Abstract
The management of atypical intraductal lesions of the breast remains controversial. In the present study, the subsequent surgical excision results and follow‐up data on 86 (3.65%) atypical intraductal lesions and 78 (3.31%) low‐grade ductal carcinoma in situ (DCIS) from a cohort of 2358 needle biopsies were examined. There were 17 cases (0.72%) of pure flat epithelial atypia (FEA), 44 (1.87%) pure atypical ductal hyperplasia (ADH), three (0.13%) pure atypical lobular hyperplasia (ALH), 18 (0.76%) combined ADH + FEA, three (0.13%) combined ALH + FEA and one (0.04%) combined ALH + FEA + ADH. Subsequent surgical excisions were done in 53 cases and revealed the following incidences of malignancy: pure FEA (1/8); pure ADH (17/31); FEA + ADH (7/10); FEA + ALH (2/3); and FEA + ALH + ADH (0/1), with pure FEA showing significantly lower incidence of malignancy. In this cohort, there were 703 carcinomas including 155 DCIS with 78 cases (50.3%) being low‐grade. FEA with ADH (and/or ALH) was present in 22 (28.2%) of these 78 cases of low‐grade DCISs at surgical excisions. Pure FEA was not detected in any of the subsequently excised surgical materials of the atypical intraductal lesions nor the low‐grade DCISs. Thus, pure FEA was very unusual in surgical specimens. When pure FEA is detected at needle biopsy, a wait and see approach can be adopted. However, when the FEA is associated with other concomitant atypical intraductal lesions, especially ADH, further excision should be contemplated. (Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02314.x, 2012)
The use of needle biopsy in evaluating calcifications detected at mammography (MMG) and/or suspicious malignant lesions detected at ultrasound has increased because of a rise in the frequency of screening. Many of these lesions represent atypical intraductal lesions such as flat epithelial atypia (FEA), atypical ductal hyperplasia (ADH) and atypical lobular hyperplasia (ALH). These are morphologically similar to early type, low grade ductal carcinoma in situ (DCIS) or lobular carcinoma in situ. For FEA, there had been previous variations in its terminology, but since the World Health Organization (WHO) working group on breast tumors,1 introduced the term FEA, this was essentially adopted by many authors.2, 3, 4, 5, 6, 7, 8, 9, 10, 11
To date the terminology is still confusing and far from uniform, sometimes FEA or ADH are used in lesions that are essentially or exclusively DCIS or are of uncertain malignant potential (indeterminate, B3),12 resulting in variations in the treatment of patients with these lesions. Although several previous reports indicated the biological and clinical significance and close genetic relationship of FEA13 and ADH,14 the management of these lesions remains controversial,4, 5, 6, 7, 8, 9, 10 and this is now a topical issue in Japan. In addition, there is limited experience of these atypical intraductal lesions reported from Asia. Thus, a detailed evaluation was undertaken from a large Asian cohort.
In the present study, needle biopsy diagnosed atypical intraductal lesions (i.e. FEA, ALH and ADH), and low‐grade DCIS, which showed similar morphology (non‐comedo type, such as the Van‐Nuys classification,15 were investigated from two perspectives: (i) the frequency and distribution of FEA, either pure or associated with other atypical intraductal and/or low‐grade DCIS that were detected at needle biopsies (core and Mammotome), and their associated and subsequent risks for malignancy based on surgical resection specimens and/or clinical follow‐up data; and (ii) the frequency and distribution of subtypes of low‐grade DCIS in biopsies (core and Mammotome); and on the presence of FEA, whether in pure form or in association with other atypical intraductal lesions (ADH,ALH), in the breast tissue adjacent to these low grade DCIS excision samples.
Materials and Methods
Definition of FEA
Flat epithelial atypia was defined as the most advanced lesion with variably distended acini lined by one to several layers of monotonous, mildly atypical, cuboidal to columnar cells growing in an exclusively “flat pattern,” with the complete absence of intraluminal proliferation with architectural atypia (Fig. 1a,b). The relatively round monomorphic appearance of the nuclei distinguished FEA from non‐atypical columnar cell lesions (columnar cell change and columnar cell hyperplasia) (Fig. 1c), which were characterized by ovoid to elongated nuclei that were regularly oriented perpendicular to the basement membrane of the involved spaces.3, 5 Low‐grade DCIS was composed of small, monomorphic cells with round nuclei, showing cribriform, solid, papillary and micropapillary architectural features,1 and corresponded to Van Nuys classification, group 1, which had low‐grade nuclei (1.0–1.5 red blood cells in diameter) with inconspicuous nucleoli and diffuse chromatin or intermediate‐grade nucleoli (1–2 red blood cells in diameter) with occasional nucleoli, coarse chromatin without comedo‐type necrosis.15 ADH was defined as lesions manifesting architectural and cytological atypia that fell short of diagnosis of low‐grade DCIS and/or the qualitative features in the ducts are similar to those of low‐grade DCIS, but the lesion is limited in the extent and small (<2 mm).6 This definition encompassed lesions that did not entirely involve a single duct. The architectural changes included focal establishment of Roman arches, cribriform pattern, or micropapillary projections (Fig. 2a–d). Thus, in a background of FEA, the presence of any single atypical intraluminal structures such as arcade, bar, roman bridge, tuft or cribriform‐micropapillary formation were considered sufficient for a diagnosis of concomitant ADH3 (Fig. 3a–c). Lesions with a flat growth pattern but with high‐grade cytologic atypia were classified as high‐grade DCIS and not as FEA, and were excluded. Cases of intracystic or encysted papillary carcinomas, papillomas with atypical epithelial hyperplasia, and mucocele‐like lesions with atypia were also excluded.
Figure 1.
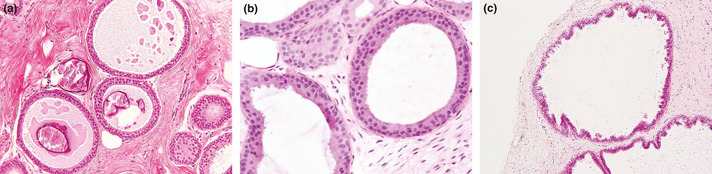
(a) Pure flat epithelial atypia (FEA). The dilated acini are lined by atypical cuboidal or columnar cells with secretions and calcifications. (b) The low‐grade, monomorphic type cytologic atypia with nuclei that are round to ovoid and relatively uniform in appearance. (c) Columnar cell hyperplasia. The columnar cells with dilated acini show stratification with foci of cellular tufting.
Figure 2.
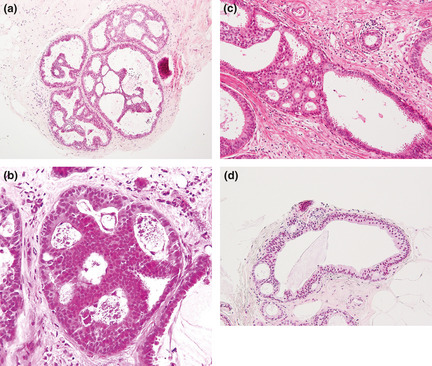
(a) Atypical ductal hyperplasia (ADH) characterized by complex architectural atypia (formation of bridges rigid bars, and micropapillary projections within the each single duct. (b) The qualitative features in the duct are similar those of low‐grade ductal carcinoma in situ (DCIS), but the lesion is limited in extent and small (<2 mm) (c) The uniform, atypical cell population involves only a portion of the single duct. (d) The arcade formation is partially seen, which is relatively round and evenly spaced consisting of monotonous cells.
Figure 3.
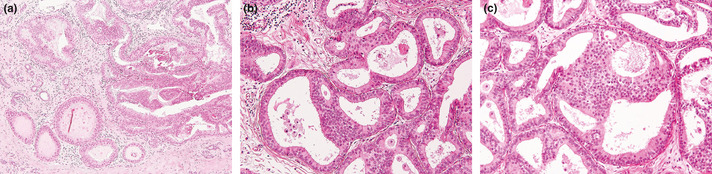
(a) Concomitant atypical ductal hyperplasia (ADH) and flat epithelial atypia (FEA). The bottom field shows FEA. The right field shows focal papillary proliferation, and the cells are cytologically identical to those of the FEA. (b) Some ducts show the characteristic features of FEA. Two spaces at the bottom show ADH with rigid bridge and cellular tufts composed of cells. (c) Atypical ductal hyperplasia is seen in the center of the field with focal cribriform pattern. The background shows FEA.
Patients and diagnosis
The patient cohort consisted of cases with needle biopsies of the breast spanning over 4 years from April 2006 to March 2010. Data were acquired over a follow‐up period of at least 1 year with imaging examination at the Social Insurance Kurume Daiichi Hospital (Kurume, Japan). This retrospective study was approved by the Social Insurance Kurume Daiichi Hospital Ethical Committee.
Overall a total of 2358 biopsies (including 1788 Mammotome biopsies using an 11‐gauge [G] vacuum‐assisted instrument) were performed. For target lesions presenting with calcifications, these were confirmed by specimen radiography of the cores; for non‐calcified target lesions (i.e. masses, hypoechoic lesions), the needle biopsies were done under ultrasound guidance and confirmation of needle penetration of the lesions. From these, patients diagnosed with either FEA, ADH, ALH, or low‐grade DCIS or any combination were selected. All of the biopsies diagnosed as low‐grade DCIS cases were confirmed as carcinoma by subsequent partial (conserving surgery) or total mastectomy.
Tissue specimens that were all obtained from surgical biopsies and subsequent partial mastectomy were cut to 4–6‐mm‐thick slices and embedded in paraffin and the 4‐μ thick sections were H&E stained. This was followed by histological examination with mapping that included the surgical margins (<5 mm). Using these tissue sections, one author made the primary diagnosis on all cases, and these cases were re‐evaluated and confirmed by a panel of three authors (original and two additional authors). Any disagreement was resolved at a multi‐head microscope and discussion. In some cases of atypical intraductal lesions and all cases of low grade DCIS, the final diagnoses were further confirmed either using tissue specimens from subsequent surgical procedures and/or follow‐up data (including imaging data) obtained at least 1 year after the needle biopsies (until March 2011). The imaging (MMG and/or ultrasound) data were evaluated using the Breast Imaging Reporting And Data System (BI‐RADS). Fundamentally, atypical lesions with BI‐RADS 4b or higher (category 4 in Japan by MMG and ultrasound guidelines) were required to undergo subsequent surgical excision; however, in some cases the patients refused further excision, and they were simply followed‐up. With the exception of some older patients, almost all patients who underwent partial mastectomy received radiotherapy (50 Gy total dose).
Statistical analysis
Data are presented as means ± SD. Statistical analysis was carried out using Fisher's exact test. A P‐value of <0.05 was considered as statistically significant.
Results
Clinicopathological data on atypical intraductal lesions from initial biopsy to subsequent surgical excision
Of the 2358 needle‐biopsies, pure FEA was diagnosed in 17 cases (0.72%), pure ADH in 44 (1.87%), and pure ALH in three (0.13%). In addition ADH + FEA were diagnosed in 18 cases (0.76%), ALH + FEA in three (0.13%) and ALH + FEA + ADH in one (0.04%). The total number of cases with atypical intraductal lesions was 86 (3.65%) (Tables 1,2). Fifty‐three out of these 86 cases were subsequently surgically excised (Table 1).
Table 1.
Clinicopathological data on 53 atypical intraductal lesions from initial biopsy to subsequent surgical excision
| Lesion type (biopsy diagnosis) | Change in clinicopathology (subsequent surgical biopsy) | Number of patients (n = 53)† | Age (years) (mean ± SD) | CNB/USMT/STMT (n) | Number of biopsies (median [range]) | Follow‐up (days) (mean ± SD)‡ | Imaging (n) > C‐4a, b (4c, 5)§ |
|---|---|---|---|---|---|---|---|
| ADH | To DCIS | 11 | 49.5 ± 15 | 1/5/5 | 4 [3–7] | 906.3 ± 327.2 | 8 (2) |
| To IDC‐NOS | 6 | 54.8 ± 11.6 | 1/2/3 | 4 [4–7] | 869.7 ± 452.2 | 6 (3) | |
| To benign | 14 | 47.1 ± 8.4 | 1/8/5 | 4 [1–5] | 1254.5 ± 505.0 | 14 (2) | |
| Total | 31 | ||||||
| Pure FEA | To DCIS | 1 | 49 | 0/0/1 | 4 | 637 | 1 (0) |
| To IDC‐NOS | 0 | – | – | – | – | – | |
| To benign | 7 | 47.0 ± 6.2 | 0/4/3 | 6 [3–12] | 634.2 ± 365.9 | 6 (1) | |
| Total | 8 | ||||||
| ADH + FEA | To DCIS | 2 | 49.5 ± 2.8 | 0/1/1 | 5.5 [5–6] | 685.0 ± 352.1 | 2 (2) |
| To IDC‐NOS | 5 | 50.8 ± 9.0 | 0/2/3 | 3 [2–5] | 988.4 ± 386.1 | 5 (3) | |
| To benign | 3 | 47.0 ± 2.0 | 0/2/1 | 3 [3–4] | 1227.3 ± 294.3 | 3 (1) | |
| Total | 10 | ||||||
| ALH + FEA | To DCIS | 1 | 50 | 0/1/0 | 2 | 1617 | 1 (0) |
| To Inv Lob | 1 | 45 | 0/0/1 | 5 | 1507 | 1 (0) | |
| To benign | 1 | 46 | 0/1/0 | 4 | 1528 | 1 (0) | |
| FEA + ALH + ADH | To benign | 1 | 51 | 0/0/1 | 6 | 928 | 1 (0) |
| Total | 4 |
†Four cases of atypical ductal hyperplasia (ADH), and one case of ADH + flat epithelial atypia (FEA) that was upgraded to carcinoma within 3 months are included in the data. ‡Follow‐up data since initial diagnosis by needle biopsy. §Imaging data is by Breast Imaging Reporting and Data System (BI‐RADS) at the initial biopsy (C, category). ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; C, category; CNB, core needle biopsy; DCIS, ductal carcinoma in situ; FEA, flat epithelial atypia; IDC‐NOS, invasive ductal carcinoma – not otherwise specified; Inv Lob, invasive lobular carcinoma; STMT, stereo‐guided Mammotome biopsy; USMT, ultrasound‐guided Mammotome biopsy.
Table 2.
Clinicopathological data on 33 atypical intraductal lesions of follow‐up cases from initial biopsy
| Lesion type | Number of patients (n = 33) | Age (years) (mean ± SD) | CNB/USMT/STMT (N) | Number of biopsies (median [range]) | Follow‐up (days) (mean ± SD)† | Imaging (n) > C‐4a, b (4c)‡ |
|---|---|---|---|---|---|---|
| ADH | 13 | 48.2 ± 10.5 | 0/3/10 | 4 [2–7] | 976.7 ± 503.0 | 13 (4) |
| Pure FEA | 9 | 48.6 ± 12.2 | 0/1/8 | 4 [2–8] | 497.0 ± 134.8 | 9 (3) |
| Pure ALH | 3 | 57.7 ± 22.1 | 1/2/0 | 5 [3–9] | 917.0 ± 420.2 | 0 (0) |
| ADH + FEA | 8 | 45.9 ± 11.1 | 0/2/6 | 3.5 [2–9] | 841.5 ± 459.1 | 7 (2) |
†Follow‐up data since initial biopsy. At least mammography and/or ultrasonography were examined per year after initial biopsy. ‡Imaging data is by Breast Imaging Reporting and Data System (BI‐RADS) at the initial biopsy. ADH, atypical ductal hyperplasia; ALH, atypical lobular hyperplasia; C, category; CNB, core needle biopsy; FEA, flat epithelial atypia; STMT, stereo‐guided Mammotome biopsy; USMT, ultrasound‐guided Mammotome biopsy.
Among the 17 cases of pure FEA, surgical excision was subsequently performed in eight. The final excision diagnoses were benign (fibrocystic changes) (seven of eight cases) and malignant (DCIS cribriform and solid type) (one of eight case). Among these, no pure FEA was found in the subsequent surgical specimens.
Of the 44 cases with pure ADH, surgical excision was subsequently performed in 31. The final excision showed 17 to be malignant, including 11 DCIS and six invasive ductal carcinoma‐not specified (IDC‐NOS); 14 were benign.
Of the 10 cases with ADH + FEA, the final excisions showed seven to be malignant, including two DCIS and five IDC‐NOS; three were benign.
Of the three cases with ALH + FEA, the final excisions showed one to be DCIS and one invasive lobular carcinoma; one was benign. One case with FEA + ALH + ADH was benign.
Pre‐excision imaging data of these 53 cases evaluated by BI‐RADS were also listed in Table 1. Most of the lesions corresponded to BI‐RADS 4a and 4b (35/53 [66.0%]) and less BI‐RADS 4c or 5 (14/53 [26.4%]). The remainders were all BI‐RADS 3.
Looking at the incidence of malignancy diagnosed from resection specimens, the incidence of malignant lesions associated with pure FEA (1/8: 12.5%) was significantly lower than those associated with pure ADH (17/31: 54.8%) (P = 0.0489) and those associated with FEA + ADH (7/10: 70%) (P = 0.0248). There was no significant difference in the associated malignancy rates between pure FEA (1/8: 12.5%) and FEA + ALH (2/3: 66.7%) (P = 0.152); and between pure ADH (17/31: 54.8%) and FEA + ADH (7/10: 70%) (P = 0.265).
Follow‐up data on atypical intraductal lesions
Follow up data were available in 33 cases including nine cases of FEA, 13 cases of ADH, three cases of ALH, and eight cases of ADH + FEA (Table 2). The nine cases of FEA were followed for 497.0 ± 134.8 days. There was no change in clinical findings, including BI‐RADS grading on the imaging findings since the initial biopsy (MMG and/or US). The 13 cases of ADH were followed for 976.7 ± 503.0 days, and there were no changes in clinical findings.
No malignant changes occurred at the end of the follow up period in cases with atypical intraductal lesions, and no recurrences occurred at the end of the follow up period after the subsequent surgical excision procedures. However, four cases out of 11 of ADH and one case out of two FEA + ADH initially diagnosed at needle biopsy showed interval changes at imaging within 3 months and surgical excisions were performed. These cases were upgraded to DCIS in the surgical excisions before the follow up period (included in Table 1).
Imaging data evaluated by BI‐RADS were also obtained in Table 2, which mainly corresponded to category 4: 4a or 4b (22/33 [66.7%]) and some of them are 4c (9/33 [27.3%]). Pure ALH corresponded only to category 3.
Clinicopathological data for low‐grade DCIS
Of the 2358 cases, 703 (29.8%) were found to have carcinoma including 155 (6.9%) DCIS. Among these 155 cases of DCIS, 78 (50.3%) were low‐grade. All patients with low‐grade DCIS were female, with an age range of 36–76 years (mean 51.7 ± 11.0 years). Of these 78 cases, one had a single biopsy, one had two biopsies, and the remaining had more than three biopsies (up to nine, median 5.0 biopsies). None of the cases of low‐grade DCIS recurred during a follow‐up period of 925.6 ± 429.9 days (Table 3).
Table 3.
Clinicopathological data for 78 low‐grade ductal carcinoma in situ (DCIS)
| Lesion type | Number of patients | Age (years) (mean ± SD) | CNB/USMT/STMT (n) | Number of biopsies (median [range]) | Follow‐up (days) (mean ± SD)† |
|---|---|---|---|---|---|
| Low‐grade DCIS | 78 | 51.7 ± 11.0 | 4/39/35 | 5.0 [1–10] | 925.6 ± 429.9 |
†Follow‐up days are since subsequent surgical excision after initial biopsy. CNB, core needle biopsy; STMT, stereo‐guided Mammotome biopsy; USMT, ultrasound‐guided Mammotome Biopsy.
Among the 78 cases with low‐grade DCIS, 17 cases (21.8%) showed single, pure DCIS subtypes (cribriform, solid, papillary or micropapillary); and 61 cases (78.2%) showed a combination of low‐grade DCIS subtypes. The most frequent pure subtype was cribriform (14.1%), and combination subtypes was cribriform + solid (15.4%). All 78 cases had excision, and 12 (15.4%) showed (micro) invasion in the excision specimens. Among these 78 cases at excision 36 (46.1%) had associated atypical intraductal lesions with ADH and/or ALH, including 22 (28.2%) that also showed FEA. No case of pure FEA without other atypical intraductal lesions was seen among these 78 excisions.
Discussion
In FEA, the strict terminology of “flat“ refers to lesions without bridging, micropapillary or a foothill‐like appearance. These lesions are relatively rare compared to other atypical intraductal lesions in needle biopsy specimens and are also extremely rare in the subsequent surgical specimens. The frequency of pure FEA detected in the present study was low (0.72%), even when compared to others that reported an incidence of about 3.7%.5, 13 One of the possible reasons for this discrepancy was the relatively recent starting in Japan (J‐start) of ultrasound guided needle biopsies in 2008, and many benign lesions were biopsied. The other reason could be ethnic differences. The percentage of cases with FEA diagnosed at needle biopsy, that were upgraded to carcinoma at surgical excision specimens (12.5%) was similar to other studies, where it ranged from 0% to 21%.4, 5, 6, 9, 10, 11, 16, 17, 18 In this study, it was shown that FEA, when present in combination with other atypical intraductal lesions (ADH, ALH), had a greater tendency to be upgraded on excisional surgical specimens. Similar findings have been reported in previous studies5, 6, 11, 17 (Table 4). Although five cases of ADH and ADH + FEA were upgraded to carcinoma at excision within 3 months, these likely represented missed detection of carcinomas at needle biopsy rather than genuine disease evolution. Rather, most pure FEA did not develop or rapidly change into carcinoma, at least over a follow‐up period of at least 1 year.
Table 4.
Summary of published data demonstrating percentage cases atypical intraductal lesions at the biopsy specimens to final diagnosis on surgical excision
| Diagnosis | ||||
|---|---|---|---|---|
| Needle biopsy | FEA | FEA | FEA + ADH | ADH |
| Surgical excision | FEA | Carcinoma | ||
| Kunju and Kleer4 | 0% (0/14) | 21% (3/14) | 11% (4/38) | 38% (3/8) |
| Martel et al.16 | 0% (0/5) | 0% (0/5) | – | – |
| Chivukula et al.6 | 0% (0/35) | 14% (5/35) | 16% (31/189) | 11% (5/45) |
| Senetta et al.18 | (?/36) | 0% (0/36) | – | – |
| Piubello et al.5 | 50% (10/20) | 0% (0/20) | 30% (3/10) | – |
| de Mascarel et al.9 | (?/24) | 0% (0/24) | – | 64% (32/50) |
|
Rakha et al.17 |
0% (0/7) | 14% (1/7) | 29% (2/7) | 40% (6/15) |
|
Rajan et al.11 |
(?/37) | 19% (7/37) | 29% (5/17) | 53% (17/32) |
| Lavoué V et al.10 | 23% (14/60) | 13% (8/60) | – | – |
| Our study (2012) | 0% (0/8) | 13% (1/8) | 70% (7/10) | 55% (17/44) |
ADH, atypical ductal hyperplasia; FEA, flat epithelial atypia.
In the literature, most reports on atypical intraductal lesions focused on their progression to carcinomas.4, 5, 6, 7, 8, 9, 10, 11, 16 Although a few reports described high percentage of pure FEA in the subsequent surgical materials,5, 10 the current findings, like many others4, 6, 9, 16, 17 demonstrated that pure FEA was rarely seen in surgical specimens excised for atypical epithelial proliferation and DCIS (Table 4). Thus, it is important to strictly distinguish between pure FEA and FEA mixed with ADH (or ALH) in needle biopsies5 or between FEA and columnar cell change and columnar cell hyperplasia.18 FEA is defined as “a presumably neoplastic intraductal alteration characterized by replacement of the native epithelial cells by a single or three to five layers of mildly atypical cells.” This definition, as detailed in the WHO classification and other previous reports,2, 13 implies that FEA should be considered a precursor to or an early stage lesion in the development of certain forms of DCIS. At the histologic diagnostic level, morphological diagnosis may sometimes be difficult, and unlike ADH,19 immunohistochemistry has not been found to be useful.
In the current study, all biopsies except one case with stand alone pure FEA (without other atypical intraductal lesions) did not progress to malignancy. Furthermore, among the low‐grade DCIS group, there was a 28% incidence of FEA in combination with other low‐grade DCIS subtypes or atypical intraductal lesions, compared to a reported 34%.3 For all the malignant cases that were excised, FEA was always associated with ADH, ALH and other DCIS subtypes, and there was no case of pure FEA coexisting with low grade DCIS. These observations may be construed as evidence that pure FEA devoid of other atypical intraductal lesions has very low risk of being associated with or progressing to malignancy. These observations were also reported by others.5, 6, 9, 16, 17 Thus, surgical excision may not be necessary when pure FEA is encountered in needle biopsies because its characteristics did not change over a follow‐up period of at least 1 year. However when FEA, together with ADH and/or other atypical lesions were detected in needle biopsy specimens, further resection would be deemed necessary and these cases should be closed monitored.
In agreement with the current findings, others reported that FEA was not associated with cancer at re‐excision, when detected alone using strict criteria at biopsy.9 Furthermore, many of the current samples were Mammotome (11 G) specimens, which represented extensive sampling (similar to other studies)5, 9 thus, these studies were potentially more likely to have sampled the lesions, compared to those using core needles biopsy (14 G) specimens.4, 6, 7, 10, 16 Moreover, even if pure FEA were present, these lesions were tiny and not extensive, because pure FEA has not been found in the surgical specimens of low grade DCIS. Furthermore, follow‐up data in the current series showed that these low grade DCIS and the atypical intraductal lesions did not recur or metastasize over a follow‐up period of at least 1 year. Thus, in dealing with this biologically indolent group of lesions, particularly pure FEA in a core biopsy, excision is not mandatory.5, 9, 16 It should also be noted that a firm diagnosis of pure FEA on needle biopsy can only be made with adequate tissue at sampling, as theoretically an inadequate sample may miss foci of other atypical intraductal lesions. This is less of a problem for Mammotome as more tissue is extracted. Most of the cases in this cohort were taken by Mammotome.
In conclusion, pure FEA rarely occurred in needle biopsy specimens, and was unusual in surgical excision specimens. Although the follow‐up period is short in the present study and further investigation is needed, pure FEA did not exhibit significant change over time, pathologically and radiologically over at least 1 year. Thus, pure FEA when encountered in needle biopsy does not warrant immediate mandatory further surgical excision. A wait‐and‐see approach with follow up may be a suitable alternative. However, cases with both FEA and ADH showed a high percentage of association with malignancies, and these cases should be further excised and closely monitored. In this context, adherence to strict diagnostic criteria for pure FEA and ADH is of utmost importance, especially in the setting of needle biopsy.
Disclosure Statement
The authors have no conflicts of interest.
Acknowledgments
We thank Ms Mari Katoh and Akiko Tanaka for their excellent technical assistance. This study was supported by the Kurume University School of Medicine Alumni Association.
References
- 1. Tavassoli FA, Hoefler H, Rosai J et al Intradectal preliferative lesions. In: Tavassoli FA, Devilee P, eds. Tumours of the Breast and Female Genital Organs. Pathology and Genetics of Tumours of the Digestive System. World Health Organization Classification of Tumours. Lyon: IARC Press, 2003; 63–73. [Google Scholar]
- 2. Schnitt SJ. Clinging carcinoma: an American perspective. Semin Diagn Pathol 2010; 27: 31–6. [DOI] [PubMed] [Google Scholar]
- 3. Collins LC, Achacoso NA, Nekhlyudov L et al Clinical and pathologic features of ductal carcinoma in situ associated with the presence of flat epithelial atypia: an analysis of 543 patients. Mod Pathol 2007; 20: 1149–55. [DOI] [PubMed] [Google Scholar]
- 4. Kunju LP, Kleer CG. Significance of flat epithelial atypia on mammotome core needle biopsy: should it be excised? Hum Pathol 2007; 38: 35–41. [DOI] [PubMed] [Google Scholar]
- 5. Piubello Q, Parisi A, Eccher A, Barbazeni G, Franchini Z, Iannucci A. Flat epithelial atypia on core needle biopsy: which is the right management? Am J Surg Pathol 2009; 33: 1078–84. [DOI] [PubMed] [Google Scholar]
- 6. Chivukula M, Bhargava R, Tseng G, Dabbs DJ. Clinicopathologic implications of “flat epithelial atypia” in core needle biopsy specimens of the breast. Am J Clin Pathol 2009; 131: 802–8. [DOI] [PubMed] [Google Scholar]
- 7. Flegg KM, Flaherty JJ, Bicknell AM, Jain S. Surgical outcomes of borderline breast lesions detected by needle biopsy in a breast screening program. World J Surg Oncol 2010; 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee TY, Macintosh RF, Rayson D, Barnes PJ. Flat epithelial atypia on breast needle core biopsy: a retrospective study with clinical‐pathological correlation. Breast J 2010; 16: 377–83. [DOI] [PubMed] [Google Scholar]
- 9. de Mascarel I, Brouste V, Asad‐Syed M, Hurtevent G, Macgrogan G. All atypia diagnosed at stereotactic vacuum‐assisted breast biopsy do not need surgical excision. Mod Pathol 2011; 24: 1198–206. [DOI] [PubMed] [Google Scholar]
- 10. Lavoué V, Roger CM, Poilblanc M et al Pure flat epithelial atypia (DIN 1a) on core needle biopsy: study of 60 biopsies with follow‐up surgical excision. Breast Cancer Res Treat 2011; 125: 121–6. [DOI] [PubMed] [Google Scholar]
- 11. Rajan S, Sharma N, Dall BJ, Shaaban AM. What is the significance of flat epithelial atypia and what are the management implications? J Clin Pathol 2011; 64: 1001–4. [DOI] [PubMed] [Google Scholar]
- 12. Ellis IO, Humphreys S, Michell M, Pinder SE, Wells CA, Zakhour HD. UK National coordinating committee for breast screening pathology; European commission working group on breast screening pathology. Best Practice No 179. Guidelines for breast needle core biopsy handling and reporting in breast screening assessment. J Clin Pathol 2004; 57: 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moinfar F, Man YG, Bratthauer GL, Ratschek M, Tavassoli FA. Genetic abnormalities in mammary ductal intraepithelial neoplasia‐flat type (“clinging ductal carcinoma in situ”): a simulator of normal mammary epithelium. Cancer 2000; 88: 2072–81. [PubMed] [Google Scholar]
- 14. Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long‐term follow‐up study. Cancer 1985; 55: 2698–708. [DOI] [PubMed] [Google Scholar]
- 15. Silverstein MJ, Poller DN, Waisman JR et al Prognostic classification of breast ductal carcinoma‐in‐situ . Lancet 1995; 345: 1154–7. [DOI] [PubMed] [Google Scholar]
- 16. Martel M, Barron‐Rodriguez P, Tolgay Ocal I, Dotto J, Tavassoli FA. Flat DIN 1 (flat epithelial atypia) on core needle biopsy: 63 cases identified retrospectively among 1751 core biopsies performed over an 8‐year period (1992–1999). Virchows Arch 2007; 451: 883–91. [DOI] [PubMed] [Google Scholar]
- 17. Rakha EA, Ho BC, Naik V et al Outcome of breast lesions diagnosed as lesion of uncertain malignant potential (B3) or suspicious of malignancy (B4) on needle core biopsy, including detailed review of epithelial atypia. Histopathology 2011; 58: 626–32. [DOI] [PubMed] [Google Scholar]
- 18. Senetta R, Campanino PP, Mariscotti G et al Columnar cell lesions associated with breast calcifications on vacuum‐assisted core biopsies: clinical, radiographic, and histological correlations. Mod Pathol 2009; 22: 762–9. [DOI] [PubMed] [Google Scholar]
- 19. Jain RK, Mehta R, Dimitrov R et al Atypical ductal hyperplasia: interobserver and intraobserver variability. Mod Pathol 2011; 24: 917–23. [DOI] [PubMed] [Google Scholar]


