Abstract
We expanded CTL specific for Tax (a human T‐lymphotropic virus type‐1‐encoded gene product) in vitro from PBMC of several adult T‐cell leukemia/lymphoma (ATL) patients, and document its potential significance as a target for ATL immunotherapy. Tax‐specific CTL responses against tumor cells were restricted by Tax‐expression and the appropriate human leukocyte antigen (HLA) type. Tax‐specific CTL recognized HLA/Tax‐peptide complexes on autologous ATL cells, even when their Tax expression was so low that it could only be detected by RT‐PCR but not by flow cytometry. Recognition resulted in interferon gamma (IFN‐γ) production and target cell lysis. This would be the first report that Tax‐specific CTL from ATL patients specifically recognized and killed autologous tumor cells that expressed Tax. The Tax‐specific CTL responded to as little as 0.01 pM of the corresponding peptide, indicating that their T‐cell receptor avidity was much higher than that of any other CTL recognizing viral or other tumor antigens. This is presumably the reason why the Tax‐specific CTL recognized and killed autologous ATL cells despite their very low Tax expression. In addition, cell cycle analyses and experiments with primary ATL cell‐bearing mice demonstrated that ATL cells present at the site of active cell proliferation, such as in the tumor masses, expressed substantial amounts of Tax, but it was minimally expressed by the tumor cells in a quiescent state, such as in the blood. The present study not only provides a strong rationale for exploiting Tax as a possible target for ATL immunotherapy but also contributes to our understanding of the immunopathogenesis of ATL.
Adult T‐cell leukemia/lymphoma (ATL) is a distinct hematologic malignancy caused by human T‐lymphotropic virus type 1 (HTLV‐1).1, 2 ATL has a long latency period of 50–60 years, so affected individuals have usually been exposed to HTLV‐1 early in their lives via agents including infected lymphocytes, mainly from mother's breast milk.3, 4 Only small subpopulations (approximately 5%) of HTLV‐1‐infected individuals progress to ATL, but there are no clear biomarkers separating those who will develop ATL from those who remain asymptomatic carriers (AC).2 There are four clinical subtypes of ATL: acute, lymphoma, chronic and smoldering.5 The two former types have more aggressive clinical courses (aggressive variants), while the latter are less aggressive (indolent variants).
Human T‐lymphotropic virus type 1 Tax, a virus‐encoded regulatory gene product, is required for the virus to transform cells,6 and is thought to be indispensable for oncogenesis. Therefore, Tax has been considered as a molecular target for immunotherapy against ATL, and many such investigations have been published.7, 8, 9, 10 However, it has been reported that the level of Tax expression in HTLV‐1‐infected cells decreases during disease progression, and Tax transcripts are detected only in approximately 40% of established ATL cases.11 Moreover, weak or absent responses to Tax have been observed in ATL patients,12 leading to controversy as to whether Tax is an appropriate target for immunotherapy of ATL. In the present study, we expanded Tax‐specific CTL in vitro from PBMC of several ATL patients, and tested their ability to respond to several ATL cell lines, HTLV‐1‐immortalized lines and to autologous ATL cells. The aim was to clarify the involvement of Tax‐specific CTL (Tax‐CTL) in the immunopathogenesis of ATL, and to confirm the significance of Tax as a potential immunotherapeutic target in ATL.
Materials and Methods
Primary adult T‐cell leukemia/lymphoma cells
Primary ATL cells were separated from PBMC using anti‐human CD4 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany). All donors provided informed written consent before sampling according to the Declaration of Helsinki, and the present study was approved by the institutional ethics committees of Nagoya City University Graduate School of Medical Sciences.
Cell lines
TL‐Su and TL‐Om1 were provided by the Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan). TCL‐Kan was kindly provided by Professor Mari Kannagi (Tokyo Medical and Dental University, Tokyo, Japan).13 HUT102, ATN‐1, MT‐2 and MT‐1 have been previously described.14, 15 MT‐4 was purchased from the Health Science Research Resources Bank (Osaka, Japan). HUT102, ATN‐1, MT‐1 and TL‐Om1 are ATL cell lines, and TL‐Su, TCL‐Kan, ILT‐#37, MT‐2 and MT‐4 are HTLV‐I‐immortalized lines. K562 is the chronic myelogenous leukemia blast crisis cell line.16
Human leukocyte antigen typing
Genotyping of HLA‐A, B and C was performed using an HLA‐typing Kit (WAKFlow HLA‐typing kit, WAKUNAGA Pharmacy, Hiroshima, Japan).
Expansion of human T‐lymphotropic virus type 1 Tax‐specific CTL
PBMC from ATL patients or HTLV‐1 AC were suspended in RPMI‐1640 supplemented with 10% autologous plasma and 0.1 μM of the corresponding Tax epitope peptides (LLFGYPVYV or SFHSLHLLF) at a cell concentration of 2.0 × 106/mL. These two synthetic peptides were purchased from Invitrogen (Carlsbad, CA, USA). The cell suspension was cultured at 37°C in 5%CO2 for 2 days, and then an equal volume of RPMI‐1640 supplemented with 100 IU/mL of IL‐2 was added. After subsequent culture for 5 days, an equal volume of ALyS505N (Cell Science & Technology Institute, Sendai, Japan) supplemented with 100 IU/mL of IL‐2 was added, and the cells were cultured with appropriate medium (ALyS505N with 100 IU/mL of IL‐2) for 7 days. Cytomegalovirus (CMV)‐pp65 specific CTL were expanded in the same manner using peptides such as NLVPMVATV or QYDPVAALF (Invitrogen). Viable cell counts were determined using the trypan blue assay.
Antibodies, tetramers and flow cytometry
Phycoerythrin‐conjugated HLA‐A*02:01/Tax11–19 (LLFGYPVYV), HLA‐A*24:02/Tax301–309 (SFHSLHLLF), HLA‐A*02:01/pp65 495–503 (NLVPMVATV) and HLA‐A*24:02/pp65 341–349 (QYDPVAALF) tetramers, and phycoerythrin‐Cyanin5‐conjugated anti‐CD8 monoclonal antibody (mAb) were purchased from Medical & Biological Laboratories, Nagoya, Japan. Allophycocyanin‐conjugated anti‐human CD45 mAb (2D1) and PerCP‐conjugated anti‐CD4 mAb (SK3) were purchased from BD Biosciences (San Jose, CA, USA). Tax expression was assessed by FITC‐conjugated anti‐Tax mAb Lt‐4.17 FITC‐conjugated anti‐interferon gamma (IFN‐γ) mAb (45.15) was purchased from Medical & Biological Laboratories. Cell cycle assessments were performed by BrdU Flow Kits (BD Biosciences). Cells were analyzed on a FACSCalibur (BD Biosciences) with the aid of FlowJo software (Tree Star, Ashland, OR, USA).
CTL assay
Cytotoxic activity was determined by a standard 4‐h chromium51 release assay as previously described.18 All values given are means of triplicate determinations.
Quantitative RT‐PCR
Tax, human CD4 and β‐actin mRNA were amplified as previously described.19 The primer set for Tax was as follows: sense, 5′‐AAGACCACCAACACCATGGC‐3′; and antisense, 5′‐CCAAACACGTAGACTGGGTATCC‐3′.
Animals
NOD/Shi‐scid, IL‐2Rγ null (NOG) mice were purchased from the Central Institute for Experimental Animals (Kawasaki, Japan). All of the in vivo experiments were approved by the Ethics Committee of the Center for Experimental Animal Science, Nagoya City University Graduate School of Medical Sciences.
Results
Expansion of Tax‐specific CTL
Expansion of Tax‐CTL was performed by stimulating PBMC from 14 ATL patients and 6 HTLV‐1 AC with synthetic peptides. PBMC from patients 1, 2, 3, 6, 8, 9 and 13 were stimulated with Tax11–19, and those from patients 4, 5, 7, 10, 11, 12 and 14 with Tax301–309 (Tables 1 and 2). Patients 1–6 were all in complete remission (CR) at the time of blood sampling. Patient 1 had achieved CR after allogeneic hematopoietic stem cell transplantation (HSCT) 5 years previously, patients 2 and 3 after systemic chemotherapy and anti‐CCR4 mAb treatment,20, 21 patient 4 after systemic chemotherapy alone, and patient 5 after allogeneic HSCT 9 months earlier (and was receiving FK506 at the time of sampling). Finally, patient 6 achieved CR after systemic chemotherapy and anti‐CCR4 mAb treatment, and was receiving prednisolone at the time of sampling. As shown in Table 1, Tax‐CTL could be expanded in vitro (fold expansion >10) by stimulation with Tax peptide in 13 of 17 ATL cases. With respect to HTLV‐1 AC, we confirmed efficient expansion (fold expansion >102) of Tax‐CTL from six of six individuals using Tax11–19 or Tax301–309 peptides in the same manner (data not shown), which are consistent with a previous report.22 Although the degree of expansion of Tax‐CTL varied among the ATL patients, there was a trend for higher rates in PBMC from those with indolent variant ATL not on any systemic treatment, or from patients with aggressive ATL in treatment‐induced remission, compared to lower or absent expansion in patients initially diagnosed with an aggressive variant. In particular, patient 8 progressed from chronic to acute subtype during the present study. Tax‐CTL could be efficiently expanded from this patient during the chronic phase, but no longer after progression to acute subtype. This was despite the finding that the percentage of HLA‐A*02:01/Tax11–19 tetramer‐positive cells in the PBMC was almost the same as before disease progression (Fig. 1). These observations collectively indicate that insufficient responses to Tax observed in ATL patients, which are also reported by other investigators,12, 23, 24 are related to disease progression from indolent to aggressive clinical variants. Subsequently, patient 8 received systemic chemotherapy but failed to achieve CR. He then received allogeneic HSCT with reduced intensity conditioning and entered partial remission. At this time, when he was not receiving immunosuppression after HSCT, his Tax‐CTL could again be efficiently expanded from PBMC. This indicates that substantial anti‐Tax responses can be restored by appropriate anti‐ATL therapies, when the patient is brought from active ATL into remission (Fig. 1). Even though patients were in CR, immunosuppressive agents such as FK506 or prednisolone were likely to have prevented CTL expansions, as observed in patients 5 and 6, consistent with reports that HTLV‐1 AC liver transplant recipients developed ATL under immunosuppression.25, 26 In patient 14, the Tax‐CTL expansion rate was drastically increased by depletion of CD4+ cells, most of which consisted of the ATL cells themselves. This suggests that Tax‐specific immune responses were suppressed by the tumor cells, consistent with our previous report that ATL cells from a subgroup of patients functioned as regulatory T (Treg) cells.27
Table 1.
Tax‐specific CTL expansion in adult T‐cell leukemia/lymphoma (ATL) patients
| Patient number | Clinical subtype | ATL status at blood sampling | Total cells (number) | Tax tetramer + cells/lymphocytes (%) | Tax tetramer + cells (number) | Expansion rate† | |||
|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | ||||
| Patient 1 | Acute | Complete remission | 4.5 × 106 | 9.5 × 106 | 0.01 | 4.51 | 4.5 × 102 | 4.28 × 105 | 951.1 |
| Patient 2 | Acute | Complete remission | 3.0 × 106 | 2.8 × 106 | <0.01 | 10.02 | <3.0 × 102 | 2.81 × 105 | 936.7 |
| Patient 3 | Chronic | Complete remission | 8.6 × 106 | 1.5 × 107 | 0.02 | 9.02 | 1.72 × 103 | 1.35 × 106 | 784.9 |
| Patient 4 | Lymphoma | Complete remission | 7.5 × 106 | 1.1 × 107 | 0.06 | 10.92 | 4.5 × 103 | 1.02 × 106 | 226.7 |
| Patient 5 | Acute | Complete remission | 3.0 × 106 | 1.0 × 107 | 0.03 | 0.15 | 9.0 × 102 | 1.50 × 104 | 16.7 |
| Patient 6 | Lymphoma | Complete remission | 4.3 × 106 | 3.5 × 106 | <0.01 | 0.62 | <4.3 × 102 | 2.17 × 104 | >50.5 |
| Patient 7 | Chronic | Watchful waiting | 2 × 107 | 1.0 × 108 | 1.32 | 12.50 | 2.64 × 105 | 1.25 × 107 | 47.3 |
| Patient 8 | Chronic | Watchful waiting | 6.5 × 106 | 9.2 × 106 | 0.01 | 7.05 | 6.5 × 102 | 6.49 × 105 | 998.5 |
| Patient 88′‡ | Acute | Before treatment | 5.26 × 106 | 5.5 × 106 | 0.02 | 0.02 | 1.05 × 104 | 1.10 × 104 | 1.05 |
| Patient 88″‡ | Acute | Partial remission | 3.5 × 106 | 6.8 × 106 | 0.06 | 26.36 | 2.1 × 103 | 1.79 × 106 | 852.4 |
| Patient 9 | Smoldering | Under systemic phototherapy for skin | 3.0 × 106 | 5.8 × 106 | 0.02 | 28.78 | 6.0 × 102 | 1.67 × 106 | 2783.3 |
| Patient 10 | Lymphoma | Initially diagnosed | 7.3 × 106 | 1.2 × 107 | <0.01 | 0.28 | <7.3 × 102 | 3.36 × 104 | >46.0 |
| Patient 11 | Acute | Initially diagnosed | 4.3 × 106 | 4.1 × 106 | <0.01 | 0.14 | <4.3 × 102 | 5.74 × 103 | >13.3 |
| Patient 12 | Acute | Initially diagnosed | 5.2 × 106 | ND | ND | ND | ND | ND | ND |
| Patient 13 | Acute | Initially diagnosed | 1.0 × 107 | ND | ND | ND | ND | ND | ND |
| Patient 14 | Acute | Diagnosed as relapse with acute type phenotype | 6.0 × 106 | 7.0 × 106 | 0.01 | 0.03 | 6.0 × 102 | 2.10 × 103 | 3.5 |
| Patient 14 (CD4‐subset)§ | 6.0 × 106 | 2.6 × 106 | 0.01 | 3.79 | 6.0 × 102 | 9.90 × 104 | 165.0 | ||
†Cell numbers of Tax tetramer + cells on day 14 was divided by that of day 0. ‡Patient 8 progressed from chronic to acute subtypes, and then he received allogeneic hematopoietic stem cell transplantation. §CD4+ cells were depleted on day 4. ATL, adult T‐cell leukemia/lymphoma; CTL, cytotoxic T lymphocytes; HTLV‐1, human T‐lymphotropic virus type‐1; ND, not detected.
Table 2.
Human leukocyte antigen (HLA) information
| HLA‐A | HLA‐B | HLA‐C | ||||
|---|---|---|---|---|---|---|
| TL‐Su | *11:01 | *24:02 | *15:01 | *40:02 | *03:04 | *04:01 |
| TCL‐Kan | *02:06 | *02:07 | *46:01 | *56:01 | *01:02 | *07:02 |
| K562 | ||||||
| HUT102 | *30:02 | *66:02 | ||||
| ATN‐1 | *11:01 | *24:02 | *54:01 | *67:01 | *01:02 | *07:02 |
| MT‐1 | *11:01 | *26:01 | *39:01 | *40:02 | *03:04 | *07:02 |
| MT‐2 | *24:02 | *24:02 | *40:02 | *51:01 | *03:03 | *14:02 |
| MT‐4 | *11:01 | *31:01 | *39:02 | *67:01 | *07:02 | *07:02 |
| TL‐Om1 | *02:01 | *02:01 | *52:01 | *52:01 | *12:02 | *12:02 |
| Patient 1 | *02:01 | *02:01 | *15:01 | *40:02 | *03:04 | *07:02 |
| Patient 3 | *02:01 | *31:01 | ||||
| Patient 4 | *24:02 | *26:01 | ||||
| Patient 5 | *02:06 | *24:02 | ||||
| Patient 6 | *02:06 | *31:01 | ||||
| Patient 7 | *02:07 | *24:02 | *46:01 | *52:01 | *01:02 | *12:02 |
| Patient 8 | *02:01 | *02:06 | *35:01 | *55:02 | *01:02 | *03:03 |
| Patient 9 | *02:01 | *31:01 | ||||
| Patient 10 | *11:01 | *24:02 | ||||
| Patient 11 | *11:01 | *24:02 | ||||
| Patient 12 | *02:06 | *24:02 | ||||
| Patient 13 | *02:03 | *31:01 | ||||
| Patient 14 | *24:02 | *31:01 | *07:02 | *40:01 | *03:04 | *07:02 |
Figure 1.

Expansion of Tax‐specific CTL from PBMC of patient 8 at different clinical stages. Flow cytometric analyses of the expanded cells are presented. The lymphocyte population was determined by FSC‐H and SSC‐H levels (left panels) and the data are plotted to show CD8 and human leukocyte antigen (HLA)‐A*02:01/Tax tetramer‐positivity (right two panels). Both CD8 and HLA‐A*02:01/Tax tetramer‐positive cells are gated, and their percentages relative to the entire lymphocyte population are indicated in each panel. Patient 8 progressed from chronic to acute stage disease. His Tax‐CTL could be efficiently expanded during the chronic phase (upper panels), but no longer after progression to acute stage (middle panels). Subsequently, he received allogeneic hematopoietic stem cell transplantation, and achieved partial remission. At this time, his Tax‐CTL could be efficiently expanded from PBMC once more (lower panels).
T‐cell receptor avidity of the expanded Tax‐specific CTL
Specific IFN‐γ production following stimulation with serial concentrations (0.01–100 pM) of Tax11–19 or Tax301–309 peptides was used as a readout to measure the T‐cell receptor (TCR) avidity of the expanded Tax‐CTL. Intracellular IFN‐γ was clearly detected specifically even at a peptide concentration of 0.01 pM in both HLA‐A*02:01‐restricted Tax‐CTL from patient 1 (Fig. 2A) and HLA‐A*24:02‐restricted Tax‐CTL from patient 7 (Fig. 2B). We also analyzed the TCR avidity of CMV‐pp65‐specific CTL expanded from the same patients. Specific IFN‐γ production by HLA‐A*02:01‐restricted pp65‐CTL was lower than Tax‐CTL at any peptide concentration. Furthermore, no specific IFN‐γ production by HLA‐A*24:02 pp65‐CTL could be detected at all at peptide concentrations of 0.01–1 pM. In general in the literature, peptide concentrations of other viral or tumor antigen epitopes that the corresponding specific CTL recognize and respond to are in the range 1 nM–10 μM, although this varies according to the antigen.28, 29, 30, 31, 32 Collectively, the results presented here indicate that the TCR avidities of these Tax‐CTL can be considered to be extremely high.
Figure 2.
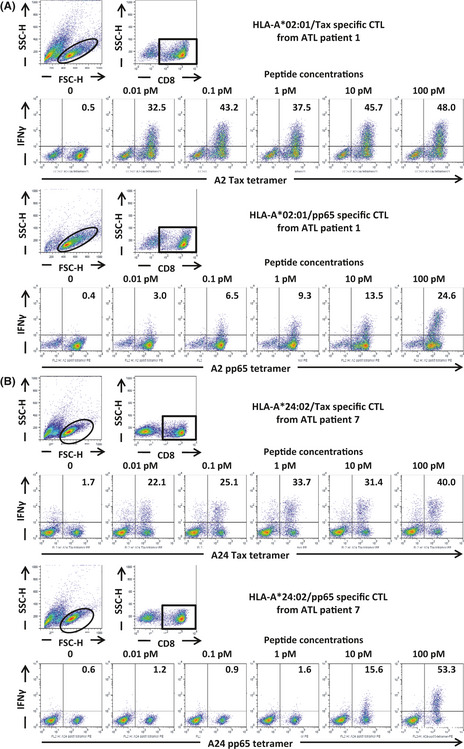
T‐cell receptor avidity of Tax‐CTL for Tax epitope peptides. (A) PBMC from adult T‐cell leukemia/lymphoma (ATL) patient 1 were stimulated by Tax11–19 peptide, and the expanded cells were then cultured with serial concentration of the cognate peptide. Flow cytometric analyses of those cells are presented. The lymphocyte population was identified by FSC‐H and SSC‐H levels, and CD8‐positive cells gated. These were then plotted according to human leukocyte antigen (HLA)‐A*02:01/Tax tetramer‐positivity and interferon gamma (IFN‐γ) production. The percentages of IFN‐γ‐producing cells relative to the entire population of HLA‐A*02:01/Tax‐positive cells are indicated in each panel (upper panels). PBMC from ATL patient 1 were also stimulated by CMV‐pp65 495–503 peptide, and then restimulated with the cognate peptide, and flow cytometric analyses of those cells are presented in the same manner as above. The percentages IFN‐γ‐producing cells relative to the entire population of HLA‐A*02:01/CMV‐pp65‐positive cells are indicated in each panel (lower panels) (B) PBMC from ATL patient 7 were stimulated with Tax301–309 peptide, and then restimulated as above: HLA‐A*24:02/Tax301–309 tetramer positivity and IFN‐γ production (upper panels). PBMC from ATL patient 7 stimulated with CMV‐pp65 495–503 peptide, and treated as above. Each result represents three independent experiments.
Expression of human T‐lymphotropic virus type 1 Tax in adult T‐cell leukemia/lymphoma cells
Given the high TCR avidity of Tax‐CTL, we next analyzed whether these CTL could recognize, respond to and kill ATL cells. To this end, Tax expression in ATL cell lines, HTLV‐1‐immortalized lines, K562 and short‐term cultured primary ATL cells was assessed (Fig. 3). Tax expression was detected both by flow cytometry and RT‐PCR in TL‐Su, TCL‐Kan, HUT102, MT‐2 and MT‐4, but not in K562, MT‐1 or TL‐Om1 by either technique. No Tax protein was seen in ATN‐1 or in short‐term cultured primary ATL cells from patients 7, 8 and 14, although Tax mRNA was present at levels 1/10–1/100th of those in TL‐Su.
Figure 3.

Expression of human T‐lymphotropic virus type 1 (HTLV‐1) Tax in adult T‐cell leukemia/lymphoma (ATL) cells. (A) Tax expression in ATL cell lines, HTLV‐1‐immortalized lines and K562 were analyzed by flow cytometry. The cells lines were stained with anti‐Tax mAb (blank histograms) or isotype control mAb (filled histograms). (B) Tax expression in short‐term cultured ATL cells from patients analyzed by flow cytometry. (C) Tax expression in the cell lines and short‐term cultured ATL cells from patients analyzed by quantitative RT‐PCR by dividing the Tax expression level by β‐actin, resulting in a Tax/β‐actin mRNA ratio with the expression level in TL‐Su set at unity. Columns, mean of triplicate experiments; bars, standard deviation.
Tax‐specific CTL responses against autologous adult T‐cell leukemia/lymphoma cells
PBMC from patient 7 were stimulated with HLA‐A*24:02 restricted Tax301–309 peptide, and the resulting CTL were expanded (Fig. 4A, upper‐left panel). In this culture, HLA‐A2‐restricted Tax11–19 specific CTL were also expanded (Fig. 4A, middle‐left panel), even though the Tax11–19 peptide was not used as a stimulator. We surmised that pre‐existing Tax‐CTL, including these HLA‐A2 Tax11–19 CTL, were stimulated by the ATL cells constitutively expressing HLA‐A2/Tax11–19 complexes, contained in the cultured PBMC. These expanded T‐cells were co‐cultured with ATL cell lines, HTLV‐1‐immortalized lines or autologous ATL cells, and their responses were evaluated by IFN‐γ production. HLA‐A*24:02/Tax301–309 tetramer‐positive fractions of these expanded CD8‐positive cells produced IFN‐γ when co‐cultured with autologous ATL cells or ATN‐1 (Fig. 4A), even though the Tax expression was so low as to be undetectable by flow cytometry, and only detectable by RT‐PCR (Fig. 3). These tetramer‐positive cells also responded to TL‐Su and MT‐2, but did not respond to the other ATL cell lines, or HTLV‐1‐immortalized lines tested. This indicates that only target cells having both HLA‐A*24:02 and Tax were recognized (Table 2 and Fig. 3). The HLA‐A*24:02/Tax301–309 tetramer‐negative fractions of these expanded CD8‐positive cells also produced IFN‐γ when stimulated with autologous ATL cells. This suggests that they recognize unidentified Tax‐derived epitopes, or antigens derived from HTLV‐1 components other than Tax, or ATL‐related tumor antigens not of viral origin. Finally, the HLA‐A*02:01/Tax11–19 tetramer‐positive fractions within these expanded CD8‐positive cells were also found to produce IFN‐γ on challenge with autologous ATL cells and TCL‐Kan, but not the other ATL cell lines or HTLV‐1‐immortalized lines. This indicates that HLA‐A2 and Tax expression were both required for recognition. HLA‐A*02:01/Tax11–19 tetramer‐negative cells also produced IFN‐γ when stimulated by TCL‐Kan. Because both patient 7 and TCL‐Kan share HLA‐B*46:01 and HLA‐C*01:02 (Table 2), the tetramer‐negative cells might be recognizing unidentified Tax‐derived epitopes, other HTLV‐1 antigens or ATL tumor antigens‐derived epitopes presented on a different shared MHC allele. These effector cells did not respond to K562 by IFN‐γ production, showing that they had no NK activity.
Figure 4.

Tax‐specific CTL responses against autologous adult T‐cell leukemia/lymphoma (ATL) cells. (A) PBMC from patient 7 were stimulated with human leukocyte antigen (HLA)‐A*24:02 restricted Tax301–309 peptide, and the resulting CTL were expanded (upper‐left panel). In this culture, HLA‐A2‐restricted Tax11–19 specific CTL were also expanded (middle‐left panel). The expanded cells were co‐cultured with autologous ATL cells, ATL cell lines, human T‐lymphotropic virus type 1 (HTLV‐1)‐immortalized lines and K562 (all CD8‐negative) for 4 h. CD8‐positive cells are plotted according to HLA‐A*24:02/Tax301–309 or HLA‐A*02:01/zax11–19 tetramer‐positivity and interferon gamma (IFN‐γ) production, and the percentages in each quadrant are presented in the panels. (B) PBMC from ATL patient 8 at chronic stage were stimulated by Tax11–19 peptide, and the expanded cells co‐cultured with the same range of cells as in (A). CD8‐positive cells are plotted by HLA‐A*0201/Tax11–19 tetramer positivity and IFN‐γ production. The HLA‐A*02:01/Tax11–19 tetramer recognized HLA‐A*02:07‐restricted Tax11–19 specific CTL. (C) PBMC from ATL patient 14 were stimulated with Tax301–309 peptide, and treated as in (A, B) above. Each result represents three independent experiments.
Next, PBMC from patient 8 at chronic stage were investigated in a similar manner, stimulated with Tax11–19 peptide (Fig. 4B, upper‐left panel). HLA‐A*02:01/Tax11–19 tetramer‐positive cells in these expanded CD8‐positive cells also produced IFN‐γ (Fig. 4B) when stimulated with Tax RT‐PCR‐positive but flow cytometry‐negative autologous ATL cells (Fig. 3). These tetramer‐positive cells responded to TCL‐Kan but not to the other ATL cell lines or HTLV‐1‐immortalized lines. Thus, their recognition was also restricted by the expression of HLA‐A2 and Tax (Table 2 and Fig. 3). HLA‐A*02:01/Tax11–19 tetramer‐negative fractions were also stimulated by autologous ATL cells, again suggesting recognition of unidentified epitopes. HLA‐A*02:01/Tax11–19 tetramer‐negative cells also produced IFN‐γ when stimulated by TCL‐Kan. Because patient 8 and TCL‐Kan are both HLA‐C*01:02‐positive (Table 2), these effector cells might be recognizing unidentified epitopes presented on this shared MHC allele. Again, there was no IFN‐γ production against K562.
We also repeated these experiments with PBMC from patient 14, and evaluated them in the same manner. In this case as well, the HLA‐A*24:02/Tax301–309 tetramer‐positive cells responded to autologous ATL cells and ATN‐1 (Fig. 4C), again despite the very low level of Tax expression. They also responded to TL‐Su, but not the other ATL cell lines or HTLV‐1‐immortalized lines, showing HLA‐A*24:02 and Tax restriction (Table 2 and Fig. 3). Once more, the HLA‐A*24:02/Tax301–309 tetramer‐negative cells were also stimulated by autologous ATL cells, indicating recognition of unidentified epitopes presented on autologous MHC molecules. HLA‐A*24:02/Tax301–309 tetramer‐negative cells also produced IFN‐γ when stimulated with TL‐Su, which shares HLA‐C*03:04 with patient 14 (Table 2). Again, no NK activity was detectable.
Lysis of autologous adult T‐cell leukemia/lymphoma cells by Tax‐specific CTL
Cells from patient 7 expanded by Tax301–309 peptide (Fig. 4A) killed TL‐Su, MT‐2, ATN‐1 and autologous ATL cells in an E/T ratio‐dependent manner, but did not lyse MT‐1 or HUT102 (Fig. 5, left panel). Lysis depended on the presence of both HLA‐A*24:02 and Tax (Table 2 and Fig. 3). Although as mentioned before, the level of Tax expression by these autologous ATL cells and ATN‐1 was so low as to be detectable only by RT‐PCR and not by flow cytometry, objective lysis of both cells was still observed. The patient 7 Tax‐CTL expanded by Tax301–309 peptide stimulation also killed TCL‐Kan. HLA‐A2‐restricted Tax11–19 CTL included in the effector subset presumably contributed to the lyses of TCL‐Kan as well as autologous tumor cells (Fig. 4A, middle‐left panel). Again, these expanded cells did not possess NK activity. The cells from patient 8 at chronic stage expanded by Tax11–19 peptide (Fig. 4B) killed TCL‐Kan and autologous ATL cells, but not TL‐Om1 (Fig. 5, middle panel) in an HLA‐A2‐restricted and Tax‐restricted manner (Table 2 and Fig. 3). Again, Tax expression by the autologous ATL cells was extremely low, but the targets were, nonetheless, killed. As with the other patients, there was no NK activity present in the expanded cells.
Figure 5.
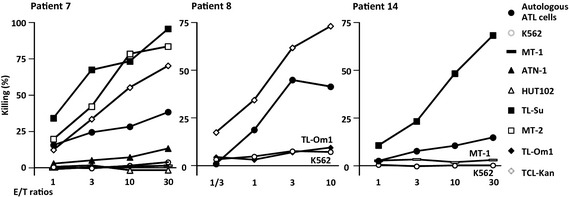
Lysis of autologous adult T‐cell leukemia/lymphoma (ATL) cells by Tax‐specific CTL. Tax301–309 peptide‐expanded cells from ATL patient 7 (left panel), Tax11–19 peptide‐expanded cells from patient 8 (middle panel) and Tax301–309‐stimulated patient 14 (right panel) were evaluated for cytotoxicity by a standard 4‐h chromium51 release assay. Lysis was restricted to human leukocyte antigen (HLA)‐A*24:02 or HLA‐A2 and Tax‐positive target cells. Each result represents three independent experiments.
Finally, cells from patient 14 stimulated by Tax301–309 peptide (Fig. 4C) killed TL‐Su and autologous ATL cells, but not MT‐1 (Fig. 5, right panel), restricted by HLA‐A*24:02 and Tax (Table 2 and Fig. 3), again with no NK activity.
Tax expression in primary adult T‐cell leukemia/lymphoma cells induced by short‐term culture
It was previously reported that although Tax expression was not detectable in primary ATL cells by flow cytometry in most cases, short‐term culture of such cells could induce Tax expression in nearly half of cases.33 Tax expression and its regulation in primary ATL cells is currently not fully understood. We tested Tax expression of primary ATL cells from patients 7, 8, 13 and 14, as listed in Table 1, and 2 additional patients, 15 and 16 (both chronic type). Tax protein was not present in any primary uncultured ATL cells isolated with anti‐human CD4 microbeads from patients' peripheral blood. In all cases, these cells were in a quiescent state, as determined by 7‐AAD staining (Fig. 6A). Cells incorporating BrdU (S phase) and those having double DNA content (G2/M phase) first appeared on culture of the primary ATL cells for several days, indicating that they had begun to cycle. At the same time, Tax‐expressing cells appeared in three of six cases (patients 7, 8 and 13) (Fig. 6B). These findings indicate that Tax expression was induced in primary ATL cells when they were actively cycling (i.e. cells not in G0 phase). Because most primary ATL cells in the peripheral blood are in a quiescent state (G0 phase), they express little or no Tax.
Figure 6.
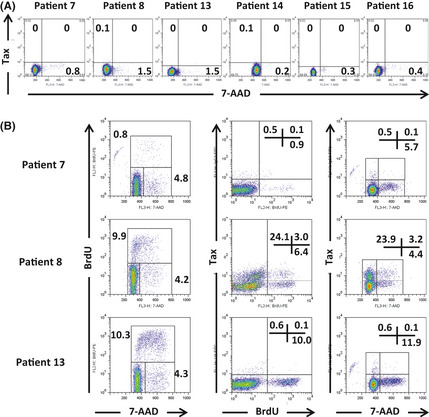
Tax expression in adult T‐cell leukemia/lymphoma (ATL) cells induced by short‐term culture. (A) Lack of Tax expression in primary ATL cells from peripheral blood of patients 7, 8, 13, 14, 15 and 16. Cells were in a quiescent state as determined by 7‐ADD staining. (B) Cell cycle status and Tax expression of short‐term cultured primary ATL cells. Tax expression was induced when cells were actively cycling. Each result represents three independent experiments.
Tax expression in primary adult T‐cell leukemia/lymphoma cell‐bearing NOG mice
NOG mice bearing primary ATL cells were established using ATL cells of patients 7, 12 and 13, as previously described.34 ATL mice from patient 7 presented with large intraperitoneal tumor masses, and tumor cells aggressively infiltrated into liver and spleen, but into the blood only to a lesser extent. Setting the Tax/human CD4 mRNA level of TL‐Su as unity, these values for blood cells, liver, spleen and tumor cell suspensions were 0.00195 ± 0.00065 (standard deviation), 0.023000 ± 0.00312, 0.00626 ± 0.00214 and 0.19533 ± 0.02185, respectively. Because there was little ATL cell infiltration into bone marrow, the Tax/human CD4 mRNA value of bone marrow cells was under the limit of detection (Fig. 7A). Tax expression in ATL cells from tumor masses was almost 100‐fold higher than in the blood.
Figure 7.

Tax expression in primary adult T‐cell leukemia/lymphoma (ATL) cell‐bearing NOG mice. Tax expression of ATL cells in each affected organ of NOG mice bearing primary ATL cells from patient 7 (A), 12 (B) and 13 (C) were evaluated. NOG mice with cells from patient 7 presented with large intraperitoneal tumor masses demarcated by the white dotted lines. Tax/human CD4 mRNA values of the cells from each organ are presented as bar graphs, where the value for TL‐Su was set at unity. Flow cytometric analysis of the cells from each organ determined by human CD45 and CD4 expression is presented. Columns, mean of triplicate experiments; bars, standard deviation. BM, bone marrow; U.L.D., under limit of detection.
ATL mice from patient 12 presented with marked hepatosplenomegaly, but few tumor cells in the blood. Tax/human CD4 mRNA values of blood cells, liver, and spleen cell suspensions were 0.01337 ± 0.00083, 0.05277 ± 0.00805 and 0.08323 ± 0.00080, respectively. Again, no Tax/human CD4 mRNA could be detected in bone marrow cells (Fig. 7B).
Adult T‐cell leukemia/lymphoma mice from patient 13 also presented with marked hepatosplenomegaly, but also with tumor infiltration into blood and bone marrow. Tax/human CD4 mRNA values of blood cells, liver, spleen cell suspensions and bone marrow cells were 0.01013 ± 0.00102, 0.12742 ± 0.01524, 0.15411 ± 0.01612 and 0.28881 ± 0.07319, respectively (Fig. 7C).
These observations are consistent with other results from the present study that Tax expression is observed predominantly in actively cycling ATL cells, whereas most primary ATL cells in the peripheral blood are in a quiescent state. Thus, only ATL cells present at the site of active cell proliferation, such as in the tumor masses, liver or spleen, strongly express Tax, but this factor is minimally expressed by the tumor cells in a quiescent state, such as in the blood.
Discussion
The significant findings in the present study are as follows. The efficiency of in vitro Tax‐CTL expansion was dependent on the stage of disease development following HTLV‐1 infection. HTLV‐1 Tax‐CTL expanded in vitro could recognize HLA/Tax‐peptide complexes on autologous ATL cells, the Tax expression of which was so low as to be detectable only by RT‐PCR and not by flow cytometry. Tax recognition resulted in the production of IFN‐γ and killing of the target cells. In an assay of TCR avidity, both HLA‐A*02:01‐restricted and HLA‐A*24:02‐restricted Tax‐CTL responded to as little as 0.01 pM of the epitope peptide, a concentration much lower than required for recognition of any other viral or tumor antigens. This documents the extremely high TCR avidity of Tax‐CTL, which is presumably one of the reasons why these CTL could recognize and kill the autologous ATL cells, despite their very low Tax expression. To the best of our knowledge, this is the first report of Tax‐specific CTL from ATL patients specifically recognizing and killing autologous tumor cells that express the Tax antigen. Earlier studies examined the responses of CD8 cells against autologous cells from ATL, HTLV‐1‐associated myelopathy/tropical spastic paraparesis patients or HTLV‐1 AC. However, in these reports, the nature of the antigens recognized by the CTL is not determined, or the target cells are HTLV‐1‐infected T‐cell lines rather than primary ATL tumor cells. In contrast, the present study clearly demonstrated that Tax antigen expressed by ATL cells was a significant target for CTL from ATL patients in an autologous setting. In addition, Tax expression was observed only in actively cycling ATL cells. This could only be noticed in primary ATL cells from patients, because established ATL cell lines or HTLV‐1‐immortalized lines, which are commonly used for many types of experiments, are, of course, continuously dividing. These findings collectively demonstrate that the main obstacle to successful immunotherapy targeting Tax, with its very limited expression in ATL cells, could be overcome. Whether primary ATL cells express Tax has been examined in several other studies using tumor cells from patients' blood.11, 12, 33 However, the present study demonstrated that most primary ATL cells in the blood are in a quiescent state, in which they express little or no Tax. Proliferating ATL cells are probably mostly to be found in lymph nodes in humans, not in the blood,35 and these should, therefore, express substantial levels of Tax. Thus, the present findings indicate that Tax is a promising molecular target for immunotherapy in ATL patients, such as adoptive T‐cell therapy and/or active vaccination.
As mentioned above, the efficiency of in vitro Tax‐CTL expansion depended on HTLV‐1 disease status. There was a trend towards superior expansion of Tax‐CTL in HTLV‐1 AC, ATL patients with the indolent variant and ATL patients who were in treatment‐induced remission compared with newly diagnosed ATL patients with the aggressive variant. These observations indicate that host immune responses against Tax play an important role in maintaining the stable status of HTLV‐1 AC, indolent ATL patients and ATL in remission. In addition, quantitative and/or functional reduction of Tax‐CTL should lead to progression from HTLV‐1 AC to ATL, or from indolent to aggressive ATL, or to relapse in ATL patients. Furthermore, the present observations suggest that restoration of substantial anti‐Tax responses in some appropriate manner will lead to improvement of ATL disease status.
The efficient expansion of Tax‐CTL from PBMC of ATL patients in remission suggests that reducing the number of tumor cells before Tax‐targeted immunotherapy could be a crucial factor for successful induction/augmentation of antigen‐specific CD8‐positive CTL. We have reported that the humanized anti‐CCR4 mAb KW‐0761 (mogamulizumab) exerted clinically significant antitumor activity in relapsed ATL patients.36, 37 In addition, consistent with the fact that CCR4 is expressed not only on Th2 cells, but also on Treg cells,21, 38, 39, 40 KW‐0761 treatment resulted in a significant and lasting decrease in CD4+CD25+FOXP3+ cells, including both the tumor cells and endogenous non‐ATL Treg cells.37 Reduction or suppression of Treg cells is expected to be a promising strategy for boosting antitumor immunity in cancer patients, as observed in studies with ipilimumab.41, 42 In fact, Tax‐CTL were efficiently expanded from PBMC of patients 2 and 3 who were in CR after KW‐0761 treatment. Thus, combining Tax‐targeted immunotherapy following reduction of ATL cells and endogenous Treg cell depletion by KW‐0761 treatment would be an ideal strategy for ATL immunotherapy.
The efficient expansion of Tax‐CTL from PBMC of patients in remission after allogeneic HSCT is consistent with the report that Tax‐specific CD8‐positive T cells contribute to graft‐versus‐ATL effects.13, 43 Therefore, Tax‐targeted immunotherapy after allogeneic HSCT should be therapeutically effective without increased graft‐versus‐host disease, which is a frequent and serious complication of this modality.
In conclusion, the present study not only provides a strong rationale for selecting Tax as a possible target for ATL immunotherapy but also contributes to our understanding of the immunopathogenesis driving progression from HTLV‐1 AC to ATL, and to devising strategies for preventing this by targeting Tax.
Disclosure Statement
Nagoya City University Graduate School of Medical Sciences has received research grant support from Kyowa Hakko Kirin for works provided by Takashi Ishida. No other conflict of interest relevant to this article is reported.
Acknowledgments
The present study was supported by Grants‐in‐Aid for Young Scientists (A) (No. 22689029, T. Ishida), Scientific Research (B) (No. 22300333, T Ishida and R. Ueda), and Scientific Support Programs for Cancer Research (No. 221S0001, T. Ishida) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grants‐in‐Aid for National Cancer Center Research and Development Fund (No. 21‐6‐3, T. Ishida), and Health and Labour Sciences Research Grants (H22‐Clinical Cancer Research‐general‐028, T. Ishida and H23‐Third Term Comprehensive Control Research for Cancer‐general‐011, T. Ishida, H Nishikawa and H. Inagaki) from the Ministry of Health, Labour and Welfare, Japan.
(Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02371.x, 2012)
References
- 1. Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T‐cell leukemia: clinical and hematologic features of 16 cases. Blood 1977; 50: 481–92. [PubMed] [Google Scholar]
- 2. Matsuoka M, Jeang KT. Human T‐cell leukaemia virus type 1 (HTLV‐1) infectivity and cellular transformation. Nat Rev Cancer 2007; 7: 270–80. [DOI] [PubMed] [Google Scholar]
- 3. Hino S, Sugiyama H, Doi H et al Breaking the cycle of HTLV‐I transmission via carrier mothers' milk. Lancet 1987; 2: 158–9. [DOI] [PubMed] [Google Scholar]
- 4. Ishida T, Ueda R. Immunopathogenesis of lymphoma: focus on CCR4. Cancer Sci 2011; 102: 44–50. [DOI] [PubMed] [Google Scholar]
- 5. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol 1991; 79: 428–37. [DOI] [PubMed] [Google Scholar]
- 6. Akagi T, Ono H, Shimotohno K. Characterization of T cells immortalized by Tax1 of human T‐cell leukemia virus type 1. Blood 1995; 86: 4243–9. [PubMed] [Google Scholar]
- 7. Kannagi M, Sugamura K, Kinoshita K, Uchino H, Hinuma Y. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia⁄lymphoma patient. J Immunol 1984; 133: 1037–41. [PubMed] [Google Scholar]
- 8. Arnulf B, Thorel M, Poirot Y et al Loss of the ex vivo but not the reinducible CD8+ T‐cell response to Tax in human T‐cell leukemia virus type 1‐infected patients with adult T‐cell leukemia⁄lymphoma. Leukemia 2004; 18: 126–32. [DOI] [PubMed] [Google Scholar]
- 9. Kannagi M, Sugamura K, Sato H, Okochi K, Uchino H, Hinuma Y. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus‐bearing cells. J Immunol 1983; 130: 2942–6. [PubMed] [Google Scholar]
- 10. Kannagi M, Harada S, Maruyama I et al Predominant recognition of human T cell leukemia virus type I (HTLV‐I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV‐I‐infected cells. Int Immunol 1991; 3: 761–7. [DOI] [PubMed] [Google Scholar]
- 11. Takeda S, Maeda M, Morikawa S et al Genetic and epigenetic inactivation of tax gene in adult T‐cell leukemia cells. Int J Cancer 2004; 109: 559–67. [DOI] [PubMed] [Google Scholar]
- 12. Kannagi M, Harashima N, Kurihara K et al Tumor immunity against adult T‐cell leukemia. Cancer Sci 2005; 96: 249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harashima N, Kurihara K, Utsunomiya A et al Graft‐versus‐Tax response in adult T‐cell leukemia patients after hematopoietic stem cell transplantation. Cancer Res 2004; 64: 391–9. [DOI] [PubMed] [Google Scholar]
- 14. Ishida T, Utsunomiya A, Iida S et al Clinical significance of CCR4 expression in adult T‐cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res 2003; 9: 3625–34. [PubMed] [Google Scholar]
- 15. Ri M, Iida S, Ishida T et al Bortezomib‐induced apoptosis in mature T‐cell lymphoma cells partially depends on upregulation of Noxa and functional repression of Mcl‐1. Cancer Sci 2009; 100: 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell line with positive Philadelphia chromosome. Blood 1975; 45: 321–34. [PubMed] [Google Scholar]
- 17. Lee B, Tanaka Y, Tozawa H. Monoclonal antibody defining tax protein of human T‐cell leukemia virus type‐I. Tohoku J Exp Med 1989; 157: 1–11. [DOI] [PubMed] [Google Scholar]
- 18. Ishida T, Iida S, Akatsuka Y et al The CC chemokine receptor 4 as a novel specific molecular target for immunotherapy in adult T‐Cell leukemia/lymphoma. Clin Cancer Res 2004; 10: 7529–39. [DOI] [PubMed] [Google Scholar]
- 19. Mori F, Ishida T, Ito A et al Potent antitumor effects of bevacizumab in a microenvironment‐dependent human lymphoma mouse model. Blood Cancer J 2012; 2: e67, doi: 10.1038/bcj.2012.12 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishii T, Ishida T, Utsunomiya A et al Defucosylated humanized anti‐CCR4 monoclonal antibody KW‐0761 as a novel immunotherapeutic agent for adult T‐cell leukemia/lymphoma. Clin Cancer Res 2010; 16: 1520–31. [DOI] [PubMed] [Google Scholar]
- 21. Ishida T, Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci 2006; 97: 1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kozako T, Arima N, Toji S et al Reduced frequency, diversity, and function of human T cell leukemia virus type 1‐specific CD8+ T cell in adult T cell leukemia patients. J Immunol 2006; 177: 5718–26. [DOI] [PubMed] [Google Scholar]
- 23. Kannagi M, Hasegawa A, Kinpara S et al Double control systems for human T‐cell leukemia virus type 1 by innate and acquired immunity. Cancer Sci 2011; 102: 670–6. [DOI] [PubMed] [Google Scholar]
- 24. Shimizu Y, Takamori A, Utsunomiya A et al Impaired Tax‐specific T‐cell responses with insufficient control of HTLV‐1 in a subgroup of individuals at asymptomatic and smoldering stages. Cancer Sci 2009; 100: 481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawano N, Shimoda K, Ishikawa F et al Adult T‐cell leukemia development from a human T‐cell leukemia virus type I carrier after a living‐donor liver transplantation. Transplantation 2006; 82: 840–3. [DOI] [PubMed] [Google Scholar]
- 26. Suzuki S, Uozumi K, Maeda M et al Adult T‐cell leukemia in a liver transplant recipient that did not progress after onset of graft rejection. Int J Hematol 2006; 83: 429–32. [DOI] [PubMed] [Google Scholar]
- 27. Yano H, Ishida T, Inagaki A et al Regulatory T‐cell function of adult T‐cell leukemia/lymphoma cells. Int J Cancer 2007; 120: 2052–7. [DOI] [PubMed] [Google Scholar]
- 28. Choi EM, Chen JL, Wooldridge L et al High avidity antigen‐specific CTL identified by CD8‐independent tetramer staining. J Immunol 2003; 171: 5116–23. [DOI] [PubMed] [Google Scholar]
- 29. Betts MR, Price DA, Brenchley JM et al The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol 2004; 172: 6407–17. [DOI] [PubMed] [Google Scholar]
- 30. Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down‐modulation by peptide: MHC molecule ligands: relationship to the quality of individual TCR signaling events. J Immunol 1999; 162: 2073–80. [PubMed] [Google Scholar]
- 31. Benlalam H, Linard B, Guilloux Y et al Identification of five new HLA‐B*3501‐restricted epitopes derived from common melanoma‐associated antigens, spontaneously recognized by tumor‐infiltrating lymphocytes. J Immunol 2003; 171: 6283–9. [DOI] [PubMed] [Google Scholar]
- 32. Yoshikawa T, Nakatsugawa M, Suzuki S et al HLA‐A2‐restricted glypican‐3 peptide‐specific CTL clones induced by peptide vaccine show high avidity and antigen‐specific killing activity against tumor cells. Cancer Sci 2011; 102: 918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kurihara K, Harashima N, Hanabuchi S et al Potential immunogenicity of adult T cell leukemia cells in vivo. Int J Cancer 2005; 114: 257–67. [DOI] [PubMed] [Google Scholar]
- 34. Ito A, Ishida T, Utsunomiya A et al Defucosylated anti‐CCR4 monoclonal antibody exerts potent ADCC against primary ATLL cells mediated by autologous human immune cells in NOD/Shi‐scid, IL‐2R gamma(null) mice in vivo. J Immunol 2009; 183: 4782–91. [DOI] [PubMed] [Google Scholar]
- 35. Umino A, Nakagawa M, Utsunomiya A et al Clonal evolution of adult T‐cell leukemia/lymphoma takes place in the lymph nodes. Blood 2011; 117: 5473–8. [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto K, Utsunomiya A, Tobinai K et al Phase I study of KW‐0761, a defucosylated humanized anti‐CCR4 antibody, in relapsed patients with adult T‐cell leukemia‐lymphoma and peripheral T‐cell lymphoma. J Clin Oncol 2010; 28: 1591–8. [DOI] [PubMed] [Google Scholar]
- 37. Ishida T, Joh T, Uike N et al Defucosylated anti‐CCR4 monoclonal antibody (KW‐0761) for relapsed adult T‐cell leukemia‐lymphoma: a multicenter phase ii study. J Clin Oncol 2012; 30: 837–42. [DOI] [PubMed] [Google Scholar]
- 38. Imai T, Nagira M, Takagi S et al Selective recruitment of CCR4‐bearing Th2 cells toward antigen‐presenting cells by the CC chemokines thymus and activation‐regulated chemokine and macrophage‐derived chemokine. Int Immunol 1999; 11: 81–8. [DOI] [PubMed] [Google Scholar]
- 39. Iellem A, Mariani M, Lang R et al Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med 2001; 194: 847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ishida T, Ishii T, Inagaki A et al Specific recruitment of CC chemokine receptor 4‐positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res 2006; 66: 5716–22. [DOI] [PubMed] [Google Scholar]
- 41. Hodi FS, O'Day SJ, McDermott DF et al Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robert C, Thomas L, Bondarenko I et al Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011; 364: 2517–26. [DOI] [PubMed] [Google Scholar]
- 43. Ishida T, Hishizawa M, Kato K et al Allogeneic hematopoietic stem cell transplantation for adult T‐cell leukemia‐lymphoma with special emphasis on preconditioning regimen: a nationwide retrospective study. Blood 2012; doi: 10.1182/blood-2012-03-414490 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


