figs 3a,4a,b) and activity (Fig. 5c) in the colonic mucosa of AOM‐treated rats compared with the untreated controls, which did not receive any carcinogen. The results of the present study also provide the first evidence that treatment with the IDO inhibitor 1‐MT effectively suppresses the development of colonic preneoplastic lesions (ACF and BCAC) induced by AOM (Fig. 2). This inhibition is considered to be associated with the inhibition of IDO activity, which is increased in AOM‐treated rats (Fig. 5a,c), because IDO‐mediated immune tolerance plays a critical role in tumor development and progression.2, 3 Therefore, 1‐MT may correct IDO‐mediated immune escape and thus suppress AOM‐induced colorectal carcinogenesis. These results suggest that upregulation of IDO, and most likely subsequent immune tolerance caused by this enzyme, is involved in the early phase of colon carcinogenesis and that targeting IDO may, therefore, be an effective strategy to prevent colorectal carcinogenesis.
The chemopreventive and anti‐cancer effects of green tea and EGCG are partially attributed to their anti‐oxidative properties, their anti‐angiogenic and anti‐mutagenic effects, and their anti‐inflammatory activities, all of which act in combination to suppress carcinogenesis. Thus, these activities are considered to be the main mechanisms underlying the anti‐cancer effects of EGCG.13, 14 Furthermore, earlier studies showed that EGCG suppresses the induction of IDO in vitro.23, 24 In the present study, EGCG inhibited the functional enzyme activity of IDO in AOM‐treated rats (Fig. 5a,c). In addition, the inhibitory effects of EGCG against AOM‐induced increases in IDO mRNA expression in the colonic mucosa were greater than those of 1‐MT (Fig. 4a,b). This may be associated with the observation that EGCG caused a greater inhibition of the total number of ACF that did 1‐MT (Fig. 2a). Therefore, these results suggest that, in addition to the previously reported multiple critical mechanisms of action underlying tumor suppression,13, 14 EGCG may prevent the early phase of colon carcinogenesis, at least in part, by inhibiting the expression and activity of IDO and thus mediating an immune response. The results of a recent study indicating that green tea catechins exert anti‐cancer effects by regulating the expression and function of both T and natural killer cells44 may also strengthen the case for EGCG modulating immune tolerance.
A recent study has revealed the possible roles of toxic tryptophan catabolites produced by IDO in cancer.45 Of these metabolites, l‐kynurenine is considered to play a critical role in the immune escape of malignant cells that occurs within the tumor and its surrounding microenvironment.4 Conversely, IDO inhibitors can impede the growth of IDO‐expressing tumors by reducing the amount of kynurenine present in the microenvironment.46 Therefore, in addition to inhibiting IDO expression, EGCG has a direct effect in inhibiting IDO enzyme activity (Fig. 6), which may have contributed to its prevention of the development of colonic preneoplastic lesions in the present study.
In the present study, IDO mRNA levels in both the epithelium and stroma decreased in rats treated with 1‐MT or EGCG; however, the ratio of l‐kynurenine/l‐tryptophan decreased only in the stroma (Fig. 4a,b,5c). These findings suggest that IDO‐induced metabolic conversion of tryptophan to kynurenine occurs mainly in the stroma. For example, in human dendritic cells constitutively expressing IDO protein, the functional activity of this enzyme is tightly regulated and requires additional triggering signals supplied during antigen presentation by CD4+ T cells.47 Many important immunoregulatory pathways, such as the IFN/JAK/signal transducer and activator of transcription (STAT) pathway and the non‐canonical nuclear factor‐κB pathway, which are controlled by immune cells in the stroma, are related to IDO expression.38 In addition, several other immune regulatory factors have been implicated as inducers of IDO, including COX‐2, which is regarded as one of the most critical inflammatory mediators in the regulation of IDO expression.42 In the present study, the AOM‐induced upregulation of COX‐2 in the colonic stroma of rats was significantly inhibited by the administration of EGCG (Fig. 7a). These findings, together with those of Basu et al.,43 who reported a suppressive role of a COX‐2 inhibitor against IDO expression in the tumor microenvironment, suggest that EGCG inhibits the expression of IDO, possibly by preventing the induction of COX‐2, although further investigations are required to clarify the effects of EGCG. Thus, combination treatment using an IDO inhibitor plus a COX‐2 inhibitor may be an effective regimen for the chemoprevention of colorectal cancer because this combination will synergistically inhibit the expression and activity of IDO.
Interferon‐γ is also thought to be a major stimulator of IDO,40, 41 and EGCG has been reported to suppress IDO expression by inhibiting STAT‐1 activation in response to IFN‐γ in vitro.23, 24 However, in the present study the expression of IFN‐γ in the colonic stroma was not affected by EGCG in the drinking water (Fig. 7b). Other novel mechanisms by which EGCG modulates the expression of IDO may exist; therefore, further studies are needed to clarify the effects of EGCG on the immunoregulatory pathways related to IDO expression.
Aberrant crypt foci have attracted attention as putative precancerous lesions of the colon in experimental models.48 Numerous molecular abnormalities, including increased expression of K‐ras and APC gene mutations, have been demonstrated in human ACF.49 In addition, BCAC, which accumulate β‐catenin protein in the nucleus and cytoplasm, are regarded as putative precursors to colorectal adenomas.50 Several rodent studies have shown that both these lesions are useful as biomarkers to evaluate the chemopreventive properties of specific agents.19, 36, 37, 51 Therefore, our findings, namely that both 1‐MT and EGCG markedly inhibit the development of ACF and BCAC, appear to be significant when considering the chemoprevention of colorectal cancer. In particular, a significant reduction of large ACF by 1‐MT and EGCG should be emphasized, because large ACF are known to have a strong correlation with the incidence of colonic adenocarcinoma.20, 21
Finally, it should be mentioned that one limitation of the present study was that the l‐kynurenine/l‐tryptophan ratio may not directly reflect IDO activity because kynurenine can be metabolized further and TDO can also produce kynurenine from tryptophan.12 However, in the present study we presumed that TDO exerted little effect on l‐kynurenine levels because the expression of TDO was not affected by AOM treatment (Fig. 4c,d). Systemic IDO activity is currently estimated by the serum l‐kynurenine/l‐tryptophan ratio,33 as in the present study, because IDO is an intracellular enzyme and circulating IDO concentrations are barely detectable.3 In fact, a method for analyzing serum IDO protein itself has not been established in experimental animals and there is only one report, published in 2012, of its detection in humans.52 This limitation needs to be addressed in future studies.
In conclusion, the escape of precancerous cells from the immune system caused by immune tolerance is involved in certain types of carcinogenesis and, therefore, may be an effective target for the implementation of chemoprevention. The results of the present study support the notion that IDO upregulation, which induces immune tolerance, contributes to the early phase of colon carcinogenesis. Furthermore, the present study is the first to provide evidence that the anti‐carcinogenic properties of 1‐MT and EGCG may be related to inhibition of IDO activity, suggesting that targeting IDO and correcting IDO‐mediated immune tolerance with EGCG or an IDO inhibitor could be a promising strategy for the prevention of colorectal cancer development in the future. Further experiments using IDO‐knockout mice would strengthen the connection between IDO activity and the development of colorectal cancer, and may prove useful in the exploration of IDO inhibitors as chemopreventive agents for colorectal cancer.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgment
The authors thank Mitsui Norin (Tokyo, Japan) for providing the EGCG.
References
- 1. Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6: 715–27. [DOI] [PubMed] [Google Scholar]
- 2. Uyttenhove C, Pilotte L, Theate I et al Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3‐dioxygenase. Nat Med 2003; 9: 1269–74. [DOI] [PubMed] [Google Scholar]
- 3. Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol 2004; 4: 762–74. [DOI] [PubMed] [Google Scholar]
- 4. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan‐derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3‐dioxygenase. J Exp Med 2002; 196: 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okamoto A, Nikaido T, Ochiai K et al Indoleamine 2,3‐dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res 2005; 11: 6030–9. [DOI] [PubMed] [Google Scholar]
- 6. Ino K, Yoshida N, Kajiyama H et al Indoleamine 2,3‐dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer 2006; 95: 1555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brandacher G, Perathoner A, Ladurner R et al Prognostic value of indoleamine 2,3‐dioxygenase expression in colorectal cancer: effect on tumor‐infiltrating T cells. Clin Cancer Res 2006; 12: 1144–51. [DOI] [PubMed] [Google Scholar]
- 8. Ninomiya S, Hara T, Tsurumi H et al Indoleamine 2,3‐dioxygenase in tumor tissue indicates prognosis in patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Ann Hematol 2010; 90: 409–16. [DOI] [PubMed] [Google Scholar]
- 9. Yoshikawa T, Hara T, Tsurumi H et al Serum concentration of l‐kynurenine predicts the clinical outcome of patients with diffuse large B‐cell lymphoma treated with R‐CHOP. Eur J Haematol 2010; 84: 304–9. [DOI] [PubMed] [Google Scholar]
- 10. Muller AJ, DuHadaway JB, Donover PS, Sutanto‐Ward E, Prendergast GC. Inhibition of indoleamine 2,3‐dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med 2005; 11: 312–9. [DOI] [PubMed] [Google Scholar]
- 11. Hou DY, Muller AJ, Sharma MD et al Inhibition of indoleamine 2,3‐dioxygenase in dendritic cells by stereoisomers of 1‐methyl‐tryptophan correlates with antitumor responses. Cancer Res 2007; 67: 792–801. [DOI] [PubMed] [Google Scholar]
- 12. Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine‐2,3‐dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer 2009; 9: 445–52. [DOI] [PubMed] [Google Scholar]
- 13. Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol 2002; 42: 25–54. [DOI] [PubMed] [Google Scholar]
- 14. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer 2009; 9: 429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)‐Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor‐2 signaling pathways in human colon cancer cells. Clin Cancer Res 2005; 11: 2735–46. [DOI] [PubMed] [Google Scholar]
- 16. Shimizu M, Deguchi A, Joe AK, McKoy JF, Moriwaki H, Weinstein IB. EGCG inhibits activation of HER3 and expression of cyclooxygenase‐2 in human colon cancer cells. J Exp Ther Oncol 2005; 5: 69–78. [PubMed] [Google Scholar]
- 17. Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin‐like growth factor‐1 receptor in human colon cancer cells. Biochem Biophys Res Commun 2005; 334: 947–53. [DOI] [PubMed] [Google Scholar]
- 18. Shirakami Y, Shimizu M, Tsurumi H, Hara Y, Tanaka T, Moriwaki H. EGCG and polyphenon E attenuate inflammation‐related mouse colon carcinogenesis induced by AOM and DSS. Mol Med Report 2008; 1: 355–61. [PubMed] [Google Scholar]
- 19. Shimizu M, Shirakami Y, Sakai H et al (−)‐Epigallocatechin gallate suppresses azoxymethane‐induced colonic premalignant lesions in male C57BL/KsJ–db/db mice. Cancer Prev Res 2008; 1: 298–304. [DOI] [PubMed] [Google Scholar]
- 20. Pretlow TP, O'Riordan MA, Somich GA, Amini SB, Pretlow TG. Aberrant crypts correlate with tumor incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis 1992; 13: 1509–12. [DOI] [PubMed] [Google Scholar]
- 21. Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett 1995; 93: 55–71. [DOI] [PubMed] [Google Scholar]
- 22. Xiao H, Hao X, Simi B et al Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane‐treated F344 rats. Carcinogenesis 2008; 29: 113–9. [DOI] [PubMed] [Google Scholar]
- 23. Jeong YI, Jung ID, Lee JS, Lee CM, Lee JD, Park YM. (−)‐Epigallocatechin gallate suppresses indoleamine 2,3‐dioxygenase expression in murine dendritic cells: evidences for the COX‐2 and STAT1 as potential targets. Biochem Biophys Res Commun 2007; 354: 1004–9. [DOI] [PubMed] [Google Scholar]
- 24. Cheng CW, Shieh PC, Lin YC et al Indoleamine 2,3‐dioxygenase, an immunomodulatory protein, is suppressed by (−)‐epigallocatechin‐3‐gallate via blocking of gamma‐interferon‐induced JAK‐PKC‐delta‐STAT1 signaling in human oral cancer cells. J Agric Food Chem 2010; 58: 887–94. [DOI] [PubMed] [Google Scholar]
- 25. Yamada Y, Yoshimi N, Hirose Y et al Sequential analysis of morphological and biological properties of beta‐catenin‐accumulated crypts, provable premalignant lesions independent of aberrant crypt foci in rat colon carcinogenesis. Cancer Res 2001; 61: 1874–8. [PubMed] [Google Scholar]
- 26. Shimizu M, Sakai H, Shirakami Y et al Preventive effects of (−)‐epigallocatechin gallate on diethylnitrosamine‐induced liver tumorigenesis in obese and diabetic C57BL/KsJ–db/db mice. Cancer Prev Res 2011; 4: 396–403. [DOI] [PubMed] [Google Scholar]
- 27. Wang ZY, Agarwal R, Bickers DR, Mukhtar H. Protection against ultraviolet B radiation‐induced photocarcinogenesis in hairless mice by green tea polyphenols. Carcinogenesis 1991; 12: 1527–30. [DOI] [PubMed] [Google Scholar]
- 28. Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim 1981; 15: 57–9. [DOI] [PubMed] [Google Scholar]
- 29. Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett 1987; 37: 147–51. [DOI] [PubMed] [Google Scholar]
- 30. Suzuki R, Kohno H, Yasui Y et al Diet supplemented with citrus unshiu segment membrane suppresses chemically induced colonic preneoplastic lesions and fatty liver in male db/db mice. Int J Cancer 2007; 120: 252–8. [DOI] [PubMed] [Google Scholar]
- 31. Sakai H, Yamada Y, Shimizu M, Saito K, Moriwaki H, Hara A. Genetic ablation of Tnfalpha demonstrates no detectable suppressive effect on inflammation‐related mouse colon tumorigenesis. Chem Biol Interact 2010; 184: 423–30. [DOI] [PubMed] [Google Scholar]
- 32. Hoshi M, Saito K, Hara A et al The absence of IDO upregulates type I IFN production, resulting in suppression of viral replication in the retrovirus‐infected mouse. J Immunol 2010; 185: 3305–12. [DOI] [PubMed] [Google Scholar]
- 33. Suzuki Y, Suda T, Furuhashi K et al Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010; 67: 361–5. [DOI] [PubMed] [Google Scholar]
- 34. Fujigaki S, Saito K, Sekikawa K et al Lipopolysaccharide induction of indoleamine 2,3‐dioxygenase is mediated dominantly by an IFN‐gamma‐independent mechanism. Eur J Immunol 2001; 31: 2313–8. [DOI] [PubMed] [Google Scholar]
- 35. Fujigaki S, Saito K, Takemura M et al The l‐Tryptophan–l‐kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon‐gene‐deficient mice: cross‐regulation between inducible nitric oxide synthase and indoleamine‐2,3‐dioxygenase. Infect Immun 2002; 70: 779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shimizu M, Shirakami Y, Iwasa J et al Supplementation with branched‐chain amino acids inhibits azoxymethane‐induced colonic preneoplastic lesions in male C57BL/KsJ–db/db mice. Clin Cancer Res 2009; 15: 3068–75. [DOI] [PubMed] [Google Scholar]
- 37. Yasuda Y, Shimizu M, Shirakami Y et al Pitavastatin inhibits azoxymethane‐induced colonic preneoplastic lesions in C57BL/KsJ–db/db obese mice. Cancer Sci 2010; 101: 1701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3‐dioxygenase in T‐cell tolerance and tumoral immune escape. Immunol Rev 2008; 222: 206–21. [DOI] [PubMed] [Google Scholar]
- 39. Dolusic E, Larrieu P, Moineaux L et al Tryptophan 2,3‐dioxygenase (TDO) inhibitors. 3‐(2‐(pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J Med Chem 2011; 54: 5320–34. [DOI] [PubMed] [Google Scholar]
- 40. Carlin JM, Borden EC, Sondel PM, Byrne GI. Interferon‐induced indoleamine 2,3‐dioxygenase activity in human mononuclear phagocytes. J Leukoc Biol 1989; 45: 29–34. [DOI] [PubMed] [Google Scholar]
- 41. Takikawa O, Tagawa Y, Iwakura Y, Yoshida R, Truscott RJ. Interferon‐gamma‐dependent/independent expression of indoleamine 2,3‐dioxygenase. Studies with interferon‐gamma‐knockout mice. Adv Exp Med Biol 1999; 467: 553–7. [DOI] [PubMed] [Google Scholar]
- 42. von Bergwelt‐Baildon MS, Popov A, Saric T et al CD25 and indoleamine 2,3‐dioxygenase are up‐regulated by prostaglandin E2 and expressed by tumor‐associated dendritic cells in vivo: additional mechanisms of T‐cell inhibition. Blood 2006; 108: 228–37. [DOI] [PubMed] [Google Scholar]
- 43. Basu GD, Tinder TL, Bradley JM et al Cyclooxygenase‐2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol 2006; 177: 2391–402. [DOI] [PubMed] [Google Scholar]
- 44. Shimizu K, Kinouchi Shimizu N, Hakamata W, Unno K, Asai T, Oku N. Preventive effect of green tea catechins on experimental tumor metastasis in senescence‐accelerated mice. Biol Pharm Bull 2010; 33: 117–21. [DOI] [PubMed] [Google Scholar]
- 45. Chung KT, Gadupudi GS. Possible roles of excess tryptophan metabolites in cancer. Environ Mol Mutagen 2011; 52: 81–104. [DOI] [PubMed] [Google Scholar]
- 46. Koblish HK, Hansbury MJ, Bowman KJ et al Hydroxyamidine inhibitors of indoleamine‐2,3‐dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO‐expressing tumors. Mol Cancer Ther 2010; 9: 489–98. [DOI] [PubMed] [Google Scholar]
- 47. Munn DH, Sharma MD, Mellor AL. Ligation of B7‐1/B7‐2 by human CD4+ T cells triggers indoleamine 2,3‐dioxygenase activity in dendritic cells. J Immunol 2004; 172: 4100–10. [DOI] [PubMed] [Google Scholar]
- 48. Raju J. Azoxymethane‐induced rat aberrant crypt foci: relevance in studying chemoprevention of colon cancer. World J Gastroenterol 2008; 14: 6632–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gupta AK, Pretlow TP, Schoen RE. Aberrant crypt foci: what we know and what we need to know. Clin Gastroenterol Hepatol 2007; 5: 526–33. [DOI] [PubMed] [Google Scholar]
- 50. Mori H, Hata K, Yamada Y, Kuno T, Hara A. Significance and role of early‐lesions in experimental colorectal carcinogenesis. Chem Biol Interact 2005; 155: 1–9. [DOI] [PubMed] [Google Scholar]
- 51. Yasui Y, Suzuki R, Kohno H et al 9trans,11trans conjugated linoleic acid inhibits the development of azoxymethane‐induced colonic aberrant crypt foci in rats. Nutr Cancer 2007; 59: 82–91. [DOI] [PubMed] [Google Scholar]
- 52. Eleftheriadis T, Antoniadi G, Liakopoulos V, Stefanidis I, Galaktidou G. Plasma indoleamine 2,3‐dioxygenase concentration is increased in hemodialysis patients and may contribute to the pathogenesis of coronary heart disease. Renal Fail 2012; 34: 68–72. [DOI] [PubMed] [Google Scholar]


