Abstract
Our retrospective study showed inhibition of the renin–angiotensin system was associated with better outcomes in patients with advanced pancreatic cancer receiving gemcitabine. The primary objective of this phase I study was to determine the recommended dose of candesartan in combination with gemcitabine in normotensive patients with advanced pancreatic cancer. Candesartan was given orally at an escalating dose (4, 8, 16, and 32 mg) q.d. daily, and gemcitabine was given 1000 mg/m2 30 min i.v. on days 1, 8, and 15, repeated every 4 weeks. Dose‐limiting toxicity (DLT) was defined as grade 4 hematological toxicities, grade 2 hypotension, abnormal creatinine or potassium, and grade 3 or 4 other non‐hematological toxicities. A standard “3+3” phase I dose‐escalation design was used. A total of 14 patients (candesartan 4 mg, three patients; 8 mg, three patients; 16 mg, six patients; 32 mg, two patients) were enrolled. One of six patients at 16 mg showed DLT of grade 4 neutropenia and two of two patients at 32 mg showed DLT of grade 2 hypotension. Response rate and disease control rate were 0% and 79%, respectively. Progression‐free survival and overall survival were 7.6 and 22.9 months, respectively. Candesartan 16 mg is the recommended dose in combination with gemcitabine in the treatment of advanced pancreatic cancer. (UMIN CTR: UMIN000002152) (Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02311.x, 2012)
Gemcitabine was shown to improve overall survival (OS) compared with 5‐fluorouracil in advanced pancreatic cancer,1 therefore, extensive research of combination therapy using gemcitabine with other cytotoxic drugs or molecular targeted drugs has been carried out. No single randomized controlled trial indicated superiority in OS of gemcitabine combined with other cytotoxic drugs compared with gemcitabine monotherapy. Recently, FOLFIRINOX,2 a multiagent regimen without gemcitabine, prolonged OS significantly but serious adverse events such as febrile neutropenia also increased. Among molecular target agents, only erlotinib in combination with gemcitabine showed modest improvement in OS3 and other agents including cetuximab,4 bevacizumab,5 and axitinib6 failed to demonstrate benefits in OS in phase III trials.
The systemic renin–angiotensin system (RAS) is associated with cardiovascular regulation and angiotensin I‐converting enzyme inhibitors (ACEIs) and angiotensin II type‐1 receptor blockers (ARBs) are the most widely used antihypertensive drugs. Since Lever et al.7 reported that the use of ACEIs was associated with a decreased incidence of cancer in a large cohort study, the potential role of the local RAS in carcinogenesis has attracted substantial attention. The local RAS reportedly promotes angiogenesis and cellular proliferation through vascular endothelial growth factor (VEGF) expression or epidermal growth factor receptor expression.8, 9 Synergistic inhibition of tumor growth in a murine pancreatic cancer has been shown with combined gemcitabine and losartan treatment through VEGF suppression.10 In addition, the inhibition of RAS is reported to induce apoptosis in pancreatic cancer cells.11, 12 Our retrospective study13 showed the inhibition of RAS by the use of ACEIs or ARBs for hypertension was associated with longer progression‐free survival (PFS) or OS in patients with advanced pancreatic cancer receiving gemcitabine. Similar results were reported in non‐small‐cell lung cancer and renal cell cancer.14 Therefore, we carried out this phase I study of combination therapy with gemcitabine and candesartan, an ARB, in normotensive patients with advanced pancreatic cancer.
Patients and Methods
Eligibility
The eligibility criteria were: (i) histologically or cytologically proven pancreatic adenocarcinoma; (ii) locally advanced or metastatic disease; (iii) no prior treatment for pancreatic cancer; (iv) ECOG performance status of 0–2; (v) age >20 years; and (vi) adequate organ function, as indicated by white blood cell count >3000/mm3, platelet count >100 000/mm3, hemoglobin >9.0 g/dl, serum creatinine within the upper limit of normal, serum potassium within the upper limit of normal, total bilirubin <3 times the upper limit of normal, and aspartate aminotransferase and alanine aminotransferase levels <5 times the upper limit of normal. The exclusion criteria were: (i) systolic blood pressure <100 mmHg or >150 mmHg; (ii) any treatment for hypertension; (iii) use of ACEIs, ARBs, or renin inhibitors; (iv) severe complications, such as active infection, cardiac or renal disease, marked pleural effusion, or ascites; (v) active gastrointestinal bleeding; (vi) active intestinal pneumonitis; (vii) severe drug hypersensitivity; (viii) active concomitant malignancy; or (ix) pregnant or lactating women.
Study design and treatment
The study was an open‐label, single‐center, non‐randomized, dose‐escalating phase I study to evaluate the tolerability of candesartan (purchased fromTakeda Pharmaceutical Company, Osaka, Japan) in combination with gemcitabine (purchased from Eli Lilly Japan, Kobe, Japan) in patients with previously untreated advanced pancreatic cancer. The primary endpoint was to determine the dose‐limiting toxicity (DLT) and the maximum tolerated dose (MTD). Secondary objectives were objective response rate, disease control rate, PFS, and OS. The study protocol was approved by the institutional review board of the University of Tokyo Hospital (Tokyo, Japan). Written informed consent was obtained from all patients.
Patients were treated with i.v. gemcitabine 1000 mg/m2 over 30 min on days 1, 8, and 15 with candesartan at escalating doses given orally day 1 to day 28. Each cycle was repeated every 4 weeks. The dose of gemcitabine was fixed and candesartan was given to three patients in each group. Four candesartan dose levels were planned: level 1, 4 mg/day; level 2, 8 mg/day; level 3, 16 mg/day; and level 4, 32 mg/day (Table 1). If a DLT was observed in one of the three patients at one dose level, an additional three patients were enrolled at this dose level. If no DLT was observed at a given dose level, three patients were enrolled at the next dose level. If only one or two of six patients experienced DLT, dose escalation would continue. The MTD was defined as one dose level below the dose that induced DLT in two of three or three of six patients. A maximum of 24 patients (six at each dose level) was planned. Dose escalation within a patient was not permitted. All treatment was given until disease progression, unacceptable toxic effects, or withdrawal of consent.
Table 1.
Dose escalation schedule and summary of dose‐limiting toxicities (DLT) and best response in patients with advanced pancreatic cancer
| Level | Gemcitabine (mg/m2) | Candesartan (mg/day) | No. patients | DLT | DLT description | Best response |
|---|---|---|---|---|---|---|
| 1 | 1000 | 4 | 3 | 0 | – | SD (n = 3) |
| 2 | 1000 | 8 | 3 | 0 | – | SD (n = 3) |
| 3 | 1000 | 16 | 6 | 1 | Grade 4 neutropenia (n = 1) |
SD (n = 5), PD (n = 1) |
| 4 | 1000 | 32 | 2 | 2 | Grade 2 hypotension (n = 2) | NE (n = 2) |
–, no DLT evident; NE, not evaluable; PD, progressive disease; SD, stable disease.
Dose‐limiting toxicities were determined during the first treatment cycle. Dose‐limiting toxicity was defined, using the National Cancer Institute's Common Toxicity Criteria (NCI‐CTC; version 3.0), as one or more of the following effects attributable to the study drug: (i) grade 4 hematologic toxicity; (ii) grade 2–4 creatinine or potassium abnormality; (iii) grade 2–4 arrhythmia or hypotension; (iv) any other grade 3–4 non‐hematologic toxicity; and (v) delay of recovery from treatment‐related toxicity for more than 4 weeks.
For the first episode of DLT, treatment was held until resolution to ≤grade 1. In cases of DLT specifically attributable to candesartan, that is, hypotension, arrhythmia, or hyperkalemia, treatment was resumed with reduction of candesartan by one dose level. Otherwise, gemcitabine was reduced by 200 mg/m2. In cases of DLT that were not specifically attributable to either drug, reduction of both drugs was allowed. Dose reduction was allowed only in cases with DLTs during cycle 1. Dose reductions during cycle 2 or thereafter were permitted when grade ≥3 hematological toxicities or grade ≥2 non‐hematological toxicities were observed. Gemcitabine administration was skipped when grade ≥3 hematological toxicities, total bilirubin level ≥3 times the upper limit of normal, aspartate aminotransferase and alanine aminotransferase levels ≥5 times the upper limit of normal, or creatinine ≥1.5 times the upper limit of normal were observed.
Response and toxicity assessment
Physical examination including blood pressure, complete blood count with differential, electrolyte levels with creatinine, and liver function tests were measured before study entry, on days 1, 8, and 15 during treatment. Carbohydrate antigen 19‐9 (CA 19‐9) was assessed on day 1 of each cycle. Pretreatment evaluation using contrast‐enhanced computed tomography (CT) was carried out within 4 weeks before the patient's enrolment. Tumor response was assessed every two cycles, using RECIST 1.0.15 Toxicity was evaluated using NCI‐CTC 3.0.
Progression‐free survival and OS were calculated using the Kaplan–Meier method. Progression‐free survival was calculated from the start of treatment to the date of either disease progression or death or censored at last follow‐up. Overall survival was defined as the time from treatment initiation to final follow‐up or until death from any cause. The final analysis was based on follow‐up information, which was received until January 2012. The JMP 9.0 statistical software program (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Patients
Fourteen patients with pathologically proven advanced pancreatic cancer were enrolled in this study between July 2009 and October 2010 (Table 2). Seven patients (50%) had locally advanced disease and performance status (PS) was 0 in 57%. The median follow‐up time was 18.3 months. All patients were evaluable for toxicity and 12 patients were evaluable for response.
Table 2.
Characteristics of patients with advanced pancreatic cancer who participated in this study (n = 14)
| Age, median (range), years | 60 (40–77) |
| Gender, male/female, n (%) | 5 (36)/9 (64) |
| ECOG performance status 0/1, n (%) | 8 (57)/6 (43) |
| Disease extent, locally advanced/metastatic, n (%) | 7 (50)/7 (50) |
| Site of primary tumor, head/body or tail, n (%) | 4 (29)/10 (71) |
| Site of metastasis, n (%) | |
| Liver | 5 (36) |
| Lung | 3 (21) |
| Lymph node | 5 (36) |
| Peritoneum | 2 (14) |
| CA19‐9, IU/L, median (range) | 1909 (17–23 290) |
Dose‐limiting toxicity and MTD
There was no DLT observed in levels 1 (4 mg candesartan) or 2 (8 mg) (Table 1). In level 3 (16 mg), one of six patients developed DLT (grade 4 neutropenia). The remaining five patients did not develop DLT. Two of two patients in level 4 developed DLT. Both patients developed grade 2 hypotension in day 5, which necessitated discontinuation of candesartan. No more patients were enrolled in level 4 and the dose of level 3 (16 mg candesartan) was determined to be the MTD and the recommended dose for further studies.
Overall toxicity and dose intensity
All 14 patients were assessable for toxicity. Dose‐limiting toxicity was seen in three patients: one grade 4 neutropenia in level 3 and two grade 2 hypotension in level 4. Toxicities throughout all cycles are shown in Table 3. The regimen was safe and tolerable, other than grade 2 hypotension in candesartan 32 mg. The regimen was stopped in these two patients, but they recovered immediately after cessation of the regimen. No treatment‐related death was observed. Leukopenia (21%) and neutropenia (50%) were the only grade 3–4 toxicities observed in this study. Hypotension was seen in five patients: grade 2 in one patient with candesartan 4 mg, grade 1 in two patients with candesartan 16 mg, and grade 2 in two patients with candesartan 32 mg. Laboratory findings as non‐hematological toxicities including renal function or hyperkalemia were not observed in any group.
Table 3.
Overall adverse effects of combination therapy using gemcitabine and candesartan in normotensive patients with advanced pancreatic cancer (n = 14)
| All grades, n (%) | Grade 3–4, n (%) | |
|---|---|---|
| Hematological | ||
| Leukopenia | 10 (71) | 3 (21) |
| Neutropenia | 8 (57) | 7 (50) |
| Anemia | 13 (93) | 0 (0) |
| Thrombocytopenia | 11 (79) | 0 (0) |
| Non‐hematological | ||
| Fatigue | 4 (29) | 0 (0) |
| Anorexia | 2 (14) | 0 (0) |
| Nausea | 2 (14) | 0 (0) |
| Constipation | 8 (57) | 0 (0) |
| Diarrhea | 3 (21) | 0 (0) |
| Rash | 2 (14) | 0 (0) |
| Stomatitis | 1 (7) | 0 (0) |
| Hypotension | 5 (36) | 0 (0) |
Efficacy
The median number of cycles delivered to all enrolled patients was eight (range, 1–28 cycles). Although assessment of tumor response was not a primary objective of the current study, patients were evaluated for tumor response. Other than two patients who discontinued treatment due to hypotension in level 4, one patient stopped treatment due to deteriorated condition without disease progression on CT scan. Disease progression on CT scan was evident in the remaining 11 patients. Seven patients were still alive with a median survival of 20.6 months at the time of analysis. The objective tumor response was not achieved but stable disease was observed in 79%. As a result, the response rate was 0% and the disease control rate was 79%, respectively. The median PFS was 7.6 months (95% confidence interval [CI], 5.4–15.4 months) (Fig. 1). The median OS was 22.9 months (95% CI, 6.4–NA months) (Fig. 1). The 1‐year survival rate was 64%. In patients with metastatic disease, the median PFS and OS were 5.9 and 11.5 months with 1‐year survival rate of 42.9%, respectively. In patients with locally advanced disease, the median PFS was 11.4 months and the 1‐year survival rate was 85.7%. The median OS was not reached at the time of analysis.
Figure 1.
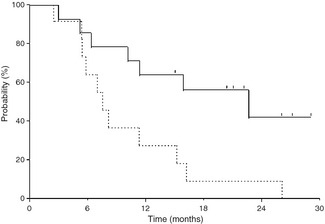
Kaplan–Meier curves of progression‐free survival (broken line) and overall survival (solid line). Notch marks denote time patients are censored.
Among 14 patients enrolled in this study, CA19‐9 was not assessed due to early termination in two patients in level 4 and normal CA19‐9 (<37 IU/L) in one patient. Of the remaining 11 patients, CA19‐9 was reduced more than 50% compared to the pretreatment level in two patients (18%).
Discussion
This phase I study showed that candesartan can be safely combined with gemcitabine in normotensive patients with advanced pancreatic cancer. The primary DLT is hypotension. Addition of candesartan did not seem to affect myelosuppression significantly, but grade 2 hypotension was observed in all two patients treated with candesartan 32 mg. The recommended dose of candesartan is 16 mg/day every 28 days in combination with gemcitabine 1000 mg/m2 on days 1, 8, and 15 of a 4‐week cycle.
Our retrospective study of gemcitabine monotherapy against advanced pancreatic cancer revealed that the use of ACEIs or ARBs as antihypertensive drugs was associated with longer PFS and OS.13 Candesartan was the most often used antihypertensive medication in the retrospective study and is reported to have the most potent selective ARB. Furthermore, when we treated a genetically engineered mouse model of pancreatic cancer16 with several ARBs, candesartan clearly inhibited VEGF expression and angiogenesis in vivo and showed a better tendency of survival extension compared with other ARBs (Motohisa Tada, Hideaki Ijichi, Koji Miyabayashi, Yoshinari Asaoka, Dai Mohri, Tsuneo Ikenoue, Keisuke Tateishi, Yasuyuki Morishita, Rintarou Mikata, Takeshi Ishihara, Fumihiko Kanai, Fumio Imazeki, Masao Omata, Harold L. Moses, Osamu Yokosuka, unpublished data, 2010). Accordingly, we selected candesartan for this phase I study.
The aim of this phase I study was to evaluate the safety of candesartan in combination with gemcitabine in normotensive patients with advanced pancreatic cancer. Even in patients with normal blood pressure, the safety of candesartan was evaluated in two large randomized controlled trials of diabetic retinopathy.17, 18 In those trials, candesartan at 16 mg for 1 month, followed by 32 mg was given orally and the discontinuation rate of candesartan due to adverse events was only 2–4%. In our trial, clinically significant hypotension was not observed in 4, 8, or 16 mg candesartan, either. The combination of gemcitabine and candesartan did not seem to increase myelosuppression, compared with gemcitabine monotherapy. Recently, meta‐analyses of randomized controlled trials suggest that ARBs are associated with an increased risk of new cancer diagnosis,19, 20, 21 although conflicting data are reported. Given the palliative nature of chemotherapy in advanced pancreatic cancer, however, the impact of this possible association would be negligibly small, if any.
Interestingly, the analysis of efficacy showed similar results with our retrospective study.13 In the retrospective analysis, although the objective response did not increase by the inhibition of RAS, PFS of 8.7 months in patients receiving ACEIs or ARBs was superior to that in patients who did not receive ACEIs or ARBs. The primary endpoint of this phase I study was safety and the analysis of efficacy was difficult with a small sample, but a disease control rate as high as 79% despite the response rate of 0%, and PFS of 7.6 months, was promising.
Many randomized controlled trials of molecular targeted agents in combination with gemcitabine have been carried out, but most studies failed to demonstrate superiority over gemcitabine monotherapy. Only erlotinib, an epidermal growth factor receptor inhibitor, in combination with gemcitabine showed modest improvement in OS.3 Vascular endothelial growth factor is reported to be upregulated and expected to be a treatment target in pancreatic cancer,22, 23, 24 but the combination of gemcitabine and VEGF inhibitors bevacizumab5 or axitinib6 failed to demonstrate better OS than gemcitabine monotherapy. The mechanism of ARBs in the treatment of pancreatic cancer has not been fully elucidated. Initially, angiotensin was reported to play an important role in angiogenesis of pancreatic cancer through VEGF upregulation25 and the inhibition of RAS in combination with gemcitabine showed synergistic inhibitory effects through VEGF downregulation.10 More recently, multiple mechanisms of ARBs in addition to inhibition of VEGF are proposed. One of these mechanisms is the tumor–stroma interaction.26 The dense fibrotic stroma of excessive connective tissue and cells, one of the histological characteristics of pancreatic cancer, is making this cancer refractory to chemotherapy and is considered a treatment target.27 Angiotensin is reported to increase the expression of various growth factors such as fibroblast growth factor and transforming growth factor‐β as well as VEGF,28 which influence the tumor–stroma interaction and may modulate the tumor microenvironment in favor of pancreatic cancer. Thus, inhibition of RAS may increase the efficacy of chemotherapy by decreasing the stromal volume and enhancing drug delivery. But the exact mechanisms of ARBs in pancreatic cancer are still to be elucidated in vitro and in vivo.
There are some limitations in this study. The study population was small and limited to normotensive patients. Although dose‐dependent antitumor effects of ARBs have been reported in basic research,12, 29 a small number of patients enrolled in this study hindered the efficacy analysis according to different doses of candesartan. We must also confirm the safety of this combination therapy with gemcitabine and candesartan in those patients with hypertension and dose modification of candesartan might be necessary in patients with hypertension whose systemic RAS is activated to various extents. Given the high prevalence in elderly people, pancreatic cancer is often diagnosed in the presence of hypertension. In this study period, 24 patients were excluded from the study due to comorbid hypertension.
In conclusion, combination therapy with gemcitabine and candesartan is feasible and safe in normotensive patients with advanced pancreatic cancer. Its efficacy is encouraging with a high disease control rate and long PFS. A multicenter phase II study is now ongoing.
Disclosure Statement
The authors have no conflicts of interest.
References
- 1. Burris HA 3rd, Moore MJ, Andersen J et al Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–13. [DOI] [PubMed] [Google Scholar]
- 2. Conroy T, Desseigne F, Ychou M et al FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–25. [DOI] [PubMed] [Google Scholar]
- 3. Moore MJ, Goldstein D, Hamm J et al Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25: 1960–6. [DOI] [PubMed] [Google Scholar]
- 4. Philip PA, Benedetti J, Corless CL et al Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group‐directed intergroup trial S0205. J Clin Oncol 2010; 28: 3605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kindler HL, Niedzwiecki D, Hollis D et al Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010; 28: 3617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kindler HL, Ioka T, Richel DJ et al Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double‐blind randomised phase 3 study. Lancet Oncol 2011; 12: 256–62. [DOI] [PubMed] [Google Scholar]
- 7. Lever AF, Hole DJ, Gillis CR et al Do inhibitors of angiotensin‐I‐converting enzyme protect against risk of cancer? Lancet 1998; 352: 179–84. [DOI] [PubMed] [Google Scholar]
- 8. Ager EI, Neo J, Christophi C. The renin‐angiotensin system and malignancy. Carcinogenesis 2008; 29: 1675–84. [DOI] [PubMed] [Google Scholar]
- 9. Khakoo AY, Sidman RL, Pasqualini R, Arap W. Does the renin‐angiotensin system participate in regulation of human vasculogenesis and angiogenesis? Cancer Res 2008; 68: 9112–5. [DOI] [PubMed] [Google Scholar]
- 10. Noguchi R, Yoshiji H, Ikenaka Y et al Synergistic inhibitory effect of gemcitabine and angiotensin type‐1 receptor blocker, losartan, on murine pancreatic tumor growth via anti‐angiogenic activities. Oncol Rep 2009; 22: 355–60. [PubMed] [Google Scholar]
- 11. Amaya K, Ohta T, Kitagawa H et al Angiotensin II activates MAP kinase and NF‐kappaB through angiotensin II type I receptor in human pancreatic cancer cells. Int J Oncol 2004; 25: 849–56. [PubMed] [Google Scholar]
- 12. Gong Q, Davis M, Chipitsyna G, Yeo CJ, Arafat HA. Blocking Angiotensin II Type 1 Receptor triggers Apoptotic cell death in human pancreatic cancer cells. Pancreas 2010; 39: 595–603. [DOI] [PubMed] [Google Scholar]
- 13. Nakai Y, Isayama H, Ijichi H et al Inhibition of renin‐angiotensin system affects prognosis of advanced pancreatic cancer receiving gemcitabine. Br J Cancer 2010; 103: 1644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mc Menamin UC, Murray LJ, Cantwell MM, Hughes CM. Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in cancer progression and survival: a systematic review. Cancer Causes Control 2012; 23: 221–30. [DOI] [PubMed] [Google Scholar]
- 15. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 16. Ijichi H, Chytil A, Gorska AE et al Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas‐specific blockade of transforming growth factor‐beta signaling in cooperation with active Kras expression. Genes Dev 2006; 20: 3147–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaturvedi N, Porta M, Klein R et al Effect of candesartan on prevention (DIRECT‐Prevent 1) and progression (DIRECT‐Protect 1) of retinopathy in type 1 diabetes: randomised, placebo‐controlled trials. Lancet 2008; 372: 1394–402. [DOI] [PubMed] [Google Scholar]
- 18. Sjolie AK, Klein R, Porta M et al Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT‐Protect 2): a randomised placebo‐controlled trial. Lancet 2008; 372: 1385–93. [DOI] [PubMed] [Google Scholar]
- 19. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin‐receptor blockade and risk of cancer: meta‐analysis of randomised controlled trials. Lancet Oncol 2010; 11: 627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bangalore S, Kumar S, Kjeldsen SE et al Antihypertensive drugs and risk of cancer: network meta‐analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 2011; 12: 65–82. [DOI] [PubMed] [Google Scholar]
- 21. Chang CH, Lin JW, Wu LC, Lai MS. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: a nationwide case‐control study. J Clin Oncol 2011; 29: 3001–7. [DOI] [PubMed] [Google Scholar]
- 22. Itakura J, Ishiwata T, Friess H et al Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res 1997; 3: 1309–16. [PubMed] [Google Scholar]
- 23. Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 2000; 88: 2239–45. [DOI] [PubMed] [Google Scholar]
- 24. Smith RA, Tang J, Tudur‐Smith C, Neoptolemos JP, Ghaneh P. Meta‐analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br J Cancer 2011; 104: 1440–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arafat HA, Gong Q, Chipitsyna G, Rizvi A, Saa CT, Yeo CJ. Antihypertensives as novel antineoplastics: angiotensin‐I‐converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll Surg 2007; 204: 996–1005. [DOI] [PubMed] [Google Scholar]
- 26. Ijichi H, Chytil A, Gorska AE et al Inhibiting Cxcr2 disrupts tumor‐stromal interactions and improves survival in a mouse model of pancreatic ductal adenocarcinoma. J Clin Invest 2011; 121: 4106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olson P, Hanahan D. Cancer. Breaching the cancer fortress. Science 2009; 324: 1400–1. [DOI] [PubMed] [Google Scholar]
- 28. Greene AS, Amaral SL. Microvascular angiogenesis and the renin‐angiotensin system. Curr Hypertens Rep 2002; 4: 56–62. [DOI] [PubMed] [Google Scholar]
- 29. Fujimoto Y, Sasaki T, Tsuchida A, Chayama K. Angiotensin II type 1 receptor expression in human pancreatic cancer and growth inhibition by angiotensin II type 1 receptor antagonist. FEBS Lett 2001; 495: 197–200. [DOI] [PubMed] [Google Scholar]


