Abstract
Hepatocellular carcinoma (HCC) is one of the most deadly human cancers because of its high incidence of metastasis. Despite extensive efforts, therapies against metastasis of HCC remain underdeveloped. Vacuole membrane protein 1 (Vmp1) was recently identified to be involved in cancer‐relevant processes; however, its expression, clinical significance and biological function in HCC progression are still unknown. Therefore, we evaluated the expression of Vmp1 in human HCC specimens. To functionally characterize Vmp1 in HCC, we upregulated its expression in HCCLM3 cells using a plasmid transfection approach, following which both in vitro and in vivo models were used to elucidate its role. A significant downregulation of Vmp1 was found in human HCC tissues and closely correlated with multiple tumor nodes, absence of capsular formation, vein invasion and poor prognosis of HCC. Such expression was verified with HCC cell lines including HepG2, MHCC97‐L and HCCLM3, and the Vmp1 expression levels negatively correlated with metastatic potential. Interestingly, upregulation of Vmp1 significantly affects proliferation, migration, invasion and adhesion of HCCLM3 cells. Using a mouse model, we demonstrated that upregulation of Vmp1 was associated with suppression of growth and pulmonary metastases of HCC. Therefore, our data suggest Vmp1 is a novel prognostic marker and potential therapeutic target for metastasis of HCC.
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of death from cancer, resulting in more than 600 000 deaths per year.1, 2 During the past decade, the long‐term survival rate remains unsatisfactory because of a high incidence of recurrence and metastasis after hepatic resection, with a 5‐year actuarial recurrence rate of 75–100% reported in the literature.3 To predict recurrence, metastasis and prognosis in patients with HCC after hepatic resection is a clinical issue of great significance. Various prognostic markers of HCC have been identified, such as vascular endothelial growth factor (VEGF), osteopontin (OPN) and transforming growth factor‐β (TGF‐β). Based on a genomic analysis, we previously reported ras homologous gene C (RhoC), high mobility group A1 (HGMA1) and Wiskott–Aldrich syndrome protein family verprolin‐homologous protein 2 (WAVE2) as potential markers for metastasis of HCC.4, 5, 6, 7 All these findings represent significant progress in this field. However, the mechanisms underlying HCC metastasis are still not fully understood, supporting a need for further studies. Thus, exploring new prognostic markers and further inhibition of invasion and metastasis is of great importance in HCC therapies.8
It is well known that reduced levels of cell–cell adhesion proteins often correlate with tumor invasion and metastasis.9 The loss of functional tight junctions would further result in changes of cytoskeletal organization, which in turn might lead to a higher invasive potential of the tumor cells.10 Vacuole membrane protein 1 (Vmp1) is a conserved putative membrane protein whose function is now beginning to be elucidated. In Drosophila, Vmp1 (known as TANGO5) was identified in a functional genomic screen and found to be required for protein secretion and Golgi organization.11 It has previously been reported that the overexpressed protein is an inhibitor of cell proliferation, anchorage‐independent growth12 and secretory membrane transport.13 Moreover, recent studies have demonstrated that Vmp1 is necessary in autophagy and its expression induces the formation of autophagosomes.14, 15 Interestingly, the expression profiling on a cDNA array and another complementary DNA microarray revealed that VMP1 was downregulated in renal cell carcinoma.16, 17 Furthermore, Vmp1 is an essential component of initial cell–cell contacts and tight junction formation where it colocalizes with zonula occludens‐1 (ZO‐1) in spots between neighboring cells. Downregulation of VMP1 by RNAi results in loss of cell adherence and increased invasion capacity.18
However, evidence for the function of Vmp1 in human malignancies is still limited, especially in the case of HCC. Therefore, we carried out the present study to determine the expression of Vmp1 in human HCC tissues as well as cell lines. Furthermore, the biological functions of Vmp1 in HCC were also elucidated in vitro and in vivo.
Materials and Methods
Ethics statement
All research involving human samples were approved by the institutional review board of Xiangya Hospital, Central South University. We obtained ethics approval for the present study from the ethics committee at our institution and at all institutions/hospitals where participants were recruited. Also, we obtained informed written consent from all participants involved in the present study.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Xiangya Hospital, Central South University. All surgery was performed under sodium pentobarbital anesthesia and all effort was made to minimize suffering.
Patients and tissue specimens
Specimens of HCC tissues were obtained from 124 consecutive HCC patients who underwent hepatic resection at the Department of Surgery, Xiangya Hospital of Central South University (CSU) from October 2005 to September 2007. These patients included 108 males and 16 females with a median age of 48 years, ranging 24–71 years. Among these 124 cases of HCC, five cases of portal vein tumor thrombosis (PVTT) samples, matched fresh specimens of HCC and paracarcinomatous liver tissue (PCLT) from 38 cases were collected. Six samples of normal liver tissues obtained from patients with cavernous hemangioma who underwent hepatic resection were also included as a control. The diagnoses were confirmed by histopathological study.
Reverse transcription and polymerase chain reaction (RT‐PCR)
The expected size of VMP1 is a 242 bp fragment. Primers synthesized by Boshang Biotechnology Company (Shanghai, China) were as follows: forward: 5′‐GTGGCTTTCATTGGTG CTGTCC‐3′; and reverse: 5′‐GAGTTCAACCGCTGCTGGA TTC‐3′. Glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) gene expression was used as a control. The level of Vmp1 mRNA expression was expressed as the relative intensity of the PCR product bands from target sequences compared with that from the GAPDH gene. The PCR experiments were done in triplicate.
Quantitative real‐time reverse transcription polymerase chain reaction (qRT‐PCR)
VMP1 and GAPDH were used as the target gene and internal loading control, respectively. Primers synthesized by Takara Biotechnology Company (Dalian, China) were as follows: VMP1‐forward: 5′‐TTTCAGGAGTACCTGGAGG CTCA‐3′; VMP1‐reverse: 5′‐CTGCTGGATTCGTTTGGCAT AA‐3′; GAPDH‐forward: 5′‐GCA.CCGTCAAGGCTGAGAA C‐3′; and GAPDH‐reverse: 5′‐TGGTGAAGACGCCA GTGG A‐3′. All amplification reactions were performed in triplicate. The PCR product quality was monitored using post‐PCR melt curve analysis. Reactions were carried out in a 96‐well plate using the ABI Prism 7300 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA).
Western blot
Total protein (100 ug) was separated using SDS‐PAGE and then transferred onto nitrocellulose membrane (Sigma, St Louis, MO, USA). The blotted membranes were incubated with mouse anti‐human Vmp1 polyclonal antibody (Abnova, Taiwan, China) diluted at 1:500. After washing, the membranes were incubated with a 1:3000 dilution of horseradish peroxidase‐linked goat anti‐mouse antibody (Biotechnology Inc., Santa Cruz, CA, USA). The blots were developed using enhanced SuperSignal West Pico chemiluminescence (Pierce, Rockford, IL, USA). Beta‐actin protein was also determined by using the specific antibody (Sigma) as a loading control. All experiments were carried out in triplicate.
Immunohistochemistry
The formalin‐fixed paraffin sections were stained for Vmp1 (Abnova, 1:200) using the Streptavidin–Peroxidase system (Zhongshan Goldenbridge Biotechnology, Beijing, China). The Vmp1 protein expression in HCC specimens was scored as 0 to 3+ using the Shimizu criteria.19 The expression levels of Vmp1 protein were thus divided into low expression (0 or 1+) and high expression (2+ or 3+). Immunohistochemical analysis and scoring were performed by two independent investigators.
Follow‐up study
Follow‐up data were obtained by reviewing the hospital records or by direct communication with the patients after hepatic resection for all 124 patients. The follow‐up period was defined from the date of surgical excision of the tumor to the date of death or last follow up. Deaths from other causes were treated as censored cases. Patients whose death was clearly documented as attributable to HCC were considered to have died of that disease; other deaths were not considered to be caused by HCC. The disease‐free survival was defined as the length of time after hepatic resection for HCC during which a patient survives with no evidence of HCC. The follow‐up time ranged 30–870 days, with a median follow‐up time of 320 days. To determine factors influencing survival after liver resection, nine conventional variables together with Vmp1 expression were tested in all 124 patients: age (≤60 years vs > 60 years), gender, HBV infection (presence vs absence), cirrhosis (presence vs absence), Edmondson–Steiner grade (I–II vs III–IV), capsule (presence vs absence), size of the tumor (≤5 cm vs > 5 cm), the number of tumor nodes (solitary vs multiple), vein invasion (absence vs presence) and Vmp1 protein expression level (high vs low).7
Cell lines and cell culture
HCCLM3 and MHCC97‐L cell lines were purchased from Liver Cancer Institute of Fudan University (Shanghai, China). HepG2 and CCL13 cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA).
Construction of plasmid pCMVtag2C‐VMP1
The human VMP1 gene was obtained by PCR using the CCL13 cell cDNA library as the template according to standard procedures. The forward and reverse primers were as follows: VMP1‐S: 5′‐AG CGGCTCCTCAAGAGTT‐3′ and VMP1‐A: 5′‐GCAGAACCC ATCCACTCC‐3′. The size of the amplified VMP1 cDNA fragment (1298 bp) was confirmed by electrophoresis in 1.5% (w/v) agarose and purified from the gel, and the bands were cloned into pMD18‐T vector (Takara) for obtaining multiple VMP1 copies.
In order to construct recombined vector pCMVtag2C‐VMP1, another pair of primers was designed: the forward primer (VMP1‐S2: 5′‐GGATCCAAATGGCAGAGAATGGAA‐3′) and reverse primer (VMP1‐A2: 5′‐GTCGACCTTATTTAGTT TTC. TCCTC‐3′) incorporated the recognition site for the BamHI and SalI site at the 5′ end, respectively. After double digestion, the fragment was ligated into pCMVtag2C (Stratagene, La Jolla, CA, USA) using the DNA Ligation Kit Ver. 2 (Takara).
Construction stable cell lines
Sixteen to twenty hours prior to transfection, exponentially growing cells were seeded (105 cells/mL) on sterile microscope cover glasses and placed in a 35 mm Petri dish. We transfected HCCLM3 cells with pCMVtag2C‐VMP1 using FuGENE 6 transfection reagent (Roche, Basel, Switzerland), according to the manufacturer's protocol. The transfected cells were selected by 400 μg G418 (Geneticin sulfate, GIBCO, Grand Island, NY, USA) per mL and maintained by 200 μg G418 per mL. After 2–3 weeks screening we established the stable transfective clone.
Wound‐healing, transwell and proliferation assays
Methods for the wound‐healing and transwell assays have been described previously.20 The proliferation of HCCLM3 was assessed using the MTT method. Briefly, HCCLM3 cells were plated in 48‐well plates at 2 × 104 per well. After incubation for 24 h, 20 μL MTT with 5 mg/mL concentration was added to the medium and cultured for another 4 h. The medium was then abandoned and 150 μL of DMSO was added into each well, rocked for 10 min and the absorbencies of each well were read using a microplate reader at a wavelength of 490 nm. Semi‐logarithmic curves were drawn with cell viability using Microsoft Excel 2003 software (Microsoft Corporation, Redmond, WA, USA).
Adhesion assay
For heterogeneity adhesion analysis, a 96‐well plate was coated with fibronectin at 37°C for 1 h and washed twice with washing buffer (0.1% BSA in DMEM). Plates were blocked with blocking buffer (0.5% BSA in DMEM) at 37°C in a CO2 incubator for 60 min. The cells were then washed with washing buffer (0.1% BSA in DMEM). When the cell count reached 1 × 105/mL, 100 μL cells were added in each well and cultured for 60, 90 or 120 min at 37°C. The medium was entirely removed and unbound cells were washed away with PBS. Cells were stained with 20 μL MTT (5 mg/mL). Cell adhesion was quantified using a colorimetric ELISA plate reader at 570 nm. Differences in the homogeneity adhesion analysis were: cells, instead of fibronectin, were used to adhere to 96‐well culture plates to form the monolayer; and adherent cells were quantified using microscopy.
In vivo HCC metastatic mouse model
A metastatic human HCC cell BALB/c nude mice model was established according to existing protocol.21 Briefly, cells (5 × 106) of HCCLM3VMP1+ or HCCLM3vector were injected subcutaneously into the right upper flank of seven male BALB/c nude mice (3–4 weeks of age). Each HCCLM3 subcutaneous tumor was measured every 5 days and mice were killed 35 days after implantation. After macroscopic examination, the subcutaneous tumor and lung were removed and fixed in 10% formalin, embedded in paraffin and cut into 4‐μm‐thick slices for standard histopathological study. The whole lung embedded in paraffin was cut into thick slices continuously for H&E staining and mice whose metastatic HCC cells were found on any slide of the lung sections were considered as lung metastasis positive. Paraffin sections embedded with the subcutaneous tumor were stained with H&E for histological examination and stained with anti‐Vmp1 antibody for evaluating transfection efficiency in vivo. The histopathological examination was carried out by a pathologist who was masked from the experimental group designation.
Co‐immunoprecipitation
HCCLM3VMP1+ cells were lysed with radioimmune precipitation assay (RIPA) lysis buffer. The cell lysates were incubated with rabbit anti‐human ZO‐1 polyclonal antibody (Santa Cruz) for 6 h at 4°C, and then the complexes of the antibodies and the target protein were precipitated with Protein A/G PLUS‐Agarose (Santa Cruz). The proteins were resolved using SDS‐PAGE (stacking gel 5% and 3.5%, separating gel 12% and 7%, respectively), and then the proteins on the gels were transferred electrophoretically to a nitrocellulose membrane (Sigma) at 100 V for 1.2 and 2.5 h, respectively. The membrane was probed with Vmp1 and ZO‐1 antibodies diluted 1:500, respectively. The cell lysate, flow through and wash before the elution were used as controls.
Statistical analysis
spss 13.0 for Windows (SPSS, Chicago, IL, USA) was used for statistical analysis. Fisher's exact test was used for statistical analysis of categorical data, whereas the independent t‐test was used for continuous data. Spearman's correlation coefficient was used to analyze the correlations between expression levels of Vmp1 mRNA and protein. Spearman correlation analysis was used to analyze the relationship between Vmp1 expression levels and clinicopathological variables of HCC. Survival curves were plotted using the Kaplan–Meier method and analyzed using the log‐rank test. In addition, the Cox proportional hazards regression model was used to identify factors that were independently associated with survival. All tests were two tailed and P < 0.05 was considered statistically significant.
Results
Vmp1 was significantly downregulated in human HCC and PVTT
To examine the expression of Vmp1 in HCC, we first used RT‐PCR to measure its mRNA levels in 38 HCC fresh tissues, paracarcinomatous liver tissues (PCLT), five portal vein tumor thromboses (PVTT) and six normal liver (NL) tissues. The HCC tissues expressed significantly lower mRNA levels of Vmp1 than PCLT (0.69 ± 0.40 vs 1.34 ± 0.53, P < 0.001) and NL (1.64 ± 0.67, P < 0.001); the level was even lower in PVTT (0.41 ± 0.13, P = 0.005; Fig. 1A) than in HCC. To obtain more precise data of the Vmp1 mRNA level in HCC, further qRT‐PCR performed in the same samples confirmed that Vmp1 mRNA in HCC was significantly lower than those in PCLT (P < 0.001) and NL (P = 0.035), and even lower in PVTT than in HCC (P = 0.027; Fig. 1B). Consistent with the mRNA expression, the expression of Vmp1 protein in HCC tissues was also significantly lower than those in the corresponding PCLT (0.64 ± 0.40 vs 0.98 ± 0.38, P < 0.001) and NL tissues (1.20 ± 0.50, P = 0.004), and even lower in PVTT (0.27 ± 0.09, P = 0.048; Fig. 1A) than in HCC. Furthermore, there was a significantly positive correlation between the expression level of Vmp1 mRNA and protein in HCC using Spearman's correlation coefficient analysis (r s = 0.872, P < 0.001; Fig. 1C).
Figure 1.

Reduced expression of vacuole membrane protein 1 (Vmp1) in hepatocellular carcinoma (HCC) tissues and HCC cells. (A) Representative results showed expression patterns of Vmp1 mRNA and protein in tissue samples determined using RT‐PCR and western blotting. The independent t test showed that the expression of Vmp1 mRNA and protein in HCC tissues was significantly lower than those in the corresponding paracarcinomatous liver tissue (PCLT) and normal liver (NL) tissues, and even lower in portal vein tumor thrombosis (PVTT) than in HCC. (B) For the quantitative real‐time PCR results, fold inductions were calculated using the formula 2−(ΔΔCT), and the average value of Vmp1 mRNA from PCLT was used to normalize (value set to 1) the corresponding average value of PVTT, HCC and NT. The result of PVTT/PCLT was 0.30, HCC/PCLT was 0.59 and NL/PCLT was 1.08. (C) Spearman's correlation coefficient was used to evaluate the correlation between mRNA and protein expression levels of Vmp1 in HCC. (D) Vmp1 mRNA and protein was examined in HepG2, MHCC97‐L and HCCLM3 HCC cell lines and the CCL13 liver cell line using RT‐PCR and western blotting.
Immunohistochemical staining showed a cytoplasmic (mostly) and membranic (in a punctate pattern) distribution of Vmp1. The Vmp1 protein was detected in 81 of 124 HCC tissues and 108 of 124 PCLT (Fig. 2). The positive expression rate of Vmp1 was significantly lower in HCC than that in PCLT (65.3% vs 87.1%, P < 0.001).
Figure 2.
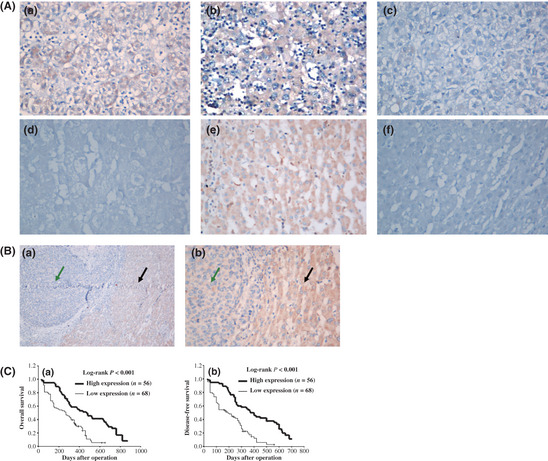
Immunohistochemistry of vacuole membrane protein 1 (Vmp1) expression in hepatocellular carcinoma (HCC) tissues and its prognostic implication. (A) Low expression of Vmp1 in HCC tissues (a–d) compared with high expression of Vmp1 in paracarcinomatous liver tissue (PCLT) (e) is shown; the negative control (f) is also included to show the specificity of the antibody. In these representative images, Vmp1 expression in the cytoplasm and membrane is scored as 3 + (a), 2 + (b), 1 + (c) and 0 (d) according to Shimizu criteria. Original magnification, ×400 (a–f). (B) Comparison of Vmp1 protein expression between HCC tissues (a) and PCLT (b). Original magnification, ×100 (a) and × 400 (b). Green arrows represent HCC and black arrows represent PCLT. (C) Kaplan–Meier survival curves of overall survival and disease‐free survival for the low Vmp1 expression group (scored as 0 and 1 +, n = 68) and the high Vmp1 expression group (scored as 2 + and 3 +, n = 56) based on the results of immunohistochemistry. The log‐rank test shows that HCC patients with low Vmp1 expression have lower overall survival (left) and disease‐free survival (right) than those with high expression of Vmp1.
Correlations of Vmp1 expression with clinicopathological characteristics and prognosis of HCC
Next we analyzed whether there was any association of Vmp1 expression with the clinicopathological characteristics of the HCC patients. Correlations between the expression levels of Vmp1 and clinicopathological variables are summarized in Table 1. The Vmp1 expression levels were found to be significantly lower in HCC with multiple nodules (P < 0.001), without capsule formation (P = 0.003) and with vein invasion (P < 0.001). There was no significant association between expression of Vmp1 and other clinicopathological parameters such as gender, HBV infection, liver cirrhosis, tumor size and tumor differentiation (P > 0.05). Furthermore, the expression level of Vmp1 protein was significantly downregulated in nodular HCC (NHCC) when compared with solitary large HCC (SLHCC) (P < 0.001) and small HCC (SHCC) (P < 0.001).
Table 1.
Correlations between Vmp1 protein expression and clinicopathological characteristics of 124 cases of HCC
| Clinicopathological parameter | n | Vmp1 expression | P | |||
|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |||
| Gender | ||||||
| Male | 108 | 35 | 24 | 38 | 11 | 0.653 |
| Female | 16 | 8 | 1 | 4 | 3 | |
| Age (years) | ||||||
| ≤60 | 95 | 33 | 20 | 30 | 12 | 0.983 |
| >60 | 29 | 10 | 5 | 12 | 2 | |
| HBV infection | ||||||
| Presence | 112 | 38 | 23 | 38 | 13 | 0.642 |
| Absence | 12 | 5 | 2 | 4 | 1 | |
| Liver cirrhosis | ||||||
| Presence | 97 | 34 | 21 | 31 | 11 | 0.650 |
| Absence | 27 | 9 | 4 | 11 | 3 | |
| Tumor size (cm) | ||||||
| ≤5 | 29 | 7 | 8 | 9 | 5 | 0.259 |
| >5 | 95 | 36 | 17 | 33 | 9 | |
| Tumor node no. | ||||||
| Multiple (≥2) | 57 | 38 | 7 | 11 | 1 | <0.001 |
| Solitary | 67 | 5 | 18 | 31 | 13 | |
| Capsular formation | ||||||
| Presence | 58 | 13 | 11 | 25 | 9 | 0.003 |
| Absence | 66 | 30 | 14 | 17 | 5 | |
| Edmondson–Steiner grade | ||||||
| I–II | 42 | 15 | 7 | 15 | 5 | 0.881 |
| III–IV | 82 | 28 | 18 | 27 | 9 | |
| Vein invasion | ||||||
| Presence | 98 | 39 | 22 | 31 | 6 | <0.001 |
| Absence | 26 | 4 | 3 | 11 | 8 | |
| Gross morphological types of HCC | ||||||
| NHCC | 54 | 38 | 6 | 9 | 1 | <0.001a |
| SLHCC | 52 | 5 | 14 | 25 | 8 | 0.226b |
| SHCC | 18 | 0 | 5 | 8 | 5 | <0.001c |
HBV, hepatitis B virus; HCC, hepatocellular carcinoma; NHCC, nodular HCC; SHCC, small HCC; SLHCC, solitary large HCC; Vmp1, vacuole membrane protein 1.
Comparison between NHCC and SLHCC.
Comparison between SLHCC and SHCC.
Comparison between NHCC and SHCC.
Based on the immunohistochemistry results, all 124 HCC patients were divided into two groups according to Vmp1 protein expression levels: the high expression group (scored as 2+ and 3+, n = 56) and the low expression group (scored as 0 and 1+, n = 68). The HCC patients in the low expression group had both poorer disease‐free survival (median disease‐free survival time, 190 vs 400 days, P < 0.001) and poorer overall survival (median overall survival time, 270 days vs 470 days, P < 0.001) than those of the high expression group (Fig. 2C). Using multivariable Cox regression analysis, low Vmp1 expression (relative risk [RR], 1.698; P = 0.037), multiple tumor node (RR, 1.934; P = 0.011) and vein invasion (RR, 1.779; P = 0.039) were found to be independent prognostic factors for overall survival (Table 2).
Table 2.
Cox regression analyses of overall survival and Vmp1 expression level, as well as clinicopathological parameters
| Clinicopathological parameter | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | ||
| Gender | |||||
| Male | 108 | 1 | 1 | 0.141 | |
| Female | 16 | 0.723 (0.395–1.324) | 0.293 | 0.622 (0.331–1.170) | |
| Age (years) | |||||
| ≤60 | 95 | 1 | 1 | 0.126 | |
| >60 | 29 | 0.837 (0.520–1.349) | 0.465 | 0.675 (0.408–1.117) | |
| HBV infection | |||||
| Absence | 12 | 1 | 1 | 0.556 | |
| Presence | 112 | 0.955 (0.515–1.772) | 0.884 | 0.815 (0.413–1.609) | |
| Liver cirrhosis | |||||
| Absence | 27 | 1 | 1 | 0.976 | |
| Presence | 97 | 1.245 (0.761–2.038) | 0.384 | 0.992 (0.587–1.676) | |
| Tumor size (cm) | |||||
| ≤5 | 29 | 1 | 1 | 0.580 | |
| >5 | 95 | 0.892 (0.553–1.439) | 0.640 | 0.867 (0.522–1.438) | |
| Capsular formation | |||||
| Presence | 58 | 1 | 1 | 0.484 | |
| Absence | 66 | 1.506 (1.009–2.248) | 0.045 | 1.200 (0.721–1.998) | |
| Edmondson–Steiner grade | |||||
| III–IV | 82 | 1 | 1 | 0.371 | |
| I–II | 42 | 0.797 (0.516–1.229) | 0.303 | 0.806 (0.503–1.292) | |
| Vein invasion | |||||
| Absence | 26 | 1 | 1 | 0.039 | |
| Presence | 98 | 2.191 (1.331–3.607) | 0.002 | 1.779 (1.029–3.076) | |
| Tumor node no. | |||||
| Solitary | 67 | 1 | 1 | 0.011 | |
| Multiple (≥2) | 57 | 2.562 (1.666–3.941) | <0.001 | 1.934 (1.161–3.222) | |
| Vmp1 expression | |||||
| High | 56 | 1 | 1 | 0.037 | |
| Low | 68 | 2.556 (1.635–3.995) | <0.001 | 1.698 (1.031–2.795) | |
CI, confidence interval; HBV, hepatitis B virus; RR, relative risk; Vmp1, vacuole membrane protein 1.
Reduced expression of Vmp1 correlates with increased metastatic potential in HCC cells
To further verify Vmp1 expression in HCC, we explored its expression in three HCC cell lines: HepG2, MHCC97‐L and HCCLM3, with a Chang liver cell line (CCL13) as a control. The results of RT‐PCR and western blot analyses confirmed the mRNA and protein expression of Vmp1 in HCC cells and showed a significantly lower expression of Vmp1 in HCCLM3 and MHCC97‐L cells than those in HepG2 and CCL13 cells (P < 0.05). Among the three cell lines analyzed, HCCLM3 cells had the lowest Vmp1 expression (P < 0.05), followed by MHCC97‐L and HepG2 (P < 0.05; Fig. 1D). Of note is that the reduced expression of Vmp1 was in agreement with the metastatic potential of these HCC cell lines,22 suggesting a potential role for Vmp1 in HCC metastasis.
Vmp1 affects proliferation, migration, invasion and adhesion of the HCCLM3 cell
To investigate the biological functions of Vmp1 in HCC, we constructed a Vmp1 stable overexpressed HCCLM3 cell line using the recombinant plasmid pCMVtag2C‐Vmp1. After 3 week selection in media supplied with G418, HCCLM3 cell lines were stably transfected with the VMP1‐expressing vector or control vector, and named as HCCLM3VMP1+ and HCCLM3vector respectively. The expression of Vmp1 mRNA and protein were markedly increased in HCCLM3VMP1+ compared with HCCLM3vector, which showed a satisfactory transfection efficiency (P < 0.01; Fig. 3).
Figure 3.

Construction of vacuole membrane protein 1 (Vmp1) stable overexpressed HCCLM3 cells. The expression of Vmp1 mRNA and protein in HCCLM3 VMP1+ and HCCLM3 vector cells was determined using RT‐PCR and western blot. Lanes 1, 2 and 3 in part (B) represent HCCLM3, HCCLM3vector and HCCLM3VMP1+, respectively.
With the Vmp1 upregulated cell line established, we began to characterize the cells by comparing proliferation rates of HCCLM3VMP1+ and HCCLM3vector cells using the MTT method. A significant difference in proliferation between the two cell lines was observed. As shown in Figure 4C, an obvious decrease of proliferation was observed in HCCLM3VMP1+ cells, which counts as a reduction of 36% at day 6 and 45% at day 7 compared with the HCCLM3vector cell (P < 0.05). Because the levels of Vmp1 expression correlated with the metastatic potentials, we investigated the effect of Vmp1 on migration and invasion of the HCCLM3 cell. As shown in Figure 4A, the wound‐healing assay showed that the closure of HCCLM3VMP1+ was significantly slower than that of HCCLM3vector, suggesting a role for Vmp1 in regulation of HCCLM3 cell migration. Next, a matrigel invasion assay in transwell culture chambers was performed to determine the effect of Vmp1 on the in vitro invasion of the HCCLM3 cell. As shown in Figure 4B, the number of HCCLM3VMP1+ cells that passed through matrigel was only 34% compared with HCCLM3vector cells (P < 0.05). Considering the close relation of Vmp1 with cellular adhesion, the adhesion test confirmed HCCLM3VMP1+ cells had significantly higher homogeneity adhesion and lower heterogeneity adhesion ability than HCCLM3vector cells (P < 0.01; Table 3). Together, these results support a critical role for Vmp1 in the adherence and metastasis of HCCLM3 cells.
Figure 4.

Vacuole membrane protein 1 (Vmp1) affects proliferation, migration and invasion of HCCLM3 cells. (A) A wound‐healing assay was used to determine the migration of HCCLM3VMP 1+ and HCCLM3vector cells. (B) Invasion assay results showed that upregulation of Vmp1 inhibited the invasion of HCCLM3. For transwell assays, HCCLM3VMP 1+ or HCCLM3vector cells were seeded into the upper chamber of the transwell and the cells that invaded through the pores to the lower surface of the filter were counted. (C) A MTT assay was performed to investigate the proliferation of HCCLM3VMP 1+ and HCCLM3vector cells. Every 24 h the absorbencies of test wells were read and semi‐logarithmic curves were drawn.
Table 3.
Effect of vacuole membrane protein 1 (Vmp1) on homogeneity adhesion (cell numbers) and heterogeneity adhesion (A 570) of HCCLM3 cells (mean ± SD, n = 6)
| Adhesion test | Time intervals | ||
|---|---|---|---|
| 60 min | 90 min | 120 min | |
| Homogeneity adhesion | |||
| HCCLM3VMP1+ | 1225 ± 65 | 1625 ± 90 | 1875 ± 125 |
| HCCLM3vector | 855 ± 45 | 1315 ± 70 | 1508 ± 85 |
| Heterogeneity adhesion | |||
| HCCLM3VMP1+ | 0.125 ± 0.032 | 0.152 ± 0.045 | 0.225 ± 0.082 |
| HCCLM3vector | 0.192 ± 0.040 | 0.275 ± 0.061 | 0.365 ± 0.102 |
Comparison of adhesion ability to homotypic cells or extracellular matrix components between HCCLM3VMP1+ and HCCLM3vector were made after the different time intervals. The HCCLM3VMP1+ cells had significantly higher homogeneity adhesion and lower heterogeneity adhesion ability than HCCLM3vector cells (P < 0.01).
In vivo inhibition of HCC proliferation and metastasis in the HCCLM3VMP1+ group
To validate the observations obtained from in vitro studies, we examined the in vivo relevance of the potential role for Vmp1 in HCC tumorigenesis and metastasis by using a mouse metastasis model. Primary subcutaneous tumors of HCCLM3 were measured every 5 days and mice were killed 35 days after implantation. We found that the average size of primary tumors in the HCCLM3VMP1+ group was dramatically smaller than that of the HCCLM3vector group (P < 0.05; Fig. 5B), which suggested that upregulation of Vmp1 in vivo could inhibit growth of HCC. The expression of Vmp1 in HCCLM3 tumors was determined to ensure the difference of Vmp1 expression in vivo. The results showed that the expression of Vmp1 was significantly increased in HCCLM3VMP1+ tumors compared with that in HCCLM3vector tumors (Fig. 5A). In light of the in vitro results implicating a role for Vmp1 in HCC cell adherence and metastasis, we examined the mice for lung metastasis of the carcinoma cells. Pulmonary metastasis was observed in the lung tissue sections of only two mice in the HCCLM3VMP1+ group (two of seven, 28.6%), significantly less than the ratio of pulmonary metastasis in the HCCLM3vector group (six of seven, 85.7%) (Fig. 5C; P = 0.031). Together, these data support an important role for Vmp1 in HCC metastasis.
Figure 5.

Upregulation of vacuole membrane protein 1 (Vmp1) inhibits tumorigenesis and metastasis of HCCLM3 cells in vivo. (A) Images a (HCCLM3VMP 1+ mice) and b (HCCLM3vector mice) were taken in the 35th day after implantation just before the mice were killed. Arrows indicate the primary tumors. H&E staining (c,d) and immunohistochemistry (e,f) examination of Vmp1 expression in HCCLM3 tumors was performed in mice of the HCCLM3VMP 1+ group (c,e) and the HCCLM3vector group (d,f). Original magnification, ×400 (c–f). (B) The primary tumor size was investigated in HCCLM3VMP 1+ and HCCLM3vector mice every 5 days after cell implantation. When the mice were all killed on the 35th day, the size of the primary tumors was measured with a caliper. (C) The pulmonary metastasis was observed in the lung tissue sections of only two mice in the HCCLM3VMP 1+ group (two of seven, 28.6%), significantly less than the ratio of pulmonary metastasis in the HCCLM3vector group (six of seven, 85.7%). (a–d) Metastatic human HCC cells in H&E‐stained lung sections from HCCLM3vector and HCCLM3VMP 1+ mice. Arrows indicate lung metastasis. Original magnification, ×100 (a,c) and × 400 (b,d).
Direct interaction of Vmp1 and ZO‐1 in HCC cells
Recent studies have indicated that Vmp1 is essential for cell–cell contacts and tight junction formation where it colocalizes with the tight junction protein ZO‐1 in spots between neighboring cells.18, 23, 24 To further confirm the subcellular localization of Vmp1 and its regulating mechanism, co‐immunoprecipitations with HCCLM3VMP1+ cell lysates were performed using a ZO‐1 capture antibody. Vmp1 was detected with a Vmp1‐specific antibody in the same lysate and eluate fractions of ZO‐1 precipitates, confirming the direct interaction of these two proteins in HCC cells (Fig. 6).
Figure 6.

Direct interaction of vacuole membrane protein 1 (Vmp1) and ZO‐1 in hepatocellular carcinoma (HCC) cells. To test the direct interaction between Vmp1 and ZO‐1, co‐immunoprecipitations with HCCLM3VMP1+ cell lysates were performed using a ZO‐1 capture antibody. The cell lysate, flow through and wash before the elution were used as controls. ZO‐1 was visible at 220 kDa in the cell lysate and eluate but not in the flow through and the wash. A band at 46 kDa corresponding to Vmp1 was detected with a Vmp1‐specific antibody in the same lysate and eluate fractions of ZO‐1 precipitates, confirming the direct interaction of these two proteins.
Discussion
Alterations in cell–cell and cell–matrix adhesion seem to have a central role in facilitating tumor cell migration, invasion and metastatic dissemination.25 The overexpressed human Vmp1 was reported to localize in the endoplasmic reticulum (ER) and Golgi complex,26 where it appeared to be required for protein secretion and Golgi organization,11 as well as organelle biogenesis and multicellular development.27 Besides its different functions including membrane traffic,13 growth12 and autophagy,14, 15, 28, 29 interestingly, recent reports showed that Vmp1 was located in the plasma membrane playing a critical role in cell–cell contact.18, 23, 24 These results suggest a role for Vmp1 in tumor relevant cellular processes, which is further supported by expression profiling data.16, 17 However, the expression profile and function of Vmp1 in HCC remains unknown, and the correlation between its expression and prognosis of patients has not been documented at present. On this basis, we aimed to define the cancer‐relevant processes Vmp1 is involved in and how this protein is associated with HCC characteristics and prognosis.
Our data revealed that both mRNA and protein levels of Vmp1 were significantly decreased in HCC tissues compared with those in the corresponding PCLT and NL tissues, and were even lower in PVTT compared with HCC (primary tumors). The prediction of recurrence, metastasis and prognosis in patients with HCC after hepatic resection is an important clinical issue, which could determine surgical therapeutic regimens. Analysis of the association of Vmp1 expression and the clinicopathological characteristics in 124 HCC patients revealed that Vmp1 downregulation was significantly correlated with multiple tumor nodes, absence of capsular formation and vein invasion of HCC, which are widely accepted factors associated with metastasis and poor prognosis of HCC. Furthermore, the expression level of Vmp1 protein was significantly downregulated in NHCC when compared with SLHCC and SHCC, which correlates with the higher metastasis potential of NHCC than that of SLHCC,30 supporting the correlation between Vmp1 expression levels and metastasis in HCC. The Kaplan–Meier analysis showed that the HCC patients with low Vmp1 expression in general had worse prognosis than those with high Vmp1 expression. A multivariable Cox regression analysis indicated that low Vmp1 expression is an independent risk factor for the prognosis of HCC patients, suggesting that Vmp1 might serve as a useful prognostic biomarker of HCC.
Of particular interest is the correlation between Vmp1 expression and the ability of HCC cells to metastasize, which was uncovered when we analyzed Vmp1 expression in three HCC cell lines (HepG2, MHCC97‐L and HCCLM3) with different spontaneous metastatic potential.22 HepG2 cells exhibited a moderate metastatic potential whereas HCCLM3 cells were highly invasive, as demonstrated by extensive metastases via both subcutaneous and orthotopic inoculation. In agreement with the difference in metastatic potentials, expression of Vmp1, both in mRNA and protein levels, was markedly lower in the HCCLM3 cell line when compared with the MHCC97‐L and HepG2 cell lines, suggesting an association of downregulation of Vmp1 with the metastatic potential of HCC. As a previous study has demonstrated, this system can serve as a useful model for the study of HCC metastasis.22
To gain insights into a role for Vmp1 in HCC metastasis, we used a plasmid transfection approach to specifically upregulate the expression of Vmp1 in HCC cells and characterized the changes. Our data show that upregulation of Vmp1 expression resulted in marked inhibition of proliferation, migration and invasion of HCCLM3 cells. The adhesion test confirmed that upregulation of Vmp1 is associated with higher homogeneity adhesion and lower heterogeneity adhesion ability, further suggesting that the expression level of Vmp1 determines the invasion capacity of cancer cells. Together, these results support a critical role for Vmp1 in the invasion and metastasis of HCCLM3 cells. Such a role for Vmp1 is further supported by our in vivo study in which we showed that upregulation of Vmp1 inhibited proliferation of primary tumors of HCC, as well as the metastatic ratio of pulmonary metastases.
Vmp1 localization in mammalian cells has been described to be in the ER, Golgi apparatus,31 autophagosomes29 and the plasma membrane.18 Our immunohistochemical staining showed Vmp1 is mostly distributed in cytoplasm and in a punctate pattern in the plasma membrane. This phenomenon is in accordance with the finding of Sauermann et al.,18 that is:
…Vmp1 precisely colocalized with Calnexin in both ER and vacuoles. However, in cells with low overexpression of Vmp1, Vmp1 was found in the plasma membrane distributed in a punctate pattern, suggesting that localization in the ER was due to accumulation of protein caused by high level of overexpression.
Vmp1 is predicted by Hirokawa et al.32 to be a seven transmembrane domain protein with major parts of the protein located towards the extracellular space. Proteins destined for the plasma membrane mostly pass through the ER and Golgi network, and it is possible that an overexpressed protein with seven transmembrane helices is not folded correctly, leading to its retention in the endoplasmic reticulum. Furthermore, recent studies indicate that Vmp1 is essential for cell–cell contacts and tight junction formation, and it colocalizes with the tight junction protein ZO‐1 in spots between neighboring HEK293 cells.18, 23, 24 Meanwhile, ZO‐1 is important for clustering of claudins and occludin, resulting in the formation of tight junctions.33, 34 To further confirm the subcellular localization of Vmp1 and its regulating mechanism, we performed co‐immunoprecipitations with HCCLM3VMP1+ cell lysates using a ZO‐1 capture antibody, confirming the direct interaction of these two proteins in HCC cells. As ZO‐1 plays an important role in cancer‐related cell biological systems,35, 36 Vmp1's direct interaction with ZO‐1 might provide some evidence on the phenomenon that Vmp1 controls HCC behaviors thorough regulation of cell–cell contacts.
Vmp1 has been associated with different functions including autophagy14, 15, 28, 29 and cell adhesion,18, 23, 24 while there are still uncertainties in its underlying mechanism of tumor suppression, whether it is functioning in autophagy (when present in the ER) or in cell adhesion (when located in the plasma membrane). Autophagy has recently emerged as a key regulator of multiple aspects of cancer biology37 and autophagy deficiency leads to tumorigenesis in the liver.37, 38 Impairment of autophagy causes accumulation of p62, which might contribute to hepatoma development.39 In previous published studies, elevated p62 was reported in human HCC,40 and immunohistochemistry staining of p62 was significantly different between the frequencies of tissues with cancer (62.5%) and paracarcinomatous tissues (0%) in patients with HCC.41 As it has been reported that p62 colocalizes with upstream autophagy factors such as VMP1,42 an interesting hypothesis is that downregulation of Vmp1 might cause suppression of autophagy, which is always accompanied by marked accumulation of p62. Also, the overexpression of p62 as a result of autophagy inhibition was shown to be important in the promotion of tumorigenesis38 through a variety of mechanisms, including deregulation of NF‐&kB signaling, accumulation of ROS and increased DNA damage.40 Additionally, p62 binds to Keap1, leading to the upregulation of NRF2,43, 44 and persistent NRF2 activation appears to be critical for anchorage independent growth of hepatocellular carcinoma cells in the context of p62 overexpression.39 Another possible mechanism is that downregulation of VMP1 and the consequent lack of Vmp1 protein (which directly interact with ZO‐1) at the plasma membrane lead to failure of cell junction assembly, resulting in loss of cell–cell adhesion and subsequent cell detachment. Disruption or failure in the formation of intercellular junctions is commonly associated with metastasis of cancer cells,45 and loss of cell–cell adhesion is a prerequisite for tumor cell invasion.46 All of these possibilities need to be explored further.
In conclusion, the present study has shown for the first time that downregulation of Vmp1 significantly correlates with a poor prognosis of HCC. Furthermore, we have demonstrated that Vmp1 inhibits metastasis of HCC by affecting cell adherence. Collectively, our data suggest Vmp1 as a novel prognostic marker and a potential therapeutic target for metastasis of HCC.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This project was supported by grants from the National Key Technologies R and D Program of China (No. 2001BA703B04, No. 2004BA703B02), National Keystone Basic Research Program of China (No. 2004CB720303), National High Technology Research and Development Program of China (No. 2006AA02Z4B2), National Natural Science Foundation of China (No. 30571826), National Natural Science Foundation for Distinguished Young Scholars of China (No. 30328028), Clinical Subjects' Key Project of Ministry of Health (2007–2009), and The Science and Technology Significantly Special–The Significant New Medicine Formulation (2009ZX09103‐681).
(Cancer Sci 2012; 103: 2110–2119)
References
- 1. Roberts LR. Sorafenib in liver cancer–just the beginning. New Engl J Med 2008; 359: 420–2. [DOI] [PubMed] [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA‐Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 3. Poon RT, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000; 232: 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang W, Wu F, Fang F, Tao YM, Yang LY. Inhibition of invasion and metastasis of hepatocellular carcinoma cells via targeting RhoC in vitro and in vivo . Clin Cancer Res 2008; 14: 6804–12. [DOI] [PubMed] [Google Scholar]
- 5. Wang W, Yang LY, Huang GW et al Genomic analysis reveals RhoC as a potential marker in hepatocellular carcinoma with poor prognosis. Br J Cancer 2004; 90: 2349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang ZG, Yang LY, Wang W et al Determination of high mobility group A1 (HMGA1) expression in hepatocellular carcinoma: a potential prognostic marker. Dig Dis Sci 2005; 50: 1764–70. [DOI] [PubMed] [Google Scholar]
- 7. Yang LY, Tao YM, Ou DP, Wang W, Chang ZG, Wu F. Increased expression of Wiskott–Aldrich syndrome protein family verprolin‐homologous protein 2 correlated with poor prognosis of hepatocellular carcinoma. Clin Cancer Res 2006; 12: 5673–9. [DOI] [PubMed] [Google Scholar]
- 8. Tateishi R, Shiina S, Yoshida H et al Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology 2006; 44: 1518–27. [DOI] [PubMed] [Google Scholar]
- 9. Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E‐cadherin in the transition from adenoma to carcinoma. Nature 1998; 392: 190–3. [DOI] [PubMed] [Google Scholar]
- 10. Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001; 2: 285–93. [DOI] [PubMed] [Google Scholar]
- 11. Bard F, Casano L, Mallabiabarrena A et al Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature 2006; 439: 604–7. [DOI] [PubMed] [Google Scholar]
- 12. Arlt D, Huber W, Liebel U et al Functional profiling: from microarrays via cell‐based assays to novel tumor relevant modulators of the cell cycle. Cancer Res 2005; 65: 7733–42. [DOI] [PubMed] [Google Scholar]
- 13. Starkuviene V, Liebel U, Simpson JC et al High‐content screening microscopy identifies novel proteins with a putative role in secretory membrane traffic. Genome Res 2004; 14: 1948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sacchetti ML, Grasso D, Lo Re AE et al Autophagy mediated by VMP1 expression is a survival mechanism in caerulein‐treated AR42J pancreas cells. Gastroenterology 2008; 134: 429. [Google Scholar]
- 15. Pardo RP, Lo Re AE, Grasso D et al The pancreatitis‐induced membrane protein VMP1 that triggers autophagy interacts with S100A10. Gastroenterology 2008; 134(Suppl. 1): A287–8. [Google Scholar]
- 16. Boer JM, Huber WK, Sültmann H et al Identification and classification of differentially expressed genes in renal cell carcinoma by expression profiling on a global human 31500‐element cDNA array. Genome Res 2001; 11: 1861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Shinghal R, Gill H et al Gene expression patterns in renal cell carcinoma assessed by complementary DNA microarray. Am J Pathol 2003; 162: 925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sauermann M, Sahin O, Sültmann H et al Reduced expression of vacuole membrane protein 1 affects the invasion capacity of tumor cells. Oncogene 2008; 27: 1320–6. [DOI] [PubMed] [Google Scholar]
- 19. Shimizu M, Saitoh Y, Itoh H. Immunohistochemical staining of Ha‐ras oncogene product in normal, benign, and malignant human pancreatic tissues. Hum Pathol 1990; 21: 607–12. [DOI] [PubMed] [Google Scholar]
- 20. Ou DP, Tao YM, Tang FQ, Yang LY. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by upregulation of matrix metalloproteinases. Int J Cancer 2007; 120: 1208–14. [DOI] [PubMed] [Google Scholar]
- 21. Li Y, Tang Y, Ye L et al Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis‐related genes through cDNA microarray. J Cancer Res Clin Oncol 2003; 129: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Tian B, Yang J et al Stepwise metastatic human hepatocellular carcinom a cell model system with multiple metastatic potentials established through consecutive in vivo selection and studies on metastatic characteristics. J Cancer Res Clin Oncol 2004; 130: 460–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arlt D, Sauermann M, Sahin O et al The role of the transmembrane protein Vmp1 in the formation of cell–cell contacts. Eur J Cell Biol 2008; 87: 43. [Google Scholar]
- 24. Arlt D, Sauermann M, Sahin O et al The invasion‐suppressive function of the novel primordial junction protein Vmp1. Eur J Cell Biol 2007; 86: 39. [Google Scholar]
- 25. Christofori G. New signals from the invasive front. Nature 2006; 441: 444–50. [DOI] [PubMed] [Google Scholar]
- 26. Bannasch D, Mehrle A, Glatting KH, Pepperkok R, Poustka A, Wiemann S. LIFEdb: a database for functional genomics experiments integrating information from external sources, and serving as a sample tracking system. Nucleic Acids Res 2004; 32: 505–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Calvo‐Garrido J, Carilla‐Latorre S, Lázaro‐Diéguez F, Egea G, Escalante R. Vacuole membrane protein 1 is an endoplasmic reticulum protein required for organelle biogenesis, protein secretion, and development. Mol Biol Cell 2008; 19: 3442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Calvo‐Garrido J, Carilla‐Latorre S, Escalante R. Vacuole membrane protein 1, autophagy and much more. Autophagy 2008; 4: 835–7. [DOI] [PubMed] [Google Scholar]
- 29. Ropolo A, Grasso D, Pardo R et al The pancreatitis‐induced vacuole membrane protein 1 triggers autophagy in mammalian cells. J Biol Chem 2007; 282: 37124–33. [DOI] [PubMed] [Google Scholar]
- 30. Yang LY, Fang F, Ou DP, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 2009; 249: 118–23. [DOI] [PubMed] [Google Scholar]
- 31. Dusetti NJ, Jiang Y, Vaccaro MI et al Cloning and expression of the rat vacuole membrane protein 1 (VMP1), a new gene activated in pancreas with acute pancreatitis, which promotes vacuole formation. Biochem Biophys Res Commun 2002; 290: 641–9. [DOI] [PubMed] [Google Scholar]
- 32. Hirokawa T, Boon‐Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 1998; 14: 378–9. [DOI] [PubMed] [Google Scholar]
- 33. Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 2006; 22: 207–35. [DOI] [PubMed] [Google Scholar]
- 34. Jamora C, Fuchs E. Intercellular adhesion, signalling and the cytoskeleton. Nat Cell Biol 2002; 4: 101–8. [DOI] [PubMed] [Google Scholar]
- 35. Bodemann BO, White MA. Ral GTPases and cancer: linchpin support of the tumorigenic platform. Nat Rev Cancer 2008; 8: 133–40. [DOI] [PubMed] [Google Scholar]
- 36. Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer 2004; 40: 2717–25. [DOI] [PubMed] [Google Scholar]
- 37. Kimmelman AC. The dynamic nature of autophagy in cancer. Genes Dev 2011; 25: 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takamura A, Komatsu M, Hara T et al Autophagy‐deficient mice develop multiple liver tumors. Genes Dev 2011; 25: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Inami Y, Waguri S, Sakamoto A et al Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol 2011; 193: 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mathew R, Karp CM, Beaudoin B et al Autophagy suppresses tumorigenesis through elimination of p62. Cell 2009; 137: 1062–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Su Y, Qian H, Zhang J, Wang S, Shi P, Peng X. The diversity expression of p62 in digestive system cancers. Clin Immunol 2005; 116: 118–23. [DOI] [PubMed] [Google Scholar]
- 42. Itakura E, Mizushima N. p62 targeting to the autophagosome formation site requires self‐oligomerization but not LC3 binding. J Cell Biol 2011; 192: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Komatsu M, Kurokawa H, Waguri S et al The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol 2010; 12: 213–23. [DOI] [PubMed] [Google Scholar]
- 44. Lau A, Wang XJ, Zhao F et al A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol Cell Biol 2010; 30: 3275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martin TA, Jiang WG. Tight junctions and their role in cancer metastasis. Histol Histopathol 2001; 16: 1183–95. [DOI] [PubMed] [Google Scholar]
- 46. Benoliel AM, Pirro N, Marin V et al Correlation between invasiveness of colorectal tumor cells and adhesive potential under flow. Anticancer Res 2003; 23: 4891–6. [PubMed] [Google Scholar]


