Abstract
The transmembrane tyrosine kinase epidermal growth factor receptor (EGFR) is considered a key player in the development of cutaneous squamous cell carcinoma (SCC), which is the second most common malignancy in white populations. Inhibition of EGFR with the small molecule tyrosine kinase inhibitor erlotinib is currently under clinical investigation in cutaneous SCC patients. In this study, we investigated the effects of EGFR activation and inhibition on normal and malignant in vitro human skin equivalents (HSEs). In healthy HSEs, increasing EGF concentrations ranging from 5 to 50 ng/mL resulted in a dramatic decrease in epidermal proliferation as immunohistochemically assessed by Ki67 and increased epidermal stress as assessed by K17 after 2 weeks of air‐exposed culture. Also, higher concentrations of EGF induced remarkable epidermal disorganization with loss of proper stratification. Similar effects were observed in HSEs generated with cutaneous SCC cell lines SCC‐12B2 and SCC‐13. Treatment of both healthy and SCC‐HSEs with 10 μM erlotinib resulted in efficient reduction of epidermal thickness from 10 to 3 viable cell layers and counteracted EGF‐induced epidermal stress. Remarkably, erlotinib treatment caused severe desquamation in healthy HSEs, reminiscent of xerosis as a known side‐effect in patients treated with erlotinib. The presented three‐dimensional organotypic SCC models appear suitable for further investigations on the morphological and functional impacts of modifying EGFR signaling in cutaneous SCC, without burdening patients or mice. The effective inhibition of epidermal growth by erlotinib in our HSEs confirms the therapeutic potential of this tyrosine kinase inhibitor for cutaneous SCC patients.
Cutaneous squamous cell carcinoma (SCC) is one of the most common malignancies in Caucasian populations, causing substantial morbidity and mortality.1, 2 Cutaneous SCCs originate from epidermal keratinocytes and are histopathologically characterized by uncontrolled advancing and often disorganized sheets of malignant epidermal cells invading the dermis.3 Squamous cell carcinoma development is driven by a misbalance between proliferation and differentiation of epidermal keratinocytes.3, 4 As a crucial player in epithelial tissue homeostasis, the transmembrane tyrosine kinase epidermal growth factor receptor (EGFR, also known as ErbB1 and HER1) is of vital importance in SCC development. In healthy epithelial tissue, EGFR signaling is involved in proliferation, differentiation and migration of epithelial cells.5 Accordingly, overexpression and activation of EGFR is found in many epithelial cancers, including colorectal carcinoma, non small cell lung carcinoma and breast carcinoma.6 In many carcinomas, EGFR overexpression is associated with more aggressive disease, poor prognosis and difficulties in treatment.7 In cutaneous SCC, EGFR overexpression, numerical aberrations, genetic amplification and overactivation have been reported in comparison to normal skin.8, 9, 10 In metastatic SCCs of cutaneous origin, EGFR overexpression is common.11 Epidermal growth factor receptor has been shown to be activated by ligand binding or by ultraviolet radiation.12 Epidermal growth factor receptor ligands include epidermal growth factor (EGF), heparin‐binding EGF (HB‐EGF), amphiregulin (AREG), betacellulin (BTC), transforming growth factor‐α (TGF‐α) and epiregulin.13 In cutaneous SCCs and surrounding stroma, increased mRNA levels were shown for AREG, HB‐EGF and TGF‐α compared to adjacent normal skin.9 Activation of EGFR results in activation of a complex network of signaling pathways, including the phosphoinositide 3 kinase (PI3K) pathway affecting the downstream Akt kinase.5
As a tyrosine kinase receptor, EGFR is a known drug target in epithelial cancers. Various small molecule EGFR inhibitors are approved for treatment of several epithelial cancers with EGFR overexpression, including colorectal and non small cell lung carcinoma.7 Treatment of cancer patients with EGFR inhibitors is often associated with discomforting skin toxicity.14 The small molecule tyrosine kinase inhibitor erlotinib has been shown to induce partial regression of cutaneous SCCs and its precursor lesion actinic keratosis.15, 16 This EGFR inhibitor is currently under clinical investigation for treatment of recurrent, late‐stage and metastatic cutaneous SCCs (www.clinicaltrials.gov: NCT00369512, NCT01198028, NCT01059305).
In the present study, we aimed to assess the effects of EGFR overactivation and EGFR inhibition on normal and transformed human skin. So far, modulation of EGFR activity in human skin cells in vitro is limited to two‐dimensional monolayer cell cultures. Here we investigated for the first time the effects of EGFR activation and inhibition on normal and malignant three‐dimensional human skin in vitro. To this end, we treated human skin equivalents (HSEs) with EGF and erlotinib. These HSEs allow for investigation of the role of EGFR in a relevant cutaneous microenvironment, as they recapitulate most of the in vivo characteristics of human skin, including a functional basement membrane and a living fibroblast‐seeded dermis harboring extracellular matrix components.3, 17 These models are therefore an excellent tool to study skin homeostasis, dermal‐epidermal interactions or to mimic and study skin diseases.18, 19, 20 In this study, we examined the effects of EGFR stimulation and inhibition in HSEs generated with both healthy and SCC keratinocytes.
Materials and Methods
Primary cells and cell lines
Healthy mamma reduction surplus skin of Caucasian women aged 37–41 years was obtained with written informed consent of the donors according to the Dutch law on medical treatment agreement. From this material, primary normal human epidermal keratinocytes (NHEKs) and primary normal human dermal fibroblasts (NHDFs) were isolated as described earlier.18 Two SCC cell lines representing the tumorigenic populations of cutaneous SCCs (SCC‐12B2 and SCC‐13) were kindly provided by Dr J.G. Rheinwald.21 These cell lines have been authenticated by short tandem repeat (STR) analysis no longer than 6 months prior to the experiments described in this work. Keratinocyte culture medium consisted of three parts of DMEM and one part of Ham's F12 (Invitrogen, Breda, the Netherlands) supplemented with 5% FBS (HyClone/Greiner, Nurtingen, Germany), 100 U/mL penicillin, 100 μg/mL streptomycin (Invitrogen), 1 μM hydrocortisone, 1 μM isoproterenol and 0.1 μM insulin (Sigma‐Aldrich, Zwijndrecht, the Netherlands). Normal human epidermal keratinocytes were used for experiments in their first passage and cultured at 37°C and 7.3% CO2. Fibroblast culture medium consisted of DMEM supplemented with 5% FBS and 10 mg/mL penicillin/streptamycin. Normal dermal fibroblasts were used for experiments in their second or third passage and cultured at 37°C and 5% CO2. For both NHEKs and NHDFs, culture medium was refreshed twice a week.
Human skin equivalents
Human skin equivalents were generated as described earlier.17 Briefly, dermal equivalents were generated by seeding rat‐tail collagen (4 mg/mL, isolated in‐house) with 1.25 x 105 fibroblasts/mL, which were incubated for 1 week under submerged conditions in fibroblast medium at 37°C and 5% CO2. Subsequently, dermal equivalents were seeded with 5 x 104 keratinocytes/cm2 (either NHEKs, SCC‐12B2 or SCC‐13). Human skin equivalents were incubated overnight under submerged conditions at 37°C and 7.3% CO2 in standard keratinocyte medium as described above. After 3 days, FBS was reduced to 1%. Two days thereafter, FBS was omitted and HSEs were cultured at the air‐liquid interface for 14 days while medium was refreshed every 3 days. Culture medium used during the air‐exposed period was keratinocyte medium as described above, but without serum and supplemented with 2 M l‐serine, 10 mM l‐carnitine, 1 μM DL‐α‐tocopherol‐acetate, 50 μM ascorbic acid, 2.4 × 10−5 M BSA and a free fatty acid supplement which contained 25 μM palmitic acid, 30 μM linoleic acid and 7 μM arachidonic acid (Sigma‐Aldrich). Culture medium was refreshed twice a week.
Epidermal growth factor treatment
Healthy HSEs based on NHEKs and HSEs with SCC‐12B2 or SCC‐13 were cultured in the presence of either 5 ng/mL, 20 ng/mL or 50 ng/mL EGF (Roche, Basel, Switzerland) during their entire air‐exposed culture period of 14 days (chronic exposure). After termination, all HSEs were processed for immunohistochemistry.
Erlotinib treatment
Erlotinib (Roche) was dissolved in DMSO to a 10 mM stock solution and kept at room temperature to prevent precipitation. Based on literature and suppliers' instructions for epithelial cells, erlotinib was further diluted in the culture medium to a final concentration of 10 μM.22, 23 Healthy HSEs based on NHEKs were cultured in the presence of 10 μM erlotinib either during their entire 14‐day air‐exposed culture period (chronic exposure), or only during the last 4 days of this period (from 10 to 14 days air‐exposed). Another set of healthy HSE cultures was supplemented with 5 ng/mL EGF during their entire air‐exposed culture period of 14 days and co‐supplemented with 10 μM erlotinib either during their entire air‐exposed culture period or only during the last 4 days of this period (from 10 to 14 days air‐exposed). Furthermore, HSEs based on SCC‐12B2 or SCC‐13 keratinocytes were cultured in the presence of 10 μM erlotinib during their entire 14‐day air‐exposed culture period. Afterwards, HSEs were terminated and processed for immunohistochemistry.
Immunohistochemistry
Human skin equivalents processed for immunohistochemistry were cut into two fragments. One part was snap‐frozen in liquid nitrogen while the other part was fixed in 4% paraformaldehyde, dehydrated and paraffin embedded. Global histological analysis was performed on 5‐μm sections through staining with H&E. Immunohistochemical analysis of involucrin (1:200, clone SY5, Santa Cruz Biotechnology, Santa Cruz, CA, USA), keratin 10 (1:50, DEK10, Labvision/Neomarkers, Fremont CA, USA), keratin 16 (1:20, clone LL025, AbD Serotec, Düsseldorf, Germany), keratin 17 (1:400, clone CKE3, Sigma, St. Louis, MO, USA) and Ki67 (1:100, clone MIB1, DAKO, Glostrup, Germany) was performed on 5 μm sections that were cut, dried o/n at 60°C, deparaffinized and rehydrated. For antigen retrieval, sections were boiled in citrate buffer (pH 6.0) in an autoclave at 110°C for 5 min. Endogenous peroxidase activity was blocked with methanol and H2O2. Sections were blocked with 5% normal goat serum in 1% BSA in PBS for 10 min at room temperature prior to incubation with the primary antibody overnight at 4°C. Next, sections were incubated with biotinylated goat anti mouse secondary antibody for 1 h at room temperature. Sections were labeled with streptavidin‐biotin in 1% BSA in PBS for 45 min at room temperature. Deaminobenzidinetetrahydrochloride was used as a chromogen. All sections were counterstained with haematoxylin.
Proliferation index
The proliferation index of HSEs was determined by the number of immunohistochemically stained Ki67 positive nuclei from a total number of 100 epidermal cells (×100%) in three different sections of each sample at a magnification of × 200. The resulting data are expressed as the mean of the three independent counts ± SD (error bars). This is a straightforward and generally accepted scoring method for proliferation determination in sections.24
Results
Epidermal growth factor results in decreased proliferation and affects differentiation
To assess the effect of EGF on three‐dimensional skin models, we exposed healthy HSEs to EGF throughout their entire air‐exposed culture period. Persistent exposure of healthy HSEs to 5 ng/mL, 20 ng/mL or 50 ng/mL EGF strongly influenced epidermal morphology, proliferation, differentiation and activation, as determined by immunohistochemical analyses. Exposure of healthy HSEs to increasing concentrations of EGF for 2 weeks resulted in substantial epidermal disorganization starting at 5 ng/mL EGF and persisting with 20 ng/mL and 50 ng/mL EGF (Fig. 1a). The proliferation rate of epidermal keratinocytes after 2 weeks of culturing slightly increased from 0.27 (±0.01) to 0.33 (±0.07, P = 0.01) upon chronic exposure to 5 ng/mL EGF, but dramatically decreased to 0.05 (±0.03, P< 0.05) at 20 ng/mL EGF, as quantified by immunohistochemical Ki67 staining (Fig. 1b). Virtually no proliferation was observed at 50 ng/mL EGF (Fig. 1b). Treatment of HSEs with increasing concentrations of EGF resulted in a disturbed early differentiation pattern, as demonstrated by immunohistochemical staining of the early differentiation marker keratin 10 (K10). This protein is normally expressed in all suprabasal layers of the epidermis, while in EGF‐treated HSEs it was found throughout the epidermis, along with the morphological disorganization at 20 ng/mL and 50 ng/mL (Fig. 1a). Terminal differentiation also appeared to be slightly disturbed, as demonstrated by immunohistochemical staining of the late differentiation marker involucrin. Involucrin staining was present in the granular layers in control HSEs and slightly expanded to the lower suprabasal layers in HSEs stimulated with EGF (Fig. 1a).
Figure 1.
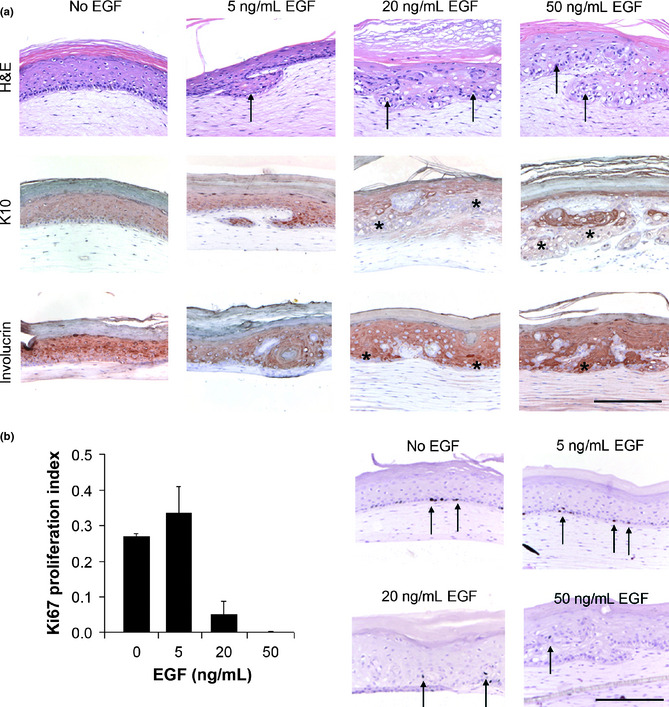
Epidermal growth factor (EGF) affects epidermal morphology, proliferation and differentiation in human skin equivalents (HSEs). (a) Shown are cross sections of HSEs chronically supplemented with increasing concentrations of EGF. Epidermal growth factor‐treated healthy HSEs stained with hematoxylin & eosin (H&E) showed global epidermal disorganization increasing with EGF‐concentration. Early differentiation of HSEs was disturbed upon exposure to increasing EGF concentrations, as reflected by a disorganized staining pattern of K10 at higher EGF concentrations. Terminal differentiation, as reflected by involucrin staining, slightly expanded over multiple cell layers in HSEs exposed to increasing concentrations of EGF. Arrows and asterisks indicate examples of significant staining patterns. Scale bar holds for all panels and indicates 200 μm. (b) Proliferation of HSEs first increased and then significantly decreased upon chronic exposure to increasing concentrations of EGF, as quantified after Ki67 staining. Representative Ki67 stainings are shown for all conditions. Arrows indicate examples of significantly stained nuclei.
Epidermal growth factor induces epidermal stress in healthy human skin models
Epidermal stress increased upon EGF stimulation, as determined by immunohistochemical staining of stress associated proteins keratin 16 (K16) and keratin 17 (K17). In non‐treated HSEs, K16 staining is predominantly observed in the stratum granulosum. Treatment with increasing concentrations of EGF resulted in intensified staining, expanding to the granular epidermal layers (Fig. 2). Staining of K17 first appears at EGF levels of 5 ng/mL EGF and dramatically increases with increasing EGF concentrations (Fig. 2).
Figure 2.
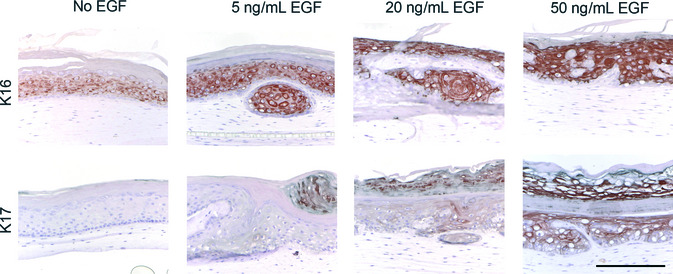
Epidermal growth factor (EGF) increases epidermal stress in human skin equivalents (HSEs). Shown are cross sections of HSEs chronically supplemented with increasing concentrations of EGF. Epidermal activation and stress in HSEs increased upon chronic exposure to increasing concentrations of EGF, as indicated by immunohistochemical staining of epidermal stress markers keratin 16 (K16) and keratin 17 (K17). Scale bar holds for all panels and indicates 200 μm.
Erlotinib reduces epidermal thickness in healthy human skin models
Upon chronic supplementation with the EGFR‐inhibitor erlotinib during the entire air‐exposed culture period, the epidermal thickness of healthy HSEs was reduced from 10 to 3 viable cell layers, while lacking a clear stratum corneum (Fig. 3a,b). To mimic the native situation, in which erlotinib is used on fully developed human skin, healthy HSEs were treated with 10 μM erlotinib during the last 4 days of their air‐exposed culture period (from 10 to 14 days air‐exposed). These cultures maintained their epidermal thickness of approximately 10 viable cell layers (Fig. 3c). Also in these cultures, the stratum corneum was absent. When the HSEs were simultaneously supplemented with 5 ng/mL EGF and 10 μM erlotinib during the last 4 days of air‐exposed culture (from 10 to 14 days air‐exposed), they showed a slightly reduced epidermal thickness of approximately 6 viable cell layers (Fig. 3d). Again, the thickness of the stratum corneum was reduced. Pre‐treatment of HSEs with 5 ng/mL EGF during the entire air‐exposed period, combined with erlotinib treatment during the last 4 days resulted in a normal epidermis of 10 viable cell layers, topped by a thick stratum corneum (Fig. 3e). Proliferation of basal keratinocytes in the epidermis of HSEs significantly decreased upon chronic erlotinib treatment, as assessed by Ki67 staining (Fig. 4a). Differentiation of HSEs was unaffected by erlotinib treatment, as indicated by K10 and involucrin (Fig. 4b). Erlotinib treatment did not result in epidermal stress, as reflected by the absence of K17 in erlotinib‐treated HSEs (Fig. 4b).
Figure 3.
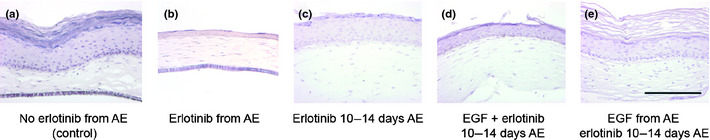
Erlotinib reduces epidermal thickness and integrity. Shown are hematoxylin & eosin (H&E) stained cross sections of healthy human skin equivalents (HSEs) treated with erlotinib during their air‐exposed (AE) culture phase, either with or without 5 ng/mL epidermal growth factor (EGF). Treatment of healthy HSEs with erlotinib resulted in a lower number of viable cell layers and an absence of stratum corneum (b–d) when compared to untreated control HSEs (a). This effect was most pronounced upon chronic supplementation of erlotinib (b) and less pronounced when co‐treated with EGF (d,e). When treated with erlotinib only in the final stage of the HSE culture (10–14 days AE), epidermal thickness was unaffected (c–e). Chronic supplementation with EGF, combined with erlotinib treatment in the final culture (10–14 days AE) phase affected neither the thickness of the epidermis nor that of the stratum corneum of the HSEs (e). Scale bar holds for all panels and indicates 200 μm.
Figure 4.
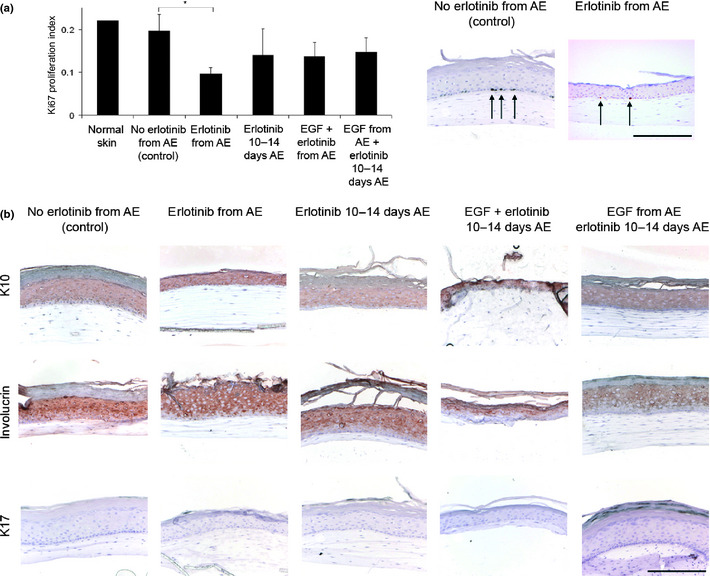
Erlotinib affects epidermal proliferation, differentiation and activation in healthy human skin equivalents (HSEs). (a) Proliferation of HSEs significantly decreased after chronic erlotinib treatment during the entire air‐exposed (AE) culture phase compared to normal skin and untreated HSEs, as quantified after Ki67 staining. Erlotinib treatment only during the last 4 days of AE culture (10–14 days AE) or simultaneous treatment with epidermal growth factor (EGF) did not significantly affect proliferation. Representative Ki67 stainings are shown for the significantly different conditions. Arrows indicate examples of significantly stained nuclei. (b) Shown are cross sections of HSEs supplemented with erlotinib, either with or without EGF. Erlotinib treatment did not affect early and terminal differentiation of HSEs, as reflected by K10 and involucrin staining, respectively. Erlotinib treatment did not result in epidermal stress, as K17 staining remained negative. Scale bar holds for all panels and indicates 200 μm.
Erlotinib efficiently reduces epidermal growth of malignant SCC cell line HSEs
When cultured in a HSE, cell line SCC‐12B2 formed a thick, strongly disorganized epidermis with reduced dermis caused by its invasive potential (Fig. 5). Upon chronic supplementation with 5 ng/mL EGF, the number of viable cell layers slightly increased while its disorganization was maintained. Higher concentrations of EGF did not result in any further morphological changes (data not shown). Chronic erlotinib supplementation of HSEs with SCC‐12B2 cells resulted in a very thin epidermis. Human skin equivalents generated with cell line SCC‐13 presented with a poorly attached but thick epidermis that is slightly disorganized, but still has a basal layer. Chronic EGF supplementation resulted in strong epidermal disorganization and reduced thickness. Upon erlotinib supplementation, HSEs generated with SCC‐13 cells showed a strong reduction in the number of viable epidermal cell layers. Furthermore, a fraction of SCC‐13 cells detached from the epidermis upon erlotinib treatment, resulting in epidermal scaling (Fig. 5).
Figure 5.
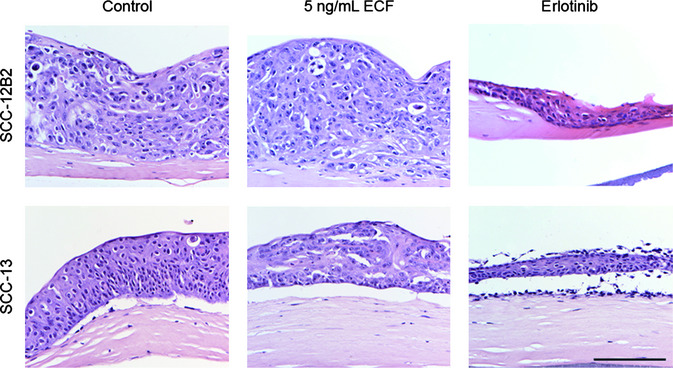
Erlotinib affects epidermal thickness in human skin equivalents (HSEs) generated with squamous cell carcinoma (SCC) cell lines SCC‐12B2 and SCC‐13. Shown are hematoxylin & eosin (H&E) stained cross sections of HSEs generated with cutaneous SCC cell lines SCC‐12B2 and SCC‐13 in the presence of epidermal growth factor (EGF) or erlotinib. SCC‐12B2 incorporated in HSEs resulted in a thick and disorganized epidermis. Human skin equivalents with SCC‐13 showed slight epidermal disorganization and poor dermal‐epidermal attachment. Upon EGF supplementation, epidermis generated with SCC‐12B2 thickened, whereas epidermis generated with SCC‐13 increased in disorganization. Erlotinib treatment resulted in strongly reduced epidermal thickness, leaving a thin disorganized epidermal SCC‐12B2 cell layer and a thin and detached but scaly SCC‐13 cell layer on top of the dermal equivalent. Scale bar holds for all panels and indicates 200 μm.
Discussion
To investigate the effects of EGFR activation and inhibition on normal and malignant human skin, we generated and exposed three‐dimensional in vitro human skin models with either NHEKs or SCC cell line keratinocytes to EGF and erlotinib. At a chronic but low concentration of 5 ng/mL, EGF treatment slightly increased epidermal thickness and proliferation in both healthy and malignant HSEs. In contrast, higher chronic EGF concentrations starting at 20 ng/mL resulted in drastically reduced epidermal proliferation in healthy HSEs without significantly affecting epidermal thickness. This is in concordance with earlier findings that EGF causes a dose‐dependent decrease in cell proliferation in SCC cell lines when cultured in a monolayer.25 Moreover, EGF‐induced EGFR activation resulted in severe epidermal disorganization and invasion, increasing with higher concentrations of EGF. This effect was not limited to healthy HSEs, as increased disorganization was also observed in HSEs generated with SCC‐13. In the literature, similar results were reported with immortalized primary esophageal cells overexpressing EGFR, which increased invasion in a three‐dimensional organotypic culture model and formed SCC‐like tumors in vivo.26 This indicates that EGFR may function as a switch between epidermal proliferation and differentiation in vitro, which is emphasized by the disturbance of both early and terminal differentiation patterns in healthy HSEs upon increasing EGF concentrations. Our findings in HSEs parallel those found in monolayer cultures in which mammalian keratinocytes were maintained in a disturbed differentiation state upon EGF stimulation.27 Furthermore, epidermal stress increased in healthy HSEs upon stimulation with higher concentrations of EGF.28 As these effects coincide with a decrease in epidermal proliferation, it might be interesting to investigate whether or not part of the epidermal cell population resides in a senescent state.
The small molecule tyrosine kinase inhibitor erlotinib is approved for treatment of several epithelial cancers and currently investigated for treatment of cutaneous SCC.7, 15 As a proof of principle, we evaluated whether our malignant SCC‐HSEs can serve as relevant in vitro readout systems to monitor effects of this small molecule tyrosine kinase inhibitor. Inhibition of EGFR by erlotinib resulted in a dramatic and significant reduction of epidermal thickness and proliferation in both healthy and SCC cell line generated HSEs compared to the unexposed controls. This was not observed upon short (4 days) exposure of established, healthy HSEs to erlotinib. So, HSEs treated from their early AE phase still need to form their final epidermal layers, which is efficiently blocked by erlotinib treatment. In established cultures, erlotinib treatment only results in breakdown of the stratum corneum. Longer treatment of these established cultures with erlotinib (beyond 14 days AE) may affect the other cell layers as well. Furthermore, in HSEs supplemented with erlotinib the epidermal stress marker K17 was absent irrespective of prior EGF stimulation, in contrast to HSEs exposed to EGF alone. The fact that erlotinib efficiently inhibited the growth of two distinct malignant cutaneous SCC cell lines in our HSEs confirms the therapeutic potential of this drug for cutaneous SCC patients.29 Remarkably, chronic supplementation of erlotinib caused severe desquamation in healthy HSEs. This was not observed upon short (4 days) exposure of HSEs to erlotinib. Desquamation also occurred in the outer cell layers of erlotinib‐treated SCC‐13 HSEs, but not in SCC‐12B2 HSEs. This may be explained by the fact that SCC‐12B2 represents poorly differentiated cells and SCC‐12B2 HSEs completely lack any stratification or terminal differentiation, whereas SCC‐13 HSEs show some stratification. In epithelial cancer patients treated with erlotinib or other EGFR inhibitors (e.g. gefitinib, lapatinib, cetuximab and panitumumab), a wide range of dermatological side‐effects is observed, including severely dehydrated, fragile skin caused by abnormal keratinocyte differentiation, resulting in significant reduction of the thickness of the stratum corneum.14, 30, 31 The loss of terminally differentiated cell layers upon erlotinib treatment in our HSEs appears to be an in vitro emulation of xerosis in patients. Future research may be directed at investigating the respective contribution of fibroblasts and keratinocytes to the eventual HSE phenotypes resulting from treatment with EGF and erlotinib. Altogether, these results prove our three‐dimensional healthy and malignant HSEs to be relevant and valid in vitro systems to study the effects of erlotinib and other anti skin cancer drugs.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was funded by a grant of the Netherlands Organization for Health Research and Development (ZonMw project number 11‐400‐0079). We hereby thank Professor Dr R. Willemze and Dr C.P. Tensen from the Department of Dermatology, Leiden University Medical Center, for critically reviewing the manuscript. Furthermore, we hereby acknowledge F. Hoffmann‐La Roche, Basel, Switzerland for providing the erlotinib hydrochloride salt.
(Cancer Sci 2012; 103: 2120–2126)
References
- 1. Erb P, Ji J, Wernli M, Kump E, Glaser A, Buchner SA. Role of apoptosis in basal cell and squamous cell carcinoma formation. Immunol Lett 2005; 100: 68–72. [DOI] [PubMed] [Google Scholar]
- 2. Williams H, Svensson A, Diepgen T et al Epidemiology of skin diseases in Europe. Eur J Dermatol 2006; 16: 212–8. [PubMed] [Google Scholar]
- 3. Kane CL, Keehn CA, Smithberger E, Glass LF. Histopathology of cutaneous squamous cell carcinoma and its variants. Semin Cutan Med Surg 2004; 23: 54–61. [DOI] [PubMed] [Google Scholar]
- 4. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401. [DOI] [PubMed] [Google Scholar]
- 5. Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res 2003; 284: 31–53. [DOI] [PubMed] [Google Scholar]
- 6. Vlahovic G, Crawford J. Activation of tyrosine kinases in cancer. Oncologist 2003; 8: 531–8. [DOI] [PubMed] [Google Scholar]
- 7. Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 2003; 21: 2787–99. [DOI] [PubMed] [Google Scholar]
- 8. Fogarty GB, Conus NM, Chu J, McArthur G. Characterization of the expression and activation of the epidermal growth factor receptor in squamous cell carcinoma of the skin. Br J Dermatol 2007; 156: 92–8. [DOI] [PubMed] [Google Scholar]
- 9. Rittie L, Kansra S, Stoll SW et al Differential ErbB1 signaling in squamous cell versus basal cell carcinoma of the skin. Am J Pathol 2007; 170: 2089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toll A, Salgado R, Yebenes M et al Epidermal growth factor receptor gene numerical aberrations are frequent events in actinic keratoses and invasive cutaneous squamous cell carcinomas. Exp Dermatol 2010; 19: 151–3. [DOI] [PubMed] [Google Scholar]
- 11. Shimizu T, Izumi H, Oga A et al Epidermal growth factor receptor overexpression and genetic aberrations in metastatic squamous‐cell carcinoma of the skin. Dermatology 2001; 202: 203–6. [DOI] [PubMed] [Google Scholar]
- 12. El Abaseri TB, Hansen LA. EGFR activation and ultraviolet light‐induced skin carcinogenesis. J Biomed Biotechnol 2007; 2007: 97939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Citri A, Yarden Y. EGF‐ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006; 7: 505–16. [DOI] [PubMed] [Google Scholar]
- 14. Li T, Perez‐Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol 2009; 4: 107–19. [DOI] [PubMed] [Google Scholar]
- 15. Amini S, Viera MH, Valins W, Berman B. Nonsurgical innovations in the treatment of nonmelanoma skin cancer. J Clin Aesthet Dermatol 2010; 3: 20–34. [PMC free article] [PubMed] [Google Scholar]
- 16. Hermanns JF, Pierard GE, Quatresooz P. Erlotinib‐responsive actinic keratoses. Oncol Rep 2007; 18: 581–4. [PubMed] [Google Scholar]
- 17. el‐Ghalbzouri A, Gibbs S, Lamme E, Van Blitterswijk CA, Ponec M. Effect of fibroblasts on epidermal regeneration. Br J Dermatol 2002; 147: 230–43. [DOI] [PubMed] [Google Scholar]
- 18. Commandeur S, de Gruijl FR, Willemze R, Tensen CP, El Ghalbzouri A. An in vitro three‐dimensional model of primary human cutaneous squamous cell carcinoma. Exp Dermatol 2009; 18: 849–56. [DOI] [PubMed] [Google Scholar]
- 19. El Ghalbzouri A, Hensbergen P, Gibbs S, Kempenaar J, van der Schors R, Ponec M. Fibroblasts facilitate re‐epithelialization in wounded human skin equivalents. Lab Invest 2004; 84: 102–12. [DOI] [PubMed] [Google Scholar]
- 20. El Ghalbzouri A, Jonkman M, Kempenaar J, Ponec M. Recessive epidermolysis bullosa simplex phenotype reproduced in vitro: ablation of keratin 14 is partially compensated by keratin 17. Am J Pathol 2003; 163: 1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rheinwald JG, Beckett MA. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultures from human squamous cell carcinomas. Cancer Res 1981; 41: 1657–63. [PubMed] [Google Scholar]
- 22. Birle DC, Hedley DW. Signaling interactions of rapamycin combined with erlotinib in cervical carcinoma xenografts. Mol Cancer Ther 2006; 5: 2494–502. [DOI] [PubMed] [Google Scholar]
- 23. Yamasaki F, Zhang D, Bartholomeusz C et al Sensitivity of breast cancer cells to erlotinib depends on cyclin‐dependent kinase 2 activity. Mol Cancer Ther 2007; 6: 2168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jonat W, Arnold N. Is the Ki‐67 labelling index ready for clinical use? Ann Oncol 2011; 22: 500–2. [DOI] [PubMed] [Google Scholar]
- 25. Ponec M, Weerheim A, Kempenaar J, Boonstra J. Proliferation and differentiation of human squamous carcinoma cell lines and normal keratinocytes: effects of epidermal growth factor, retinoids, and hydrocortisone in vitro . Cell Dev Biol 1988; 24: 764–70. [DOI] [PubMed] [Google Scholar]
- 26. Okawa T, Michaylira CZ, Kalabis J et al The functional interplay between EGFR overexpression, hTERT activation, and p53 mutation in esophageal epithelial cells with activation of stromal fibroblasts induces tumor development, invasion, and differentiation. Genes Dev 2007; 21: 2788–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature 1977; 265: 421–4. [DOI] [PubMed] [Google Scholar]
- 28. El Ghalbzouri A, Lamme E, Ponec M. Crucial role of fibroblasts in regulating epidermal morphogenesis. Cell Tissue Res 2002; 310: 189–99. [DOI] [PubMed] [Google Scholar]
- 29. Uribe P, Gonzalez S. Epidermal growth factor receptor (EGFR) and squamous cell carcinoma of the skin: molecular bases for EGFR‐targeted therapy. Pathol Res Pract 2011; 207: 337–42. [DOI] [PubMed] [Google Scholar]
- 30. Tan EH, Chan A. Evidence‐based treatment options for the management of skin toxicities associated with epidermal growth factor receptor inhibitors. Ann Pharmacother 2009; 43: 1658–66. [DOI] [PubMed] [Google Scholar]
- 31. Tsimboukis S, Merikas I, Karapanagiotou EM, Saif MW, Syrigos KN. Erlotinib‐induced skin rash in patients with non‐small‐cell lung cancer: pathogenesis, clinical significance, and management. Clin Lung Cancer 2009; 10: 106–11. [DOI] [PubMed] [Google Scholar]


