Abstract
The aims of the present study were to: (i) develop a clinically useful prognostic classification in Asian patients with metastatic renal cell carcinoma (RCC) by combining metastatic features with several pretreatment parameters; and (ii) evaluate the validity of this prognostic classification. Baseline characteristics and outcomes were collected for 361 patients who underwent interferon‐α‐based therapy between 1995 and 2005. Relationships between overall survival (OS) and potential prognostic factors were assessed using Cox's proportional hazard model. The predictive performance of the model was evaluated using bootstrap resampling procedures and by using an independent dataset obtained from randomly selected institutions. The predictive accuracy was measured using the concordance index (c‐index). Four factors were identified as independent prognostic factors: time from initial diagnosis to treatment, anemia, elevated lactate dehydrogenase (LDH), and poor prognostic metastatic group (liver only, bone only, or multiple organ metastases). Each patient was assigned to one of three risk groups: favorable risk (none or one factor; n = 120), in which median OS was 51 months; intermediate risk (two factors; n = 101), in which median OS was 21 months; and poor risk (three or four factors; n = 102), in which median OS was 10 months. The c‐index was 0.72 in the original dataset and 0.72 in 500 random bootstrap samples. In the independent dataset for external validation, the c‐index was 0.73. Thus, the new prognostic classification is easily applicable for Asian patients with previously untreated metastatic RCC and should be incorporated into patient care, as well as clinical trials performed in Asia.
With the advent of molecular targeted therapy, treatment for metastatic renal cell carcinoma (RCC) has changed markedly. At present, drugs that should be used for molecular targeted therapy are usually selected on the basis of the Memorial Sloan‐Kettering Cancer Center (MSKCC) risk classification.1, 2 This results from the fact that clinical trials of molecular targeted drugs, such as sunitinib and temsirolimus, have been performed by selecting subjects on the basis of this risk classification and their usefulness has been clarified.3, 4 In Japan, the MSKCC risk classification is widely used not only in clinical trials, but also in daily clinical practice.5, 6 However, whether this risk classification is useful for predicting outcomes in Japanese patients with metastatic RCC is not unclear. Recently, we performed a retrospective study on the MSKCC risk classification in patients with metastatic RCC and reported its applicability to Japanese patients.7 However, when this risk classification was used in Japanese RCC patients, the percentage of patients in the poor risk group was high compared with that in Western series and the survival period of the poor risk group was twice as long in Japanese patients as that in patients in Western countries.7, 8 This may be because multiple organ metastases are less frequently observed in Japanese patients and the status of metastases is not used as a prognostic variable in the MSKCC risk classification. The importance of the number of organs showing metastasis and metastatic sites on survival in patients with metastatic RCC has been pointed out by several investigators.9, 10 Thus, the aims of the present study were to develop a clinically available prognostic classification in Asian patients with metastatic RCC, who are still frequently treated with interferon (IFN)‐α‐based therapy, by combining metastatic features with several pretreatment parameters and to evaluate the validity of this prognostic classification by internal and external validation assessments.
Materials and Methods
Patient population
A total of 361 patients treated with IFN‐α‐based therapy as their initial treatment in Hokkaido University Hospital and hospitals of the Hokkaido Immunotherapy Research Group (Appendix I) between 1995 and 2005 were the subjects of the present retrospective analysis. Patients were included in the study if they had a clinical and pathological diagnosis of RCC, clinical confirmation of the presence of metastasis, no previous treatment of metastatic lesions, and course observation for ≥3 months, except for early fatal cases. The exclusion criteria were the presence of other clinical cancer, insufficient clinical data before and after treatment, and withdrawal from the study by the patient and/or his/her family. The study was approved by the Internal Review Boards of the participating institutes.
Clinical, pathological, and survival data were collected for each patient. The performance status was determined on the basis of the Eastern Cooperative Oncology Group Performance Status (ECOG‐PS) scale. Tumor staging was according to the 1997 TNM classification of the Union Internationale Contre le Cancer (UICC).11 Laboratory data included hemoglobin (Hb), serum lactate dehydrogenase (LDH), corrected Ca (cCa), and C‐reactive protein (CRP) at the initial diagnosis of metastasis. The LDH values were standardized against the upper limit of normal (ULN) in each participating hospital when appropriate. The pathological grade was determined on the basis of the General Rules for Clinical and Pathological Studies on Renal Cell Carcinoma in Japan.12 Tumor histology was classified into three groups: clear cell carcinoma, clear cell carcinoma with sarcomatoid features, and non‐clear cell carcinoma.
Statistical analysis
Patient characteristics are shown as the median for continuous variables and the number of patients with percentages for categorical variables. The endpoint of the present study was OS, which was calculated from the date when IFN‐α‐based therapy started to the date of death as a result of any cause (or censored at the date of the last follow‐up). Molecular targeted therapeutic drugs were administered during the treatment course in a few patients; in these patients, the day before the initiation of molecular targeted drugs was regarded as the final observation day. Survival distributions were estimated using the Kaplan–Meier method. Median and 2‐year OS, along with 95% confidence intervals (CI), are reported. Associations between OS and potential prognostic factors were assessed using the log‐rank test and Cox's proportional hazards model in univariate analysis. If the prognostic factor had P < 0.05, then it could be considered as relevant and was included in multivariate analysis. Then, the preselected prognostic factors were included in the multivariate Cox's proportional hazard model using a stepwise procedure, with a significance level of 0.10 for entering and removing variables. Once the prognostic factors were identified and the final model was formed, a risk group variable was created by counting the number of unfavorable features presented for each patient. The discrimination of the prognosis after 2 years of the prognostic classification was measured using the concordance index (c‐index).13
For internal validation, we assessed the predictive performance of the final model by using bootstrap resampling procedures.14 Five hundred bootstrap samples were generated randomly with replacement from the original study population (n = 371). The stepwise Cox regression procedure was used for each sample with the same selection criteria as the original modeling. We calculated the frequency of each variable that was included in the resulting models from the 500 bootstrap samples. Furthermore, for each of the samples, we refitted Cox's regression model by using the variables selected in the final model and we calculated the regression parameters and hazard ratios. The means and 95% CI were computed from the 500 samples and were compared with the model by using the original study population. The c‐index analysis was also calculated using the bootstrap resampling procedures. The 95% CI for the c‐index was calculated on the basis of 500 bootstrap resamplings. Minimum and maximum resampled estimates of the c‐index are also reported.
For external validation, we assessed the predictive activity of the present risk classification by using the 137 metastatic RCC Japanese patients who were independently treated with IFN‐α‐based therapy in four other institutes. By using the present prognostic classification, the patients were again classified into risk groups, as mentioned above, and median OS along with 95% CI is reported for each risk group. Discrimination of the prognosis after 2 years of the risk classification was evaluated with the c‐index, which was compared with the c‐index obtained from the original dataset.
For all statistical analyses, P < 0.05 was considered significant. All statistical analyses were performed using SAS version 9 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics and outcome
The distribution of baseline characteristics for the 361 patients is presented in Table 1. At the time of analysis, 114 patients (32%) were still alive, 240 patients (66%) had died of RCC, and seven patients (2%) had died of other causes. The median follow‐up was 21.5 months (range: 1–177 months). Three hundred and forty‐five patients received natural IFN‐α, whereas the remaining 16 patients were treated with recombinant IFN‐α. Eighty‐nine patients (25%) underwent metastasectomy during the treatment course. The median OS for the entire cohort of 361 patients was 26 months, with a 2‐year OS rate for the entire cohort of 52% (95% CI 47–57%; Fig. 1).
Table 1.
Patient characteristics
| Gender (%) | |
| No. men | 262 (73) |
| No. women | 99 (27) |
| Median (range) age (years) | 63 (32–85) |
| Metastasis at initial diagnosis of RCC (%) | |
| Yes | 205 (57) |
| No | 156 (43) |
| ECOG‐PS (%) | |
| 0 | 207 (57) |
| 1 | 88 (24) |
| ≥2 | 56 (16) |
| Unknown | 10 (3) |
| Prior nephrectomy (%) | 293 (81) |
| No. metastatic sites (%) | |
| 1 | 214 (59) |
| 2 | 104 (29) |
| ≥3 | 43 (12) |
| Sites of metastasis (%) | |
| Lung | 251 (70) |
| Bone | 104 (29) |
| Lymph nodes | 73 (20) |
| Liver | 43 (12) |
| Histology (%) | |
| CCRCC only | 231 (64) |
| CCRCC with sarcomatoid | 24 (7) |
| Non CCRCC | 40 (11) |
| Unknown | 66 (18) |
Unless indicated otherwise, data show the number of patients in each group with percentages given in parentheses. CCRCC, clear cell renal cell carcinoma; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; RCC, renal cell carcinoma.
Figure 1.
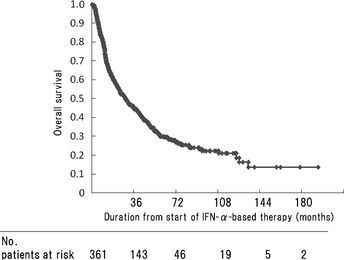
Overall survival of 361 patients with metastatic renal cell carcinoma. At the time of the last follow‐up, 114 patients were alive. IFN‐α, interferon‐α.
Survival analysis
Relationships between potential prognostic variables and the OS were investigated (Table 2). Median OS was significantly shorter in patients with liver‐only or bone‐only metastasis than in those with lung‐only, lymph node‐only, or other organ‐only metastasis. The median OS of the former group (liver‐only or lung‐only) was almost compatible with that of patients with multiple organ metastases. Therefore, we grouped liver‐only metastasis and bone‐only metastasis together with multiple organ metastases as a possible prognostic variable. Clinical and laboratory features that were significantly associated with poor OS included metastasis at the initial diagnosis of RCC, poor prognostic metastatic group, no prior nephrectomy, time from initial diagnosis to treatment <1 year, an ECOG‐PS >0, anemia, hypercalcemia, elevated LDH, and elevated CRP. The significant pretreatment prognostic variables on univariate analysis were selected for the multivariate analysis (Table 3). Because previous nephrectomy was strongly correlated with time from initial diagnosis of RCC to the start of IFN‐α‐based therapy, the latter was selected for multivariate analysis. Because there were a lot of missing data for serum CRP values, this variable was omitted. Of the significant prognostic variables on univariate analysis, four variables were identified as independent poor prognostic factors: time from initial diagnosis to treatment <1 year, anemia, elevated LDH, and poor prognostic metastatic group.
Table 2.
Univariate survival analysis
| Factor | n (%) | Median OS (95% CI) | Hazard ratio | P‐value |
|---|---|---|---|---|
| Age (years) | ||||
| ≥65 | 162 (45) | 25 (18–34) | 1.180 | |
| <65 | 199 (55) | 27 (21–36) | 1 | 0.197 |
| Metastasis at initial diagnosis of RCC | ||||
| Yes | 205 (57) | 14 (12–20) | 2.234 | |
| No | 156 (43) | 46 (37–65) | 1 | <0.001 |
| No. metastatic sites | ||||
| ≥2 | 147 (41) | 14 (11–22) | 1.451 | |
| 1 | 214 (59) | 33 (25–40) | 1 | 0.004 |
| Metastatic sites | ||||
| Lung | ||||
| Yes | 251 (70) | 25 (18–31) | 1.187 | |
| No | 110 (30) | 33 (19–45) | 1 | 0.218 |
| Lung only | 129 (36) | 32 (22–42) | 0.811 | |
| Others | 232 (64) | 22 (16–30) | 1 | 0.123 |
| Lymph nodes | ||||
| Yes | 73 (20) | 14 (11–35) | 1.403 | |
| No | 288 (80) | 27 (22–36) | 1 | 0.023 |
| Lymph nodes only | 14 (4) | 38 (12–59) | 0.956 | |
| Others | 347 (96) | 25 (20–32) | 1 | 0.885 |
| Bone | ||||
| Yes | 104 (29) | 18 (12–22) | 1.708 | |
| No | 257 (71) | 33 (24–42) | 1 | <0.001 |
| Bone only | 35 (10) | 21 (14–27) | 1.540 | |
| Others | 326 (90) | 27 (20–36) | 1 | 0.024 |
| Liver | ||||
| Yes | 43 (12) | 12 (9–13) | 1.875 | |
| No | 318 (88) | 31 (22–37) | 1 | <0.001 |
| Liver only | 11 (3) | 12 (8–24) | 1.653 | |
| Others | 350 (97) | 27 (21–35) | 1 | 0.136 |
| Prognostic metastatic group | ||||
| Liver only or bone only or >2 sites | 193 (53) | 16 (12–22) | 1.800 | |
| Others | 168 (47) | 41 (32–53) | 1 | <0.001 |
| Previous nephrectomy | ||||
| No | 68 (19) | 8 (6–9.) | 4.872 | |
| Yes | 293 (81) | 37 (29–44) | 1 | <0.001 |
| Time from initial diagnosis to treatment | ||||
| <12 months | 251 (70) | 15 (12–20) | 3.095 | |
| ≥12 months | 110 (30) | 65 (49–162) | 1 | <0.001 |
| ECOG‐PS | ||||
| ≥1 | 144 (40) | 12 (10–16) | 2.322 | |
| 0 | 207 (57) | 41 (32–51) | 1 | <0.001 |
| Unknown | 10 (3) | 99 (7–69) | ||
| Hemoglobin (mg/dL) | ||||
| <13.0/11.5 | 184 (51) | 15 (11–19) | 2.036 | |
| ≥13.0/11.5 | 140 (39) | 44 (32–53) | 1 | <0.001 |
| Unknown | 37 (10) | 36 (18–44) | ||
| Lactate dehydrogenase | ||||
| >1.5× ULN | 23 (6) | 9 (6–10) | 2.226 | |
| ≤1.5× ULN | 299 (83) | 28 (22–36) | 1 | <0.001 |
| Unknown | 39 (11) | 29 (12–44) | ||
| Corrected Ca (mg/dL) | ||||
| >10 | 24 (7) | 11 (5–13) | 2.278 | |
| ≤10 mg/dL | 301 (83) | 27 (21–35) | 1 | <0.001 |
| Unknown | 36 (10) | 36 (18–60) | ||
| C‐Reactive protein (mg/dL) | ||||
| >0.3 | 174 (48) | 14 (11–18) | 3.031 | |
| ≤0.3 mg/dL | 78 (22) | 53 (48–118) | 1 | <0.001 |
| Unknown | 109 (30) | 29 (20–40) | ||
CCRCC, clear cell renal cell carcinoma; CI, confidence interval; ECOG‐PS, Eastern Cooperative Oncology Group Performance Status; OS, overall survival; RCC, renal cell carcinoma; ULN, upper limit of normal.
Table 3.
Multivariate analysis based on pretreatment factors in 361 patients who received interferon‐α‐based therapy
| Factor | Poor prognostic category | Hazard ratio | P‐value |
|---|---|---|---|
| Time from initial diagnosis to treatment | <12 months | 2.596 | <0.001 |
| Hb | <13.0/11.5 mg/dL | 1.681 | <0.001 |
| LDH | >1.5× ULN | 1.883 | 0.012 |
| Prognostic metastatic group | Liver only, bone only multiple metastases | 1.479 | 0.005 |
Hb, hemoglobin; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Prognostic classification model
We established the following new prognostic classification (Japanese Metastatic Renal Cancer [JMRC] prognostic classification) of patients into three groups using these four factors: favorable risk group (no. poor prognostic factors = 0 or 1), intermediate risk group (no. poor prognostic factors = 2), and poor risk group (no. poor prognostic factors = 3 or 4). This prognostic classification was applied to all 361 patients and the survival time was compared among the risk groups (Fig. 2). There were 120 (33%), 101 (28%), and 102 (28%) patients in the favorable, intermediate, and poor risk groups, respectively; 39 patients (11%) could not be classified. The median OS periods were 51, 21, and 10 months in the favorable, intermediate, and poor risk groups, respectively (Table 4). The OS rates at 2 years were 75% (95% CI 66–83%), 47% (95% CI 37–57%), and 25% (95% CI 16–33%) in the favorable, intermediate, and poor risk groups, respectively. The c‐index was 0.72 when the JMRC prognostic classification was applied to these 361 patients.
Figure 2.
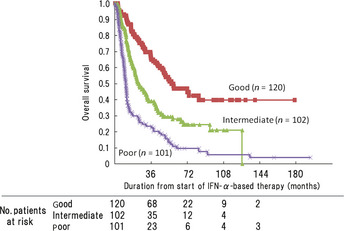
Overall survival stratified according to Memorial Sloan‐Kettering Cancer Center (MSKCC) risk classification (n = 323) into good, intermediate, and poor risk groups. Thirty‐eight patients for whom one or more of the risk factors were missing were excluded from the analysis. IFN‐α, interferon‐α.
Table 4.
Survival outcome in each risk group classified by the Japanese Metastatic Renal Cancer prognostic classification (original data)
| Risk group | n (%) | Median OS (months) | 2‐year OS (95% CI) |
|---|---|---|---|
| Favorable | 120 (33) | 51 | 77 (72–81) |
| Intermediate | 101 (28) | 21 | 50 (44–55) |
| Poor | 102 (28) | 10 | 29 (25–34) |
| Unclassified | 38 (11) | 36 | 63 (58–68) |
Favorable risk, poor prognostic factor = 0 or 1; intermediate risk, poor prognostic factor = 2; poor risk, poor prognostic factor = 3 or 4; CI, confidence interval; OS, overall survival.
These 361 patients were also classified according to the MSKCC risk classification1 (Table 5). There were 48 (13%), 166 (46%), and 104 (29%) patients in the favorable, intermediate, and poor risk groups, respectively; 43 patients (12%) could not be classified. The median OS was not reached in the favorable risk group and was 26 and 10 months in the intermediate and poor risk groups, respectively. As shown in Table 5, 70 (42%) and 24 (14%) of 166 patients who were classified as being at intermediate risk by the MSKCC classification were considered to have favorable risk and poor risk by the JMRC classification, respectively. There were significant differences in OS among these three risk groups (P = 0.002). Although 28 (27%) of 104 patients who were considered to be at poor risk according to the MSKCC risk classification were classified as being at intermediate risk by the JMRC classification, there was no significant difference in the OS among these patients.
Table 5.
Comparison of Japanese Metastatic Renal Cancer prognostic classification and Memorial Sloan‐Kettering Cancer Center risk classification
| MSKCC risk group | JMRC risk groups | |||||
|---|---|---|---|---|---|---|
| Favorable | Intermediate | Poor | ||||
| No. patients | Median OS (months) | No. patients | Median OS (months) | No. patients | Median OS (months) | |
| Favorable | 48 | NR | 0 | 0 | ||
| Intermediate | 70 | 34 | 72 | 26 | 24 | 11 |
| Poor | 0 | 28 | 12 | 76 | 10 | |
A total of 43 patients were unclassified by either the Japanese Metastatic Renal Cancer (JMRC) prognostic classification or the Memorial Sloan‐Kettering Cancer Center (MSKCC) risk classification. NR, not reach; OS, overall survival.
We further applied the JMRC classification to 129 patients with pulmonary‐only metastasis. There were 67 (52%) and 48 (37%) patients in the favorable and intermediate risk groups, respectively, and 14 patients could not classified. The median OS periods were 43 and 18 months in the favorable and intermediate risk groups, respectively.
Internal validation of the JMRC prognostic classification using the bootstrap method
Using original datasets in 361 patients, the validity of the JMRC prognostic classification was determined using the bootstrap method. Table 6 shows the results of multivariate analysis using the bootstrap method. The hazard ratio was 2.654 for the time from initial diagnosis to treatment, 1.680 for Hb, 2.042 for LDH, and 1.482 for poor metastatic site. These results were similar to the hazard ratios for the original dataset. The c‐index based on bootstrap samples was 0.72, which was similar to that for the original dataset.
Table 6.
Multivariate analysis based on pretreatment factors in bootstrap dataset
| Factor | Poor prognostic category | Hazard ratio | 95% CI |
|---|---|---|---|
| Time from initial diagnosis to treatment | <12 months | 2.654 | 1.444–3.515 |
| Hb | <13.0/11.5 mg/dL | 1.680 | 1.089–2.220 |
| LDH | >1.5× ULN | 2.042 | 0.310–2.934 |
| Prognostic metastatic group | Liver only, bone only multiple metastases | 1.482 | 0.981–1.925 |
CI, confidence interval; Hb, hemoglobin; LDH, lactate dehydrogenase; ULN, upper limit of normal.
External validation of the JMRC prognostic classification using an independent dataset from four Japanese institutions
For external validation, the JMRC prognostic classification was applied to 136 patients with metastatic RCC treated with IFN‐α‐based therapy during the same period in four institutions across Japan and OS evaluated (Table 7). All 136 patients received natural IFN‐α. There were 34 (25%), 50 (37%), and 48 (35%) patients in the favorable, intermediate, and poor risk groups, respectively, and four patients (3%) could not be classified. The median OS was 56, 28, and 12 months in the favorable, intermediate, and poor risk groups, respectively. The OS rates at 2 years were 79% (95% CI 73–86%), 60% (95% CI 52–68%), and 40% (95% CI 31–48%) in the favorable, intermediate, and poor risk groups, respectively. The c‐index was 0.73 when the JMRC prognostic classification was applied to independent dataset.
Table 7.
Survival outcome in each risk group classified by the Japanese Metastatic Renal Cancer prognostic classification (independent data set)
| Risk group | n (%) | Median OS (months) | 2‐year OS (95% CI) |
|---|---|---|---|
| Favorable | 34 (25) | 56 | 79 (73–86) |
| Intermediate | 50 (37) | 28 | 60 (52–68) |
| Poor | 48 (35) | 12 | 40 (31–48) |
| Unclassified | 4 (3) | 9 | 20 (13–27) |
Favorable risk, poor prognostic factor = 0 or 1; intermediate risk, poor prognostic factor = 2; poor risk, poor prognostic factor = 3 or 4; CI, confidence interval; OS, overall survival.
Discussion
The present study shows that time from initial diagnosis to treatment <1 year, anemia, elevated LDH, and poor prognostic metastatic group are important factors indicating a poor prognosis and associated with OS. By combining these four factors, we developed a JMRC prognostic classification for patients with metastatic RCC. The validity of this prognostic classification was confirmed by internal validation using the bootstrap method, as well as by external validation using data from four randomly selected institutions across Japan.
Some risk classifications have been proposed for the evaluation of the outcomes of metastatic RCC. Of these, the most widely used system is the MSKCC risk classification, which was established on the basis of data from 400 patients who underwent cytokine therapy between 1975 and 1996. The MSKCC system consists of five poor prognostic factors (time from initial diagnosis to treatment <1 year, Karnofsky performance status <80%, anemia, elevated LDH, and elevated cCa).1 External validation of this risk classification was performed by the Cleveland Clinic Foundation (CCF) group, and its appropriateness has been confirmed.9 External validation of this risk classification system was also performed in Japan7 and the MSKCC risk classification system was shown to be applicable to Japanese RCC patients. However, when this classification was applied to Japanese patients, patients in the favorable risk group and those in the poor risk group accounted for 10% and 40% of all patients, respectively, a high percentage in the poor risk group compared with that in Western countries. In addition, even the poor risk group, which accounted for a high percentage of the total, had a survival period of 10 months, suggesting a marked difference between Japanese patients and patients in Western countries, even for those in the same poor risk group.7
Previous studies have demonstrated the effectiveness of cytokine therapy against pulmonary and lymph node metastases.15, 16 Mekhail et al.9 reported that the number of distant metastatic lesions was one of the significant prognostic factors in patients who underwent cytokine therapy. Negrier et al.10 reported that the number of metastases and the number of organs showing metastasis were prognostic factors. In the present study, liver‐only metastasis, bone‐only metastasis, and multiple organ metastases were included as possible poor prognostic variables for analysis. Multivariate analysis showed that poor prognostic metastatic group is an important factor for poor prognosis in addition to time from initial diagnosis to treatment <1 year, anemia, and elevated LDH in the MSKCC risk classification system.
Using these four factors, the JMRC prognostic classification system was developed and the system was tested to determine whether it allows for appropriate classification of the outcomes of the different patient groups. Approximately one‐third of patients were in each of the favorable, intermediate, and poor risk groups, with median survival periods of 51, 21, and 10 months, respectively. Comparing the JMRC classification system with the MSKCC system shows that our system can equally classify patients into three groups while overcoming the problem of the disproportion number of patients belonging to each risk group that is seen when the MSKCC risk classification system is applied to Japanese patients. This is because the JMRC classification system reclassifies the subset of patients identified by the MSKCC as having intermediate risk, but who actually have a better outcome, as having favorable risk, whereas those with a poorer prognosis are classified as having poor risk.
The JMRC prognostic classification may be applicable to many patients with metastatic RCC, but its consistency needs to be determined. Therefore, for internal validation, the bootstrap method was used with the original dataset and the hazard ratio for each poor prognostic factor used in the present study was determined and the c‐index confirmed. The hazard ratio obtained using bootstrap samples was similar to that using the original dataset, and the c‐index was 0.72, which was no different. These results suggest that there is no problem with the internal consistency of the JMRC prognostic classification. Subsequently, external validation of the risk classification system was performed. Using results of 137 patients who were treated in four institutions across Japan revealed no marked differences from the results using the original dataset. The c‐index for the 137 patients was 0.73, which was only 0.1 higher than that obtained using the original dataset. These results confirm the external consistency of the JMRC prognostic classification.
The results of the present study suggest that the JMRC prognostic classification is useful for Asian patients treated with cytokine therapy. However, because the present study was a retrospective analysis, there is the possibility of various biases (e.g. selection bias, treatment dose and duration of IFN‐α‐based therapy). Future prospective studies are needed to confirm the findings of the present study. In addition, it is not clear whether the JMRC prognostic classification system is applicable to patients undergoing molecular targeted therapy and this needs to be addressed in future studies.
Disclosure Statement
The authors declare that they have no conflicts of interest.
Acknowledgment
The authors thank Y. Shibasaki (Otsuka Pharmaceutical Co., Tokyo, Japan) for assistance on statistical analysis with the manuscript.
Appendix 1.
The members of the Hokkaido Immunotherapy Research Group are as follows: Yoshihiro Sasaki, Obihiro Kosei Hospital; Tango Mochizuki, Sapporo City Hospital; Akira Kashiwagi, Teine Keijinkai Hospital; Kouichi Kanagawa, Asahikawa City Hospital; Kinya Matsumura, Jinyukai Urological Hospital; Tatsuya Mori, Asahikawa Kosei Hospital; Yuichiro Shinno, Otaru City Hospital; Haruo Seki, Hokkaido Urological Memorial Hospital; Kunihiko Tsuchiya, Hakodate Central Hospital; Masashi Murakumo, Kushiro Rosai Hospital; Hidenori Katano, Iwamizawa City Hospital; Junri Shindo, Kushiro City Hospital; Ichiro Takeuchi, Tomakomai City Hospital; Sosyu Sato, Ebetsu City Hospital; Takanori Yamashita, Nayoro City Hospital; Shinji Kamoda, Abashiri Kosei Hospital; Shin Suzuki, Konan Medical Center; Rintaro Machino, KKR Tonan Medical Center; Kazushi Hirakawa, Keiyukai Cancer Hospital; Osamu Nonaka, Chitose City Hospital.
(Cancer Sci, 2012; 103: 1695–1700)
References
- 1. Motzer RJ, Bacik J, Murphy BA et al Interferon‐alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol 2002; 20: 289–96. [DOI] [PubMed] [Google Scholar]
- 2. Hudes G, Carducci M, Choueiri T et al NCCN Task Force report: optimizing treatment of advanced renal cell carcinoma with molecular targeted therapy. J Natl Compr Canc Netw 2011; 9 (Suppl): S1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Motzer RJ, Hutson TE, Tomczak P et al Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27: 3584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hudes G, Carducci M, Tomczak P et al Temsirolimus, interferon alfa, or both for advanced renal‐cell carcinoma. N Engl J Med 2007; 356: 2271–81. [DOI] [PubMed] [Google Scholar]
- 5. Tomita Y, Shinohara N, Yuasa T et al Overall survival and updated results from a phase II study of sunitinib in Japanese patients with metastatic renal cell carcinoma. Jpn J Clin Oncol 2010; 40: 1166–72. [DOI] [PubMed] [Google Scholar]
- 6. Yuasa T, Tsuchya N, Urakami S et al Clinical efficacy and prognostic factors for overall survival in Japanese patients with metastatic renal cell cancer treated with sunitinib. BJU Int 2012; 109: 1349–54. [DOI] [PubMed] [Google Scholar]
- 7. Shinohara N, Abe T, Mochizuki T et al Is Memorial Sloan‐Kettering Cancer Center risk classification appropriate for Japanese patients with metastatic renal cell carcinoma in the cytokine era? Urol Oncol 2011; doi: 10.1016/j.urolonc.2011.08.009 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8. Naito S, Yamamoto N, Takayama T et al Prognosis of Japanese metastatic renal cell carcinoma patients in the cytokine era: a cooperative group report of 1463 patients. Eur Urol 2010; 57: 317–25. [DOI] [PubMed] [Google Scholar]
- 9. Mekhail TM, Abou‐Jawde RM, BouMerhi G et al Validation and extension of the Memorial Sloan‐Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005; 23: 832–41. [DOI] [PubMed] [Google Scholar]
- 10. Negrier S, Escudier B, Gomez F et al Prognostic factors of survival and rapid progression in 782 patients with metastatic renal carcinomas treated by cytokines: a report from the Groupe Francais d'Immunotherapie. Ann Oncol 2002; 13: 1460–8. [DOI] [PubMed] [Google Scholar]
- 11. Sobin LH, Wittekind CH, eds. International Union Against Cancer (UICC). TNM Classification of Malignant Tumours, 5th edn New York: John Wiley & Sons, Inc., 1997. [Google Scholar]
- 12. Japanese Urological Association, The Japanese Society of Pathology and Japanese Radiological Society . General Rules for Clinical and Pathological Studies on Renal Cell Carcinoma, 4th edn Tokyo: Kanehara, 2011. [Google Scholar]
- 13. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–87. [DOI] [PubMed] [Google Scholar]
- 14. Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall, 1993. [Google Scholar]
- 15. Miyake H, Kurahashi T, Takenaka T, Inoue T, Fujisawa F. Clinical outcome of combined immunotherapy with interferon‐α and low‐dose interleukin‐2 for Japanese patients with metastatic renal cell carcinoma. Urol Oncol 2009; 27: 598–603. [DOI] [PubMed] [Google Scholar]
- 16. Akaza H, Kawai K, Tsukamoto T et al Successful outcomes using combination therapy of interleukin‐2 and interferon‐α for renal cell carcinoma patients with lung metastasis. Jpn J Clin Oncol 2010; 40: 684–9. [DOI] [PubMed] [Google Scholar]


