Abstract
Adult T‐cell leukemia/lymphoma (ATLL) is a peripheral T cell neoplasm that is associated with infection by the human T‐cell leukemia virus type I (HTLV‐1). Although the high incidence of ATLL in HTLV‐1‐endemic areas is well known, population‐based evidence concerning the incidence of ATLL in non‐endemic areas is scarce. To answer this, we estimated the age‐standardized incidence of ATLL from 1993 to 2006 for Japan and 1993 to 2008 for the US and assessed its trend using data from a population‐based cancer registry in Japan and Surveillance Epidemiology and End Results (SEER) in the US. The Japanese data were collected from 15 prefectures. A total of 2055 patients in the three prefectures in Kyushu and 1380 patients in the 12 prefectures in Honshu were diagnosed with ATLL in the study period. In the US, a total of 140 patients were diagnosed with ATLL. The results showed that the age‐standardized incidence in non‐endemic areas in Japan and in the US significantly increased during this period (annual percent change [95%CI]; Japan‐Honshu: +4.6% [1.1, 8.2]; US: +6.2% [1.5, 11.1]), while in the endemic areas of Japan there was no change (annual percent change [95%CI]; Japan‐Kyushu: 0.0% [−1.6, 1.7]). This result indicates that the disease has been spread by carriers to non‐endemic areas, and suggests the necessity of establishing a standard preventive strategy.
Adult T‐cell leukemia/lymphoma (ATLL) is defined as a peripheral T cell neoplasm associated with infection by the human T‐cell leukemia virus type I (HTLV‐1).1 Human T‐cell leukemia virus type I infects an estimated 10–20 million people worldwide,2 and is primarily transmitted by breastfeeding, blood transfusion, sharing of needles, and sexual intercourse. Infection with HTLV‐1 and cases of ATLL are endemic in several regions of the world, including southwestern Japan (Kyushu), the Caribbean basin, and parts of Central Africa.1 Sporadic cases in non‐endemic areas have also been described, but the affected patients had often migrated from endemic regions and were usually exposed to the virus early in life.3 The virus has long latency, resulting in ATLL after several decades (median onset of disease is around 60 years) and the cumulative risk of developing the disease is reported to be 2–5%.4
Although virology and treatment strategies have progressed over recent decades,5, 6, 7, 8, 9, 10 trends in the incidence of ATLL in non‐endemic areas have not been epidemiologically evaluated. Here, we report trends in the incidence of ATLL in Kyushu, an endemic area of Japan, Honshu, a non‐endemic area of Japan, and the United States (US).
Material and Methods
We used the population‐based cancer registry data of Japan and the US for these analyses. The Japanese data were collected from 15 prefectures (Akita, Yamagata, Miyagi, Niigata, Tochigi, Chiba, Kanagawa, Fukui, Aichi, Tottori, Okayama, Hiroshima, Saga, Nagasaki and Kumamoto; three from Kyushu, 12 from Honshu), which were included in the Monitoring of Cancer Incidence in Japan (MCIJ) project.11 The MCIJ project, data source of our analysis, was started in 2007 as a national project aiming to collect and unite the cancer registry data of each prefecture by the standardized protocol. Currently, national estimates for incidence are based on 15 out of 32 prefectures, which provided data to the MCIJ project. This selection is based on the international rules for the cancer incidence in population‐based registry data that evaluates the quality index for completeness of registry.12 As for data in the US, the SEER nine registries that are Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco‐Oakland, Seattle‐Puget Sound, and Utah, was used.13, 14 The data of the MCIJ project consists of 33.7% of the Japanese population,11 while that of SEER9 consists of 9.5% of the US population.13, 14
The period covered in these analyses was 1993–2006 in Japan and 1993–2008 in the US. In the Japanese and US cancer registry systems, disease incidence data are constructed and collected according to the International Classification of Diseases – Oncology (ICD‐O). Adult T‐cell leukemia/lymphoma was coded as 9827 in ICD‐O‐3 and we identified the number of cases from the database. Incidence rates were standardized by age‐adjustment according to the world standard population,15 and were calculated as newly diagnosed cases of ATLL per 100 000 person‐years. We estimated the annual percent change (APC) by joinpoint regression analysis and evaluated the trend, as described in detail elsewhere.16 The standard error of the age‐standardized rate was estimated for each year. All computations were performed with STATA version 11 (STATA Corporation, College Station, TX, USA) except for the joinpoint regression analysis, for which we used the Joinpoint Regression Program version 3.3 (US National Cancer Institute, Bethesda, MD, USA). For the joinpoint regression analysis, two‐sided P‐values < 0.05 were considered statistically significant.
Results
A total of 2055 patients in the three prefectures of Kyushu and 1380 patients in the 12 prefectures of Honshu in this study were diagnosed with ATLL between 1993 and 2006. In the US, a total of 140 patients were diagnosed with ATLL in the nine registries in this study between 1993 and 2008. There was a slight male predominance in the incidence of ATLL (male/female ratio of incidence, Honshu: 1.39, Kyushu: 1.52, US: 1.26). When we looked at the ethnicities of the US patients, 68 patients (49%) were white, 45 patients (32%) were African‐American, 17 patients (12%) were Japanese and 10 patients (7%) were other ethnicities. The incidence of ATLL in the study periods in each area is summarized in Table 1. To see the overall trend in the incidence in each area, we estimated age‐standardized incidences that combined male and female as shown in Fig. 1. The age‐standardized modeled incidence estimated by joinpoint regression analysis is shown as a line in Fig. 1. The incidence of ATLL in Honshu and in the US significantly increased during the study period (Table 2). In contrast, the incidence in Kyushu showed no change.
Table 1.
Age‐standardized incidence rates of Adult T‐cell leukemia/lymphoma
| Incidence rate (95%CI) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Area | Year | |||||||||||||||
| 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | |
| Kyushu | ||||||||||||||||
| Male | ||||||||||||||||
| Age‐standardized rate (95%CI) | 2.53 (2.46–2.59) | 2.61 (2.54–2.67) | 2.54 (2.48–2.60) | 2.22 (2.17–2.28) | 2.33 (2.27–2.39) | 2.26 (2.20–2.31) | 1.98 (1.93–2.03) | 2.09 (2.04–2.14) | 2.47 (2.42–2.53) | 2.40 (2.34–2.45) | 3.08 (3.01–3.14) | 2.38 (2.33–2.43) | 2.41 (2.36–2.46) | 2.09 (2.04–2.13) | ||
| Female | ||||||||||||||||
| Age‐standardized rate (95%CI) | 1.66 (1.61–1.70) | 1.67 (1.63–1.71) | 1.57 (1.53–1.62) | 1.58 (1.53–1.62) | 1.25 (1.21–1.29) | 1.60 (1.56–1.64) | 1.35 (1.31–1.39) | 1.70 (1.65–1.74) | 1.88 (1.84–1.93) | 1.24 (1.20–1.28) | 2.07 (2.01–2.12) | 1.35 (1.31–1.38) | 1.56 (1.52–1.60) | 1.67 (1.63–1.71) | ||
| Honshu | ||||||||||||||||
| Male | ||||||||||||||||
| Age‐standardized rate (95%CI) | 0.10 (0.10–0.11) | 0.10 (0.10–0.11) | 0.15 (0.14–0.15) | 0.20 (0.19–0.20) | 0.18 (0.17–0.18) | 0.24 (0.24–0.25) | 0.22 (0.21–0.23) | 0.20 (0.19–0.20) | 0.29 (0.28–0.29) | 0.20 (0.20–0.21) | 0.24 (0.24–0.25) | 0.29 (0.28–0.29) | 0.28 (0.27–0.29) | 0.21 (0.21–0.22) | ||
| Female | ||||||||||||||||
| Age‐standardized rate (95%CI) | 0.09 (0.08–0.09) | 0.09 (0.08–0.09) | 0.09 (0.08–0.09) | 0.19 (0.18–0.19) | 0.14 (0.13–0.14) | 0.19 (0.18–0.20) | 0.18 (0.17–0.18) | 0.13 (0.12–0.13) | 0.18 (0.17–0.18) | 0.12 (0.11–0.12) | 0.19 (0.19–0.20) | 0.19 (0.18–0.19) | 0.20 (0.20–0.21) | 0.14 (0.13–0.14) | ||
| United States | ||||||||||||||||
| Male | ||||||||||||||||
| Age‐standardized rate (95%CI) | 0.03 (0.03–0.04) | 0.01 (0.01–0.02) | 0.00 (0.00–0.01) | 0.03 (0.02–0.03) | 0.02 (0.01–0.02) | 0.04 (0.03–0.04) | 0.01 (0.01–0.01) | 0.02 (0.02–0.02) | 0.03 (0.03–0.03) | 0.02 (0.02–0.03) | 0.03 (0.03–0.03) | 0.04 (0.04–0.05) | 0.06 (0.05–0.06) | 0.03 (0.03–0.03) | 0.01 (0.01–0.01) | 0.01 (0.01–0.01) |
| Female | ||||||||||||||||
| Age‐standardized rate (95%CI) | 0.01 (0.00–0.01) | 0.01 (0.01–0.01) | 0.02 (0.01–0.02) | 0.02 (0.01–0.02) | 0.01 (0.01–0.02) | 0.01 (0.01–0.02) | 0.02 (0.02–0.02) | 0.02 (0.02–0.02) | 0.02 (0.02–0.02) | 0.03 (0.02–0.03) | 0.02 (0.02–0.02) | 0.01 (0.01–0.01) | 0.05 (0.04–0.05) | 0.03 (0.03–0.04) | 0.01 (0.01–0.01) | 0.04 (0.03–0.04) |
Incidences are standardized by world populations (/100 000). CI, confidence interval.
Figure 1.
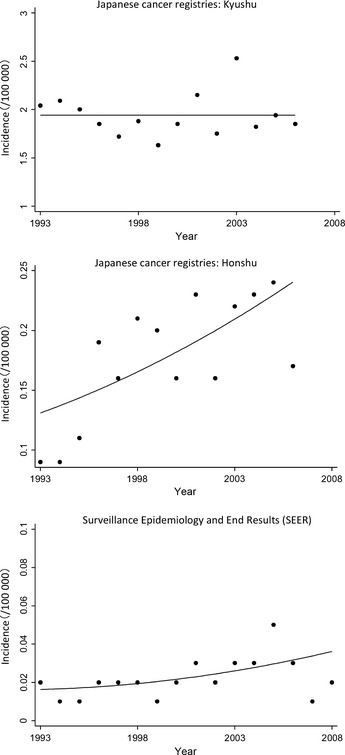
Incidence of adult T‐cell leukemia/lymphoma in Japan and US. Circles indicate observed age‐standardized incidence rates and lines indicate age‐standardized incidence rate estimated by joinpoint regression analysis.
Table 2.
Trend of age‐standardized incidence rates with joinpoint analyses
| Areas | Trend | |
|---|---|---|
| Year | APC (95%CI) | |
| Kyushu | 1993–2006 | 0.0 (−1.6, 1.7) |
| Honshu | 1993–2006 | 4.6 (1.1, 8.2)a |
| United States | 1993–2008 | 6.2 (1.5, 11.1)a |
APC is significantly different from zero (two‐sided P < 0.05, calculated using a t‐test.). APC, annual percent change; CI, confidence interval.
Discussion
We found that there was a significant increase in the incidence of ATLL in non‐endemic areas in Japan and the US, while the incidence in the endemic area (Kyushu) showed no change. In the past decades, population distribution has accelerated with economic growth, suggesting that more carriers have moved and are moving to non‐endemic areas. The nationwide survey conducted in 2006–2007 to evaluate the prevalence of HTLV‐1 carriers among first‐time blood donors revealed that the number of HTLV‐1 carriers is increasing in non‐endemic areas in Japan.17 Our result supports this previous report that HTLV‐1 spreads by migration of HTLV‐1 carriers from endemic to non‐endemic areas, based on the incidence and trend of ATLL.
Although the incidence of ATLL in the US is still significantly lower than that in Japan, the annual percentage increase in the US was 6.2%, indicating a doubling of the incidence in the next decade if the trend does not change. Half of the patients diagnosed in the US are non‐white including a substantial number of Japanese, which suggests the immigration of carriers from the endemic areas of Japan. Adult T‐cell leukemia/lymphoma in US‐born citizens is still rare. However, as the lifetime cumulative incidence of ATLL in carriers was <5%, there may be many more HTLV‐1 carriers than patients with ATLL. When the carriers immigrated and became settled in the US, the virus could have spread by horizontal infection, resulting in the increase in ATLL cases in the next generation. Since the interval between HTLV‐1 infection and onset of ATLL is several decades, a change in the distribution of carriers would result in a change in incidence in the distant future. ATLL is no longer restricted to endemic areas.
Human T‐cell leukemia virus type I is most commonly transmitted by breastfeeding, and can also be sexually transmitted. The overall rate of vertical transmission ranged between 15% and 25%, and in subgroups of children who received prolonged breastfeeding, these rates were even higher.18 Refraining from breastfeeding blocked approximately 80% of mother‐to‐child transmissions of HTLV‐1.7 Strong efforts have been made to prevent HTLV‐I infection in the Kyushu area since the detection of HTLV‐1. Although the effect of these prevention strategies has yet to be reflected as a decrease in the incidence of ATLL in Kyushu, the Ministry of Health, Labour and Welfare of Japan decided to start checking the HTLV‐1 status of all pregnancies from 2011. This screening consists of an initial check for antibody by the particle agglutination method or the chemiluminescent enzyme immunoassay method before 30 weeks of pregnancy, with positive results subsequently confirmed by Western blot analysis. Pregnant women who are sero‐positive are recommended to refrain from breastfeeding. The effect of this intervention will take decades to manifest itself, but may decrease the incidence of ATLL in Japan even in non‐endemic areas.
Although our results are consistent with the previous results that showed an increase in the number of HTLV‐1 carriers in non‐endemic areas, we should interpret this increase in the incidence in non‐endemic areas with some caution since the change in diagnostic accuracy of ATLL over time may also have contributed to the increase in part. Adult T‐cell leukemia/lymphoma sometimes resembles other T‐cell lymphomas,19 suggesting the possibility of misdiagnosed cases in the past especially in the non‐endemic areas where the diagnostic strategy was not well‐known.
Regarding Japanese analysis, our results are based on the data from limited prefectures (33.7% of all Japanese population) not including metropolitan areas like Tokyo or Osaka. This is because of data unavailability (1993–2006) and/or data quality, therefore, there remains uncertainty in the generalizability of our results to the entire population of Japan. The same is true of the US evaluation (covering 9.5% of the US population). But, considering the fact that 12 prefectures exclusive of Kyushu analyzed in Japan and the US are definite non‐endemic areas of HTLV‐I,20 at least it is true that ATLL incidence increased in the HTLV‐I non‐endemic area. We are speculating that an increasing trend in these areas could be a result of the diffusion of carriers that has occurred to non‐endemic areas from endemic areas, which is reported for Japanese by Satake et al.17
In conclusion, we found evidence that the incidence of ATLL is increasing in non‐endemic areas of Japan, and in the US. Given the increase in migration over the past few decades, this trend may not change in the next decades. Considering the efficacy of HTLV‐1 transmission prevention programs in conjunction with the increasing incidence of ATLL in Honshu and the US, more attention should be paid to prevention even in non‐endemic areas and consideration should be given to the establishment of a worldwide standard preventive strategy.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We thank all of the staff of the MCIJ and SEER project. This study was partly supported by the 3rd‐term Comprehensive Ten‐year Strategy for Cancer Control and by the Research Funding for Longevity Sciences (22‐9) from the National Center for Geriatrics and Gerontology (NCGG), Japan.
Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02373.x, 2012
References
- 1. Swerdlow S, Campo E, Harris N et al WHO classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: International Agency for Research on Cancer (IARC), 2008. [Google Scholar]
- 2. de The G, Bomford R. An HTLV‐I vaccine: why, how, for whom? AIDS Res Hum Retroviruses 1993; 9: 381–6. [DOI] [PubMed] [Google Scholar]
- 3. Tajima K, Tominaga S, Suchi T, Fukui H, Komoda H, Hinuma Y. HTLV‐I carriers among migrants from an ATL‐endemic area to ATL non‐endemic metropolitan areas in Japan. Int J Cancer 1986; 37: 383–7. [DOI] [PubMed] [Google Scholar]
- 4. Tokudome S, Tokunaga O, Shimamoto Y et al Incidence of adult T‐cell leukemia/lymphoma among human T‐lymphotropic virus type I carriers in Saga, Japan. Cancer Res 1989; 49: 226–8. [PubMed] [Google Scholar]
- 5. Yamamoto K, Utsunomiya A, Tobinai K et al Phase I study of KW‐0761, a defucosylated humanized anti‐CCR4 antibody, in relapsed patients with adult T‐cell leukemia‐lymphoma and peripheral T‐cell lymphoma. J Clin Oncol 2010; 28: 1591–8. [DOI] [PubMed] [Google Scholar]
- 6. Tsukasaki K, Utsunomiya A, Fukuda H et al VCAP‐AMP‐VECP compared with biweekly CHOP for adult T‐cell leukemia‐lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol 2007; 25: 5458–64. [DOI] [PubMed] [Google Scholar]
- 7. Hino S, Katamine S, Miyata H, Tsuji Y, Yamabe T, Miyamoto T. Primary prevention of HTLV‐1 in Japan. Leukemia 1997; 11(Suppl 3): 57–9. [PubMed] [Google Scholar]
- 8. Yoshida M. Mechanism of transcriptional activation of viral and cellular genes by oncogenic protein of HTLV‐1. Leukemia 1994; 8(Suppl 1): S51–3. [PubMed] [Google Scholar]
- 9. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol 1991; 79: 428–37. [DOI] [PubMed] [Google Scholar]
- 10. Seiki M, Hattori S, Hirayama Y, Yoshida M. Human adult T‐cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci USA 1983; 80: 3618–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Matsuda T, Marugame T, Kamo KI, Katanoda K, Ajiki W, Sobue T. Cancer incidence and incidence rates in Japan in 2006: based on data from 15 population‐based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2011; 42: 139–47. [DOI] [PubMed] [Google Scholar]
- 12. Curado M, Edwards B, Shin H et al, eds. Cancer Incidence in Five Continents, Vol. IX Lyon: IARC Scientific Publications, 2007. [Google Scholar]
- 13. Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2009) Surveillance Research Program. Released January 2011 edn. National Cancer Institute, DCCPS; Cancer Statistics Branch.
- 14. Surveillance, Epidemiology, and End Results (SEER) Program. Surveillance Research Program. Released April 2011 edn: National Cancer Institute, DCCPS; Cancer Statistics Branch.
- 15. Bray F, Guilloux A, Sankila R, Parkin DM. Practical implications of imposing a new world standard population. Cancer Causes Control 2002; 13: 175–82. [DOI] [PubMed] [Google Scholar]
- 16. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335–51. [DOI] [PubMed] [Google Scholar]
- 17. Satake M, Yamaguchi K, Tadokoro K. Current prevalence of HTLV‐1 in Japan as determined by screening of blood donors. J Med Virol 2012; 84: 327–35. [DOI] [PubMed] [Google Scholar]
- 18. Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T‐lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis 2007; 7: 266–81. [DOI] [PubMed] [Google Scholar]
- 19. Ohshima K. Pathological features of diseases associated with human T‐cell leukemia virus type I. Cancer Sci 2007; 98: 772–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mueller N. The epidemiology of HTLV‐I infection. Cancer Causes Control 1991; 2: 37–52. [DOI] [PubMed] [Google Scholar]


