Abstract
Malignant tumors are often associated with denervation, suggesting the functional implication of axonal guidance molecules in tumor growth. Here, we assessed the role of semaphorin 3C (sema3C) in the progression of gastric cancer. Immunohistochemistry of human samples revealed that sema3C was strongly expressed in neoplastic cells, especially at the invasion front. Stable transfection of target sequences of sema3C miRNA did not affect the in vitro proliferative activity of human gastric cancer AZ‐521 cells. However, when the tumor growth was examined in vivo using an orthotopic model in nude mice, primary stomach tumors as well as metastatic liver tumors were significantly suppressed by sema3C silencing with the reduction of microvessel density. Immunostaining of primary tumor indicated the rate of Ki‐67 positive carcinoma cells was decreased, whereas that of apoptotic cells was significantly increased in sema3C‐silenced tumor. In addition, capillary‐like tubular formation was reduced by the addition of culture media of sema3C miRNA cells compared with the media of control miRNA cells. Semaphorin 3C is positively expressed in gastric cancer cells and may be involved in tumor progression, presumably through the stimulation of angiogenesis.
Modern pathology has shown the lack of innervation in malignant tumors,1, 2 suggesting the functional involvement of the nervous system in tumor biology. In particular, the sympathetic nerve system has been shown to play an important role in tumor growth. Anatomical sympathectomy is reported to inhibit tumor growth in rodents.3, 4, 5, 6 Dopamine, a representative adrenergic neurotransmitter, had inhibitory effects on angiogenesis through the suppression of the vascular endothelial growth factor (VEGF) receptor signal in mature endothelial cells and endothelial precursor cells, and the pharmacological ablation of peripheral dopaminergic nerves has been shown to induce VEGF‐dependent angiogenesis and stimulate tumor growth.7, 8, 9 These facts strongly suggest that the denervation in malignant tissue may critically affect the behavior of malignant tumors. In fact, the loss of sympathetic nerve fiber was described in gastrointestinal cancer tissue10, 11 and the ratio of sympathetic denervation was inversely correlated with patient outcome in gastric cancer.12
Recent studies in the field of neurology have identified four types of axonal guidance molecules, the semaphorin, ephrin, netrin, and slit families, that regulate the outgrowth of axonal cones.13 Among them, class 3 semaphorins have been shown to be highly expressed in various types of tumor cells and to be correlated with tumor development and progression.14, 15 Most class 3 semaphorins (i.e., semaphorins 3A, 3B, 3D, 3F, and 3G) inhibit cell migration and seem to be endowed with antitumor properties.16 Among semaphorins, semaphorin 3B (sema3B), sema3C, and sema3F are repellents of the sympathetic nerve fibers17, 18 and sema3B and sema3F are known to function as tumor suppressors.15 In contrast to these semaphorins, sema3C promotes tumor migration and was highly expressed in metastatic tumor cells.19, 20 Semaphorin 3C also promotes endothelial cell proliferation, migration, and tube formation in vitro and might be involved in angiogenesis.21 From these findings, sema3C is supposed to assist tumor progression. However, the mechanisms of the regulation of cancer progression by sema3C have not been fully clarified. Here, we investigated the expression of sema3C in human gastric cancer cell lines and tissue specimens, and characterized its role in the progression of gastric cancer.
Materials and Methods
Materials
The cDNA probes of sema3C, neuropilin‐1 (nrp‐1), neuropilin‐2 (nrp‐2), and β‐actin (ACTB) were purchased from Applied Biosystems (Foster City, CA, USA). Rabbit polyclonal anti‐human sema3C antibody (ab39300), semaphorin 3C peptide (ab39299), and rabbit polyclonal anti‐Ki67 antibody (ab15580) were purchased from Abcam (Cambridge, UK). Rabbit polyclonal anti‐human neuropilin‐2 antibody (H‐300) (sc‐5542) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal anti‐CD34 antibody (clone QBend10) was purchased from Dako (Kyoto, Japan). Mouse monoclonal anti‐D2‐40 antibody (SIG‐3730) was purchased from Covance (San Diego, CA, USA). Rat monoclonal anti‐CD31 antibody was purchased from BD Pharmingen (San Diego, CA, USA).
Cell culture and animals
The human gastric cancer cell lines MKN‐1, MKN‐28, MKN‐45, MKN‐74, HGC‐27, AZ‐521, and KATOIII were obtained from Riken Cell Bank (Tsukuba, Japan). These cells were maintained in DMEM supplemented with 5% FBS (Sigma, St. Louis, MO, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco‐BRL, Grand Island, NY, USA) at 37°C in a humidified 5% CO2 incubator. Primary HUVEC were established in our laboratory and used after three passages. Immunodeficient BALB/c nu/nu mice at 4–5 weeks of age, obtained from Oriental Kobo Laboratories (Tokyo, Japan), were used for the model of orthotopic tumor of the stomach. Mice were treated in accordance with the policies of the Animal Ethics Committee of the University of Tokyo (Tokyo, Japan).
RNA isolation and quantitative real‐time RT‐PCR
Total RNA was extracted from each cell line using an RNeasy Mini kit (Qiagen, Dusseldorf, Germany), according to the manufacturer's instructions. Total RNA was then reverse‐transcribed to form the first‐strand cDNAs using a High Capacity cDNA reverse transcription kit (Applied Biosystems). The expression of sema3C mRNA was determined by real‐time PCR using TaqMan technology and the 7500 Fast Real‐Time PCR System (Applied Biosystems). ACTB was used as an internal control gene. Primers (sema3C primer product number, Hs00170762_m1; ACTB, Hs_m1; both from Applied Biosystems) were added at a final concentration of 900 nM. The thermal cycler conditions were 95°C for 20 min, and 40 cycles of 95°C for 3 s and 60°C for 30 s. All experiments were independently carried out twice.
Immunohistochemical study
Sections were deparaffinized in xylene, hydrated through a gradually diluted ethanol series, and heated in a microwave oven for three 5‐min cycles (500 W). After rinsing the sections in PBS, endogenous peroxidase activity was inhibited by incubation with 0.3% hydrogen peroxide in 100% ethanol for 25 min. After washing three times in PBS, non‐specific reactions were blocked by incubation with PBS containing 5% BSA for 30 min at room temperature. The sections were incubated overnight at 4°C in humidified chambers with the primary antibody to sema3C (1:3200), neuropilin‐2 (1:500), CD34 (1:100), D2‐40 (1:100), and CD31 (1:200). The specificity of immunostaining was confirmed by the blocking of staining by the presence of peptide of the sema3C specific sequence. As negative control, control rabbit IgG (Nichirei, Tokyo, Japan) was used.
To determine whether the staining was specific for sema3C, an immunizing peptide blocking experiment was carried out. The antibody was incubated with a twofold excess of blocking peptide derived from within residues 700 to the C‐terminus of Human sema3C. Immunostaining was carried out with the neutralized antibody. Antibody binding was visualized with a ChemMate EnVision detection kit (cat. No. K5027; Dako) according to the manufacturers' instructions, as follows. After washing three times with PBS, the sections were incubated with peroxidase‐labeled polymer containing goat anti‐rabbit immunoglobulin for 30 min. After washing again with PBS, the slides were developed by immersion in hydrogen peroxide and 3,3′‐diaminobenzidine tetrahydrochloride for 10 min, followed by light counterstaining with Mayer's hematoxylin.
For immunostaining of murine tumor, the prediluted polyclonal anti‐Ki67 antibody (1:1000 dilution) was incubated overnight in a humidified chamber. The positive rate was defined as the percentage of Ki67‐positive nuclei per 100 tumor cells. In situ DNA fragmentation was established using the terminal deoxyribonucleotidyl transferase‐mediated TUNEL in paraffin‐embedded sections. We used the In Situ Cell Death Detection Kit (cat. no. 11684817; Roche GmbH, Mannheim, Germany). Staining procedures were carried out following the manufacturer's recommendations. Apoptotic count was carried out using a light microscope, counting stained apoptotic tumor cells per high‐powered field (magnification, ×400). Corresponding H&E sections were analyzed to avoid miscounting necrotic cells.
Transfection
AZ‐521 cells were used for the knockdown experiment of sema3C. We designed the miRNAs for the sema3C targeting sequence: sema3C‐miRNA (5′‐TGACTTGGCACTCTACAAGTATA). Those miRNA gene double‐strands were ligated with Block‐iT pol II miR RNAi Expression Vector (Invitrogen, Carlsbad, CA, USA). Then the vector with sema3C‐miRNA or control miRNA plasmids were stably transfected into AZ‐521 cells using Lipofectamine‐2000 (Invitrogen) according to the manufacturer's instructions. We selected the stably transfected cells with 5 μg/mL blasticidin. The mRNA expression of sema3C was determined by real‐time PCR analysis.
Proliferation assay
Cells were seeded in 96‐well plates at a density of 1 × 103/well. These cells were incubated in DMEM supplemented with 5% FBS. The 3‐(4, 5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium, inner salt (MTS) assay was used to monitor the number of viable cells according to the manufacturer's instructions (CellTiter 96 Non‐Radioactive Cell Proliferation Assay; Promega, Madison, WI, USA). The proliferative activity at 24, 48, 72, 96, and 120 h after seeding was determined. All experiments were independently done three times.
Capillary‐like tube formation
The formation of vascular‐like structures by HUVEC on the Matrigel Basement Membrane Matrix (Becton Dickinson, Bedford, MA, USA) was assessed. A 96‐well flat‐bottomed plate was coated with Matrigel (50 μL/well), and HUVEC seeded at 2 × 104 cells/well in 100 μL endothelial basal medium (EBM) (0.5% FBS) by the addition of the same volume of the culture media of control miRNA cells or sema3C miRNA cells in DMEM (0.1% BSA). After incubation at 37°C for 12 h, the wells were observed using a phase contrast microscope. Images were captured with a digital camera. The length of capillary‐like tube formation was measured using ImageJ software (http://rsb.info.nih.gov/ij/). Each experiment was carried out at least twice.
Orthotopic model of gastric cancer
A total of 1.5 × 105 AZ‐521 cells in 100 μL PBS were subserosally inoculated into the gastric walls of BALB/c nu/nu mice under deep inhalation anesthesia with ether. Eight weeks after inoculation, the mice were killed for macroscopic and microscopic evaluation (n = 8 per group). The experiment was repeated three times.
Determination of microvessel density in orthotopic gastric tumor
The stomach of the mice was excised, washed in PBS containing 10% sucrose at 4°C, embedded in optimal cutting temperature compound (Tissue‐Tek; Sakura Finetek, Torrance, CA, USA), and snap frozen in 2‐methylbutane cooled with liquid nitrogen for immunohistochemical examination. The 5‐μm cryostat sections of frozen samples were immunostained with a rat mAb to mouse CD31 (1:200 dilution) (BD Pharmingen) to detect blood vessels. Subsequently, specimens were incubated with the corresponding secondary antibodies labeled with Alexa Fluor 594 (Invitrogen) at 1:200 dilution. Cell nuclei were counterstained with DAPI. CD31 and DAPI were imaged using a red and blue filter, respectively, under a fluorescence stereomicroscope (BZ8000; Keyence, Osaka, Japan). Microvessels of individual slides were then counted at a high magnification (×200) in three randomly selected spots. In all of the samples, the average of the number of microvessels was calculated and determined as the microvessel density.
Statistical analysis
Results are expressed as mean values with SEM. Each value was analyzed using Student's t‐test using a commercially available software (JMP 8; SAS Institute, Cary, NC, USA), and differences with a P‐value <0.05 were considered statistically significant.
Results
Expression of sema3C and neuropilin‐2 in specimens from gastric cancer patients
Semaphorin 3C expression was immunohistochemically examined in the specimens from gastric cancer patients. Semaphorin 3C was weakly stained in epithelial cells of normal gastric mucosa, although the staining intensity differed among cases (Fig. 1A). In gastric cancer tissue, a strong cytoplasmic staining was observed in most of the neoplastic cells (Fig. 1B). In particular, carcinoma cells at the invasive front (Fig. 1C,D) or those invading the lymphatic vessels (Fig. 1E). Lymphatic vessels were confirmed by immunohistochemical staining of D2‐40 in continuous tissue sections (Fig. 1F). Neuropilin‐2, which is the main receptor of sema3C, was positively expressed in CD31‐positive endothelial cells around the tumor (Fig. 1G,H). In most cases, however, neuropilin‐2 was not stained in carcinoma cells.
Figure 1.
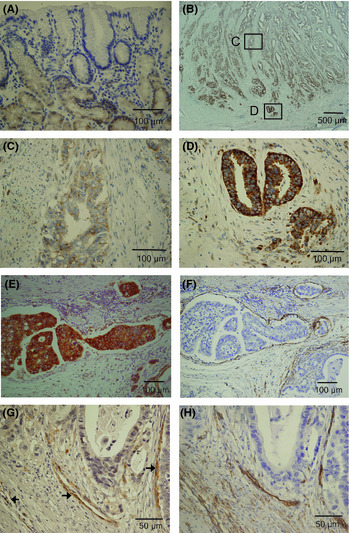
Immunohistochemical staining of semaphorin 3C (sema3C) in human gastric cancer specimens. (A) Staining of normal mucosa adjacent to gastric cancer. (B–D) Semaphorin 3C immunoreactivity was strongly observed in carcinoma cells at the invasive front (D) compared with the central area (C) of gastric cancer tissue. (E) Strong expression of sema3C in carcinoma cells invading lymphatic vessels. (F) Lymphatic vessels were immunostained by mAb to D2‐40 in continuous section. Expression of neuropilin‐2 (G) and CD31 (H) in continuous sections of a gastric cancer specimen.
Semaphorin 3C expression in gastric cancer cell lines
Next, we examined the mRNA expression of sema3C and neuropilin‐2 in gastric cancer cell lines using real‐time PCR. In all seven gastric cancer cell lines analyzed, sema3C was expressed at variable levels, but in most of the cell lines tested, the expression levels were higher than that found in HUVEC, used as the positive control (Fig. 2A). Among the cell lines analyzed, AZ‐521 cells showed the highest level of sema3C expression, and selected as the most appropriate cell type to examine the effect of sema3C on tumor progression by means of mRNA silencing. In contrast, the expression of neuropilin‐2 in most gastric cancer cell lines was relatively low compared with HUVEC, which was compatible with the results of immunohistochemical staining of human gastric cancer specimens (Fig. 2B).
Figure 2.
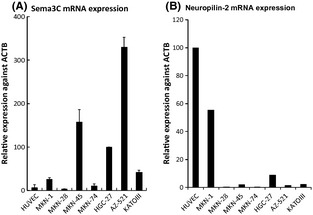
Real‐time PCR analysis of mRNA expression of semaphorin 3C (sema3C) (A) and neuropilin‐2 (B) in gastric cancer cell lines. RNA was extracted from the cells and analyzed for the expression levels of sema3C mRNAs. β‐actin (ACTB) was used as an internal control gene.
Knockdown of sema3C does not affect tumor growth in vitro but accelerates tumor growth in vivo
To investigate the effect of sema3C on tumor growth, we generated sema3C‐silenced AZ‐521 cells as well as control AZ‐521 cells through stable transfection of plasmid with target sequences of sema3C miRNA and control miRNA after selection with blasticidin. As shown in Figure 3(A), transfection of miRNA for sema3C in AZ‐521 significantly suppressed the mRNA expression of sema3C to approximately one‐third of that in the control transfectant. The proliferation of these two cell lines was compared by MTS assay, but no significant difference in proliferative activity was found (Fig. 3B). In addition, sema3C knockdown did not affect the adhesion and invasion activity of AZ‐521 (data not shown). Those data suggest that the silencing of sema3C does not affect the cellular activity of AZ‐521 in vitro.
Figure 3.
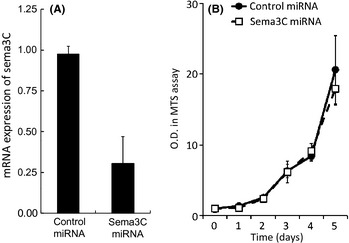
(A) Downregulation of semaphorin 3C (sema3C) in AZ‐521 cells by the stable transfection of sema3C–miRNA. Pre‐miRNA double‐strand oligo was inserted into the miRNA expression vector‐pcDNA 6.2‐GW/EmGFP‐miR. Expression levels of AZ‐521 cells transfected with sema3C–miRNA compared to control‐miRNA cells, by real‐time PCR. (B) Proliferation of AZ‐521 cells transfected with sema3C–miRNA or control‐miRNA. O.D., optical density; MTS, 3‐(4, 5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium, inner salt.
The sema3C‐silenced AZ‐521 cells were then inoculated in the gastric wall as an orthotopic model, and tumor growth was compared with control AZ‐521. As shown in Figure 4, the tumor growth in gastric wall was significantly suppressed in sema3C‐silenced AZ‐521 as compared to control transfectant. AZ‐521 is a cell line known to metastasize to the liver after orthotopic inoculation into the gastric wall. Although the number of liver metastasis found in the three series of experiments showed a large variation, the rates of mice with liver metastases as well as the weights of the livers tended to be lower in sema3C‐silenced AZ‐521 in all series (Table 1). This result indicates that sema3C may play a positive role in metastasis as well as in tumor growth in the stomach wall.
Figure 4.
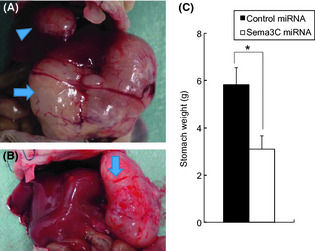
Effect of semaphorin 3C (sema3C)–miRNA on the growth of human gastric cancer AZ‐521 cells orthotopically innoculated in the stomach wall of BALB/c nu/nu mice. Representative gastric tumor (arrow) and hepatic metastasis (arrow head) of AZ‐521 cells transfected with control‐miRNA (A) or sema3C–miRNA (B). (C) Weights of the stomachs inoculated with control‐miRNA cells and sema3C‐miRNA cells were compared. Data show the mean ± SD of three different series of experiments. *P = 0.00015.
Table 1.
Development of liver metastasis in mice inoculated with AZ‐521 human gastric cancer cells with semaphorin 3C knockdown (sema3C KO)
| Rate of mice with metastasis | Weight of the liver (g) | P‐value (for liver weight) | |
|---|---|---|---|
| Exp. 1 | |||
| CTL | 5/6 | 4.50 ± 2.55 | 0.083 |
| sema3C KO | 7/8 | 2.71 ± 1.69 | |
| Exp. 2 | |||
| CTL | 2/10 | 0.40 ± 0.97 | 0.223 |
| sema3C KO | 0/10 | 0.00 | |
| Exp. 3 | |||
| CTL | 2/4 | 4.21 ± 1.12 | 0.046 |
| sema3C KO | 1/5 | 2.83 ± 0.96 | |
AZ‐521(1.5 × 105) cells transfected with miRNAs of sema3C targeting sequence or control miRNAs were subserosally inoculated into the gastric walls of BALB/c nu/nu mice. The mice were killed after 8 weeks and livers evaluated. CTL, control; Exp., experiment.
Proliferation and apoptosis of AZ‐521 cells in gastric tumor
Immunohistochemical staining of Ki‐56 and TUNEL staining of AZ‐521 gastric tumors were carried out to detect the rate of proliferative and apoptotic cells, respectively. As shown in Figure 5 and Table 2, the ratio of Ki‐56(+) cells was markedly reduced, whereas that of TUNEL(+) cells was significantly increased in sema3C‐silenced AZ‐521 tumors. This suggests that production of sema3C in tumor tissue stimulates proliferation while suppressing apoptosis of AZ‐521 in vivo, supporting the growth retardation of stomach tumor by sema3C silencing.
Figure 5.
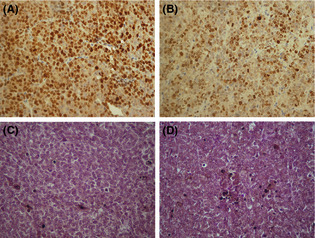
Immunostaining of Ki‐67 in gastric tumors of AZ‐521 cells transfected with control‐miRNA (A) or semaphorin 3C (sema3C)–miRNA (B). TUNEL staining of gastric tumors of AZ‐521 cells transfected with control‐miRNA (C) or sema3C–miRNA (D).
Table 2.
Rate of cell proliferation and apoptotis in primary stomach tumor in mice inoculated with AZ‐521 human gastric cancer cells
| Cells | Ki‐67(+) cells | P‐value | TUNEL(+) cell | P‐value |
|---|---|---|---|---|
| Control miRNA | 53.8 ± 7.6 | 0.015 | 6.8 ± 2.1 | 0.019 |
| Sema3C miRNA | 38.3 ± 8.2 | 9.4 ± 6.1 |
Proliferative and apoptotic cells were detected by immunostaining with anti‐Ki‐67 mAb and TUNEL assay, respectively. Data represent the mean ± SD of the cell number per high‐powered field of each tumor in two different series of experiments. Sema3C, semaphorin 3C.
Effect of sema3C knockdown on microvessel density
As neuropilin‐2, the receptor of sema3C, was positively expressed in endothelial cells of human gastric cancer tissue, we next examined the number of microvessels in gastric tumors in the orthotopically implanted tumors of mice. Intratumoral vessels were clearly detected by the immunohistochemical staining of CD31 in both tumors (Fig. 6A). As expected, the density of CD31(+) microvessels of sema3C‐silenced AZ‐521 tumor was significantly reduced compared to the control AZ‐521 (Fig. 6; P = 0.0054).
Figure 6.
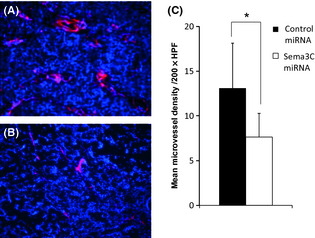
Microvessel immunostaining of gastric tumors of AZ‐521 cells transfected with control‐miRNA (A) or semaphorin 3C (sema3C)–miRNA (B). Vessels were stained red using anti‐CD31 mAb and the nuclei were counterstained with DAPI. (C) The microvessel density of each tumor is shown. Data represent the mean ± SD of two different series of experiments. HPF, high‐powered field. *P = 0.0054.
Effect of sema3C knockdown on tube formation of endothelial cells
We added the culture supernatant of AZ‐521 to HUVEC cultures to evaluate the in vitro effect on endothelial cells. Capillary‐like tube formation of HUVEC on collagen gel was significantly reduced by the addition of supernatant of sema3C‐silenced AZ‐521 compared with control miRNA‐silenced AZ‐521 (Fig. 7; P = 0.0016).
Figure 7.
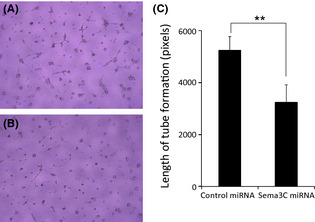
Representative pictures of HUVEC tube formation treated with endothelial basal medium (EBM) (0.5% FBS) + control miRNA supernatant (DMEM [0.1% BSA]) (A), and EBM (0.5%FBS) + semaphorin 3C (sema3C) miRNA (B). (C) Length of tube formation of HUVECs treated with supernatant of AZ‐521 cells transfected with control‐miRNA or sema3C‐miRNA. Data represent the mean ± SD of two different series of experiments. *P = 0.016.
Discussion
In this study, we investigated the expression and possible role of sema3C in gastric cancer. Semaphorin 3C was highly expressed on gastric cancer cells of human tissue as well as on the gastric cell lines. Although at different levels, sema3C was also weakly expressed on normal gastric epithelium, as confirmed by immunohistochemistry. Previous studies have shown that sema3C is positively expressed in inflammatory diseases such as Crohn's disease and rheumatoid arthritis. Therefore, the different levels of expression in normal gastric mucosa in our series might be dependent on the presence of inflammatory conditions, such as chronic gastritis accompanied with Helicobacter pylori infection.
More importantly, in most cases, the expression levels on neoplastic cells were markedly enhanced compared with normal counterparts. In particular, the expression of sema3C was prominent in carcinoma cells at the invasion front as well as in tumors invading the lymphatics. Previous studies have suggested the positive correlation between sema3C expression and tumor invasiveness. Herman and Meadows showed that in prostate cancer cells, sema3C transfected cells showed increased invasive and adhesive characteristics with upregulation of E‐cadherin expression, downregulation of β‐catenin expression, and greater cellular membrane expression of α‐integrins.20 More recently, Esselens et al.19 have shown that the cleavage of sema3C induced by ADAMTS‐1 promotes the release of sema3C from the ECM and the migration of breast cancer cells. The aberrant expression of sema3C protein was detected in recurrent squamous cell carcinomas after extensive radiochemotherapy.22 Those results are consistent with our finding and support the hypothesis that sema3C expression is related to the malignant potential of gastric carcinoma cells, including the lymphatic metastatic potential.
We then examined the effect of sema3C on the growth of gastric cancer in vivo using miRNA targeting sema3C. The cell line with the highest mRNA expression level of sema3C, AZ‐521, was chosen for the mRNA silencing experiment. Our results indicate that silencing of sema3C significantly suppressed not only the tumor growth in primary sites but also the development of liver metastasis. Ki‐67 immunostaining and TUNEL assay revealed that sema3C silencing reduced the proliferation and accelerated the apoptosis of carcinoma cells in vivo. However, the silencing did not affect the proliferation and migration activity of AZ‐521 in vitro. From these facts, it is supposed that sema3C may accelerate tumor progression not by a direct effect on cancer cells, but dependent on the interaction with the surrounding cells of the tumor microenvironment. Semaphorin 3C has been shown to be secreted from endothelial cells and to promote cell proliferation and migration in an autocrine manner,21 indicating that sema3C might promote tumor growth through angiogenesis. In our experiment, microvessel density was markedly reduced in sema3C‐silenced AZ‐521 tumors, and neuropilin, the main receptor of sema3C, was highly expressed in the vessels found in human gastric cancer tissue. Moreover, tube formation of HUVEC was significantly reduced by the addition of supernatant of sema3C‐silenced AZ‐521 compared with control AZ‐521. Those facts strongly support our hypothesis and it can be speculated that the sema3C–neuropilin system is associated in the process of tumor invasion and metastasis of human gastric cancer.
In summary, we showed that the expression of sema3C in gastric cancer may support tumor progression, possibly through the stimulation of angiogenesis. Semaphorin 3C is known to be a potent chemorepellent factor of sympathetic nerve fibers17, 23 and dopamine, the representative mediator of the sympathetic nerve, inhibits the proliferation or migration of endothelial cells induced by VEGF and angiogenesis.7, 8, 9 From these facts, sema3C is supposed to be one of the important factors regulating angiogenesis induction in tumor tissue, at least partly, through the sympathetic denervation in malignant gastric tumor. As we have recently found that loss of sympathetic nerve fibers in gastric cancer is strongly correlated with patient outcome,12 regulation of sema3C could lead to a novel anti‐angiogenic therapy for solid tumors.
Disclosure Statement
The authors have no conflict of interest.
Abbreviations
- CTL
control
- Exp.
experiment
- Sema3C
semaphorin 3C
Acknowledgments
This study was supported in part by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ministry of Health, Labor and Welfare of Japan.
(Cancer Sci 2012; 103: 1961–1966)
References
- 1. RA W. The Spread of the Tumors in the Human, 3rd edn London: Butterworths, 1973; 121–5. [Google Scholar]
- 2. Mitchell BS, Schumacher U, Kaiserling E. Are tumours innervated? Immunohistological investigations using antibodies against the neuronal marker protein gene product 9.5 (PGP 9.5) in benign, malignant and experimental tumours. Tumour Biol 1994; 15: 269–74. [DOI] [PubMed] [Google Scholar]
- 3. Grzanna R, Frondoza CG, Otten U. Sympathectomy inhibits growth of a murine plasmacytoma tumor. J Auton Nerv Syst 1985; 13: 149–60. [DOI] [PubMed] [Google Scholar]
- 4. Horn PT, Mirkin BL. Modulation of in situ murine neuroblastoma tumor growth by the adrenergic nervous system: differential response of clonal cell lines to chemical sympathectomy. Life Sci 1990; 47: 2251–9. [DOI] [PubMed] [Google Scholar]
- 5. Romeo HE, Colombo LL, Esquifino AI, Rosenstein RE, Chuluyan HE, Cardinali DP. Slower growth of tumours in sympathetically denervated murine skin. J Auton Nerv Syst 1991; 32: 159–64. [DOI] [PubMed] [Google Scholar]
- 6. Raju B, Haug SR, Ibrahim SO, Heyeraas KJ. Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience 2007; 149: 715–25. [DOI] [PubMed] [Google Scholar]
- 7. Basu S, Nagy JA, Pal S et al The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med 2001; 7: 569–74. [DOI] [PubMed] [Google Scholar]
- 8. Basu S, Sarkar C, Chakroborty D et al Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor‐mediated angiogenesis. Cancer Res 2004; 64: 5551–5. [DOI] [PubMed] [Google Scholar]
- 9. Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Catecholamines regulate tumor angiogenesis. Cancer Res 2009; 69: 3727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashraf S, Crowe R, Loizidou MC, Turmaine M, Taylor I, Burnstock G. The absence of autonomic perivascular nerves in human colorectal liver metastases. Br J Cancer 1996; 73: 349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chamary VL, Robson T, Loizidou M, Boulos PB, Burnstock G. Progressive loss of perivascular nerves adjacent to colorectal cancer. Eur J Surg Oncol 2000; 26: 588–93. [DOI] [PubMed] [Google Scholar]
- 12. Miyato H, Kitayama J, Ishigami H, Kaisaki S, Nagawa H. Loss of sympathetic nerve fibers around intratumoral arterioles reflects malignant potential of gastric cancer. Ann Surg Oncol 2011; 18: 2281–8. [DOI] [PubMed] [Google Scholar]
- 13. Chilton JK. Molecular mechanisms of axon guidance. Dev Biol 2006; 292: 13–24. [DOI] [PubMed] [Google Scholar]
- 14. Vachkov IH, Huang X, Yamada Y et al Inhibition of axonal outgrowth in the tumor environment: involvement of class 3 semaphorins. Cancer Sci 2007; 98: 1192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 2008; 8: 632–45. [DOI] [PubMed] [Google Scholar]
- 16. Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment–two sides of a coin. J Cell Sci 2009; 122: 1723–36. [DOI] [PubMed] [Google Scholar]
- 17. Chen H, He Z, Bagri A, Tessier‐Lavigne M. Semaphorin‐neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron 1998; 21: 1283–90. [DOI] [PubMed] [Google Scholar]
- 18. Klostermann A, Lohrum M, Adams RH, Puschel AW. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J Biol Chem 1998; 273: 7326–31. [DOI] [PubMed] [Google Scholar]
- 19. Esselens C, Malapeira J, Colome N et al The cleavage of semaphorin 3C induced by ADAMTS1 promotes cell migration. J Biol Chem 2010; 285: 2463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Herman JG, Meadows GG. Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. Int J Oncol 2007; 30: 1231–8. [PubMed] [Google Scholar]
- 21. Banu N, Teichman J, Dunlap‐Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J 2006; 20: 2150–2. [DOI] [PubMed] [Google Scholar]
- 22. Yamada T, Endo R, Gotoh M, Hirohashi S. Identification of semaphorin E as a non‐MDR drug resistance gene of human cancers. Proc Natl Acad Sci USA 1997; 94: 14713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mark MD, Lohrum M, Puschel AW. Patterning neuronal connections by chemorepulsion: the semaphorins. Cell Tissue Res 1997; 290: 299–306. [DOI] [PubMed] [Google Scholar]


