Abstract
Angiomodulin (AGM/IGFBP‐rP1), a glycoprotein of about 30 kDa, is overexpressed in tumor vasculature as well as some human cancer cell lines, but it has been suggested to be a tumor suppressor. To elucidate roles of angiomodulin (AGM) in tumor progression, we here examined distribution of AGM in three types of human cancer tissues by immunohistochemistry. The results showed that AGM was overexpressed in the stroma as well as the vasculature surrounding tumor cells in the human cancer tissues. AGM and α‐smooth muscle actin (α‐SMA) as an activated fibroblast marker were often colocalized in cancer‐associated fibroblasts (CAFs). In vitro analysis indicated that transforming growth factor (TGF)‐β1 might be an important inducer of AGM in normal human fibroblasts. AGM strongly stimulated the expression of fibronectin and weakly that of α‐SMA in normal fibroblasts. AGM significantly stimulated the proliferation and migration of fibroblasts. The AGM‐induced expression of fibronectin and α‐SMA was blocked by a TGF‐β signal inhibitor but neither the stimulation of cell growth nor migration. These results imply that AGM activates normal fibroblasts by TGF‐β‐dependent and independent mechanisms. These findings also suggest that AGM and TGF‐β1 cooperatively or complementarily contribute to the stromal activation and connective tissue formation in human cancer tissues, contributing to tumor progression. (Cancer Sci 2012; 103: 691–699)
It has recently been accepted that tumor microenvironment plays critical roles in tumor progression.1, 2, 3 Fibroblasts, vascular endothelial cells, inflammatory cells and their secreted protein products including extracellular matrix proteins are major components present in the tumor microenvironment. The behavior of tumor cells is regulated by complex interaction between the tumor cells and surrounding stromal cells and secreted factors.2, 3
Angiomodulin (AGM), a secretory glycoprotein of about 30 kDa, is a member of the insulin‐like growth factor binding protein (IGFBP) superfamily.4 However, the amino acid identity of AGM to IGFBPs is as low as about 20%, and its affinity for IGFs is far lower than that of IGFBPs.4, 5, 6 Therefore, the names “IGFBP‐related protein‐1 (IGFBP‐rP1)” and “IGFBP‐7” are often used for this protein. AGM was originally identified as tumor‐derived cell adhesion factor (TAF) secreted by human bladder carcinoma cells7 and as prostacyclin‐stimulating factor (PSF) from human fibroblasts.8 The cDNA of AGM was cloned as mac25, which was expressed in normal human leptomeningeal cells but scarcely in meningiomas.9 Because AGM is highly expressed in tumor blood vessels, the name “angiomodulin (AGM)” was proposed.10 A recent study has shown that AGM is mainly expressed in developmental and adult vascular systems and plays a synergistic role with VEGF in angiogenesis.11 The AGM message or protein is detected in a wide range of normal tissues such as the heart, spleen, ovary, small intestine and colon12 and cells such as vascular endothelial cells, high endothelial cells, smooth muscle cells, fibroblasts and cancer cells.4, 7, 8, 9, 10
There are many contradictory reports on the pathological roles of AGM in cancer. Although our earlier studies showed that AGM is highly expressed in some cancer cell lines,13 invading tumor cells in colon cancer14 and tumor vasculature,10 other studies showed that AGM may be a tumor‐suppressing protein.4 Expression of AGM is associated with cell senescence in normal human mammary epithelial cells.15, 16 In breast,17 prostate18 and lung19 cancers, reduced expression of AGM is correlated with worse prognosis of patients compared to higher expression. Forced expression of exogenous AGM in breast,16 lung19 and prostate20, 21 cancer cells inhibited the cell growth by inducing senescence or apoptosis in culture. Moreover, forced expression of exogenous AGM in colon carcinoma,22 lung carcinoma19 and melanoma23 cell lines suppressed the tumor growth in xenograft models. In the case of melanoma cells, the administration of recombinant AGM protein suppresses tumor cell growth by inducing apoptosis both in vivo and in vitro.23, 24 Thus, AGM might play positive and negative roles in tumor progression depending on some unknown conditions.
As past studies have not clearly shown the distribution of AGM in human cancer tissues, here we again analyzed expression and distribution of AGM in human cancer tissues of the lung, colon and uterus by immunohistochemistry. We found that AGM was overexpressed in not only the vasculature but also stromal fibroblasts of the cancers. The biological activity of AGM towards human fibroblasts was also investigated in vitro.
Materials and Methods
Materials
Human tissue specimens of 10 lung cancers (five squamous cell carcinomas and five adenocarcinomas), five adenocarcinomas of the colon, and five adenocarcinomas of the uterus were provided by Human Cancer Tissue Center of Kanagawa Cancer Research and Information Association (KCRIA), Kanagawa, Japan. The study protocol was approved by the Ethical Committees of both KCRIA and Kihara Institute for Biological Research, Yokohama City University and performed according to the guidelines of the 1995 declaration of Helsinki. Written informed consent was obtained from each patient. Human recombinant transforming growth factor‐β1 (TGF‐β1) and Smad signal inhibitor (SB431542) were purchased from Wako (Osaka, Japan). Mouse monoclonal antibody against human AGM/TAF/IGFBP‐rP1 (clone 88), which is available from Cosmo‐Bio (Tokyo, Japan), was previously reported.10 Other antibodies used were mouse monoclonal antibody against fibronectin from Takara (Shiga, Japan), ones against β‐actin and against vimentin from Sigma‐Aldrich (St. Louis, MO, USA), goat polyclonal antibody against IGFBP‐rP1 (R&D, Minneapolis, MN, USA), and rabbit polyclonal antibody against α‐smooth muscle actin (α‐SMA) from Abcam (Cambridge, UK).
Cell culture
Human natal dermal fibroblasts (HDFs) were obtained from Lifeline Cell Technology (Los Angeles, CA, USA). Human fetal lung fibroblast cell line WI38 and human bladder carcinoma cell line T24 (EJ‐1 strain) were provided from Japanese Collection of Research and Bioresources (JCRB, Osaka, Japan). One clone of T24 cells (clone 8) was previously isolated in our laboratory and used in this study.22 These kinds of cells were maintained in DMEM/F12 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS) (JRH Bioscience, Lenexa, KS, USA) at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Purification of AGM
Angiomodulin was purified from the serum‐free conditioned medium of T24 cell line by the previously reported method with some modifications.13 Briefly, the conditioned medium was collected every 2 or 3 days from confluent cultures of T‐24 cells. AGM in the conditioned medium was concentrated by heparin affinity chromatography and then purified by immuno‐affinity chromatography on a column conjugated with the anti‐AGM antibody (clone 88).
Immunohistochemistry of human cancer tissues
Five‐μm serial frozen sections were obtained from KCRIA and subjected to immunohistochemistry as described previously.25 The sections were treated with the first antibody, biotin‐conjugated second antibody (Vector, Burlingame, CA, USA) and then streptoavidin‐conjugated peroxidase (Vector). The resultant immunocomplexes were visualized with 3,3‐diaminobenzidine (DAB) using the Histofine kit (Nichirei, Tokyo, Japan). The evaluation of immunosignal intensity was performed independently by three coworkers in a blind fashion as to the origins of cancer samples, using standard stained sections of grade 0, +1, +2 and +3. The average score was recorded for each sample. In immunofluorescence staining, cy3‐labeled anti‐mouse‐IgG antibody, FITC‐labeled anti‐rabbit‐IgG antibody, or FITC‐labeled anti‐goat‐IgG antibody was used as the second antibody (Vector).
Preparation of cell lysates and conditioned media for protein analysis
To examine protein expression in fibroblasts in response to AGM or TGF‐β1, HDF or WI38 cells were inoculated at a density of 3 × 105 cells per 35‐mm dish and incubated overnight. The cultures were washed twice with the serum‐free DMEM/F12 and incubated in the medium supplemented with test samples for 2 days. The resultant conditioned medium was collected and clarified by centrifugation. For the analysis of AGM, proteins in the medium were concentrated by precipitation with 10% (w/v) trichloroacetic acid (TCA), washed with cold ethanol and dissolved in 0.03 mL of the SDS sample buffer for immunoblotting. Fibronectin was analyzed using unconcentrated conditioned medium. The cells remaining on the dishes were washed with PBS and then harvested by incubating in 5 mM EDTA/PBS. The total cell number in each dish was determined with a cell counter (Sysmex, Hyogo, Japan), and the remaining cells were collected by centrifugation and dissolved in 150 μL of the SDS sample buffer.
SDS‐PAGE and immunoblotting
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) was performed on 12.5% or 6% gels under reducing or non‐reducing conditions. For analyzing specific proteins, cell lysates or conditioned media were separated on the gels, transferred to PVDF membranes, and detected by immunoblotting using specific antibodies and the ECL detection reagents (GE Healthcare, Buckinghamshire, UK).
Cell proliferation assay
Human natal dermal fibroblast (HDFs) were inoculated at a density of 5 × 103 cells per well containing 500 μL of DMEM/F12 plus 5% FCS on 24‐well culture plates (Becton Dickinson, Franklin Lakes, NJ, USA) and incubated with test samples for indicated lengths of time. The grown cells were counted with a Sysmex cell counter. To test effects of signal inhibitors on cell growth, HDFs (1 × 103 cells per well) were incubated with test samples on 96‐well plastic culture plates. To quantify the grown cells, each well was fixed and stained with 0.1% (w/v) crystal violet in 20% methanol. The dye was extracted with isopropanol and measured for absorbance at 544 nm.
Cell migration assay
Migration of HDF cells through a membrane filter with 8‐μm pores was analyzed using a xCELLigence System Real‐Time Cell Analyzer (Roche, Basel, Switzerland) according to the manufacturer's instruction manual. Test samples mixed in DMEM/12 plus 1% heat inactivated FCS were placed into lower chambers of the CIM‐Plate 16. HDF cells were inoculated at a density of 5 × 104 cells per 100 μL of the same medium into upper chambers and incubated at 37°C. The cell migration from the upper chambers to the lower ones was monitored for 3 h.
Statistical analysis
Statistical significance was evaluated with an unpaired Student's T test. A P‐value of <0.05 was considered significant.
Results
Expression of AGM in human cancer tissues
Expression of AGM protein in a colon cancer tissue was examined by immunohistochemistry with the anti‐AGM monoclonal antibody (clone 88). In a single stained section (3 × 3 mm) of the adenocarcinoma, connective tissues surrounding normal glands showed faint or little immunoreactivity, while strong signals were detected throughout the stroma surrounding tumor cells with stronger signals in the vasculature (Fig. 1). To examine common expression of AGM in the cancer stroma, we further analyzed the following human cancer tissues from different patients, as well as corresponding normal tissues as controls: 10 carcinomas of the lung (five squamous cell carcinomas and five adenocarcinomas), five adenocarcinomas of the colon and five adenocarcinomas of the uterus. In normal lung and colon tissues, AGM was weakly detected in the vasculature and smooth muscle but not in the epithelial tissues and underlying connective tissues (Fig. 2a,b). Exceptionally, AGM was clearly detected in the basement membranes of normal endometrial glands of the uterus (Fig. 2c). This localization was consistent with the past study, which showed the localization of AGM in the endometrium and stroma of the uterus and its implication in endometrial receptivity.26 In all cases of the cancer tissues tested, blood vessels always showed strong immunoreactivity for AGM (Fig. 3–d). In addition, all cases of the cancer tissues showed more or less positive signals for AGM in the stroma surrounding tumor cells, most likely in fibroblasts (Fig. 3–d and Table 1). Strong expression of AGM in the tumor stroma was found in all cases of the colon cancers and some cases of the other types of cancer. AGM was also detected in tumor cells of some invasive cancer tissues (Fig. 3b; Table 1).
Figure 1.
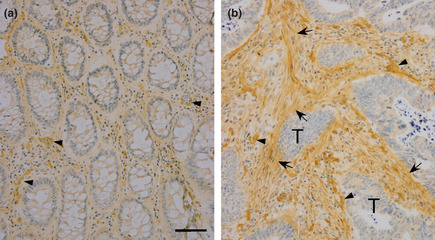
Immunohistochemical analysis of angiomodulin (AGM) expressed in normal epithelial tissues and cancer tissues of colon. A single frozen section of a colon carcinoma (approximately 3 × 3 mm square size) was immunostained for AGM as described in Materials and Methods. (a) Normal glandular tissue, (b) stroma adjacent to tumor cells. Arrowheads, AGM‐positive micrrovessels; arrows, AGM‐positive stroma. Scale bars, 50 μm. When non‐immune mouse IgG was used as a negative control instead of the first antibody, no staining was found in the same conditions.
Figure 2.
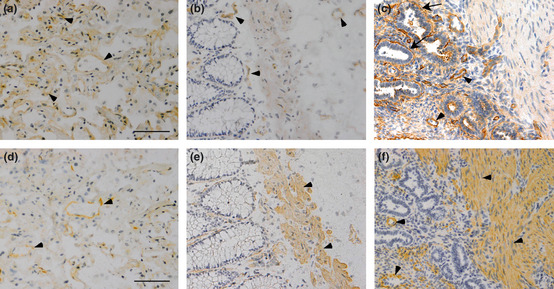
Two close sections containing normal epithelial tissues of the lung (a,d), colon (b,e), and uterus (c,f) were immunostained for angiomodulin (AGM) (a–c) and α‐smooth muscle actin (α‐SMA) (d–f). Arrowheads, AGM‐positive signals; arrows, endometrial basement membrane positive for AGM in (c). Scale bars, 50 μm.
Figure 3.
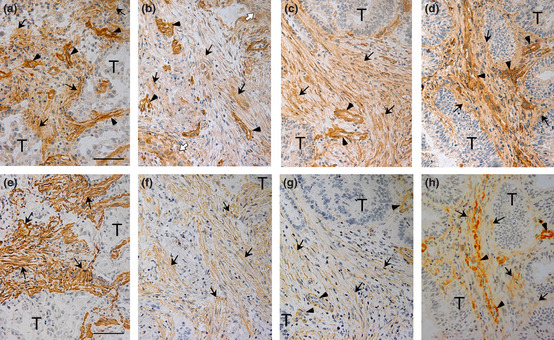
Two close sections containing tumor cells (T) of lung adenocarcinoma (a,e), lung squamous cell carcinoma (b,f), colon adenocarcinoma (c,g) and uterus adenocarcinoma (d,h) were immunostained for angiomodulin (AGM) (a–d) and α‐smooth muscle actin (α‐SMA) (e–h). The colon cancer sections were derived from a different cancer sample from Figure 1. Arrows, positive signals for AGM or α‐SMA in stroma; arrowheads, AGM‐ or α‐SMA‐positive blood vessels; open arrowheads, AGM‐positive invading tumor cells in (b). Scale bars, 50 μm.
Table 1.
Summary of AGM expression in three types of human cancer tissues
| Organs | Total no. | Vasculaturea | Stromab | Tumor cells | |
|---|---|---|---|---|---|
| (+3) | (+2/+3) | (+1) | (+1/+2) | ||
| Lung | 10 | 10 | 5 | 5 | 4 |
| Colon | 5 | 5 | 5 | 0 | 0 |
| Uterus | 5 | 5 | 2 | 3 | 1 |
The intensity of immunostaining was classified into 0, +1 (low), +2 (intermediate) and +3 (high) as described in Materials and Methods.
Endotheilal cells, pericytes and the basement membranes.
Connective tissues and cells except vasculature.
As activated cancer‐associated fibroblasts (CAFs), or myofibroblasts, are known to express α‐smooth muscle actin (α‐SMA), its expression was also analyzed using close sections of the same tissue specimens as used for the AGM analysis. In normal colon and lung tissues α‐SMA was weakly detected in smooth muscle and vasculature (Fig. 2–e), while it was highly detected in the myometrium of normal uterus (Fig. 2f). In cancer tissues, α‐SMA was more or less detected in the AGM‐positive stroma (Fig. 3–h). Double immunofluorescence staining analysis showed that most α‐SMA‐positive cells were positive for AGM but not vice versa (Fig. 4a–c). On the other hand, AGM was well colocalized with the fibroblast marker vimentin as analyzed by double immunofluorescent staining (Fig. 4d–f). These results demonstrated that AGM was widely overexpressed in not only vasculature but also activated fibroblasts, or myofibroblasts, in the cancer tissues.
Figure 4.
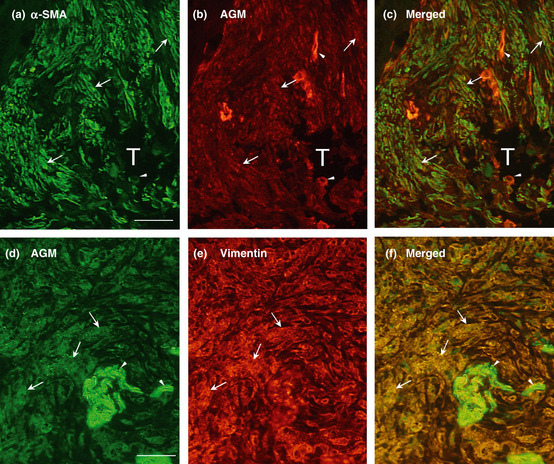
Double immunofluorescence staining of cancer tissues for angiomodulin (AGM) and α‐smooth muscle actin (α‐SMA) or vimentin. (a) A section of colon adenocarcinoma, which derived from a tumor specimens different from those used in Fig. 1, was subjected to double immunofluorescence staining with a rabbit anti‐α‐SMA polyclonal antibody (a: green) and the anti‐AGM monoclonal antibody (clone 88) for AGM (b; red) as described in Materials and Methods. The two images (a and b) were merged in (c). (d–f) A section of lung adenocarcinoma, different from the tumor specimens used in Fig. 1, was immunostained with a goat anti‐AGM polyclonal antibody (d; green) and a mouse anti‐vimentin monoclonal antibody (e; red). The two images (d and e) were merged in (f). Arrowheads, positive signals for AGM in blood vessels; arrows, positive signals for AGM, α‐SMA or vimentin in stromal tissues; T, tumor cell mass. Scale bar, 50 μm.
Induction of AGM by TGF‐β in cultured fibroblasts
It is well known that TGF‐β plays critical roles in the phenotypic changes of CAFs. TGF‐β activates fibroblasts to induce expression of α‐SMA and ECM molecules such as fibronectin and interstitial collagen.3, 27, 28, 29 Therefore, we tested whether or not TGF‐β1 stimulates AGM expression in two types of fibroblasts in vitro. When primary HDFs were incubated with TGF‐β1, AGM secretion into culture medium was weakly enhanced (Fig. 5a). As expected, TGF‐β1 greatly enhanced the expression of fibronectin and α‐SMA in HDFs. The induction of AGM by TGF‐β1 was more clearly shown when human lung fibroblast cell line WI38 was used in the assay (Fig. 5b). These data suggest that AGM is induced in CAFs at least in part by TGF‐β.
Figure 5.
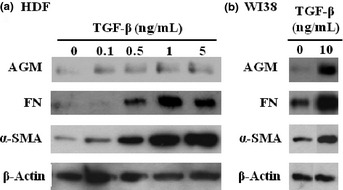
Effect of transforming growth factor‐β1 (TGF‐β1) on expression of angiomodulin (AGM), fibronectin (FN) and α‐smooth muscle actin (α‐SMA) in two kinds of cultured human fibroblasts. Human natal dermal fibroblasts (HDFs) (a) and WI38 cells (b) were incubated with the indicated concentrations (ng/mL) of TGF‐β1 in serum‐free medium for 2 days. From each culture, the conditioned medium and cell lysates were prepared, as described in Materials and Methods. AGM and fibronectin were analyzed with the conditioned media, while α‐SMA and β‐actin as an internal loading control were done with the cell lysates. The results were reproduced in at least three separate experiments.
Activation of fibroblasts by AGM in vitro
To understand pathological roles of AGM in cancer tissues, effects of purified AGM on HDFs were examined in comparison of the effects of TGF‐β1. AGM appeared to slightly increase the α‐SMA level though the induction was far lower than that by TGF‐β1 (Fig. 6a). However, AGM significantly enhanced fibronectin secretion from fibroblasts (Fig. 6b). The induction level of fibronectin by AGM was nearly comparable with that induced by TGF‐β1, although the effective dose of AGM was far higher than that of the latter. In WI38 cells, the induction of fibronectin and α‐SMA by AGM was very low or insignificant (data not shown).
Figure 6.
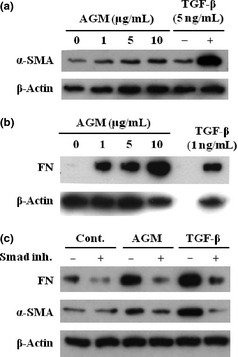
Effects of angiomodulin (AGM) and transforming growth factor‐β1 (TGF‐β1) on expression of fibronectin and α‐smooth muscle actin (α‐SMA) in human fibroblasts in presence or absence of Smad signal inhibitor. (a,b) Human natal dermal fibroblasts (HDFs) were incubated with the indicated concentrations of AGM or TGF‐β1 as a positive control in serum‐free medium for 2 days. Expression of α‐SMA (a) and fibronectin (b; FN) in the cultures were analyzed, as described in Fig. 4. (c) HDFs were pre‐incubated for 1 h with (+) or without (−) 10 μM Smad signal inhibitor SB431542 (Smad inh.) and then treated without (Cont.) or with 5 ng/mL TGF‐β1 or 5 μg/mL AGM. Fibronectin (FN) and α‐SMA expressed in the cultures were analyzed as above. Other experimental conditions are described in Materials and Methods.
The activity of TGF‐β1 is mainly mediated by the Smad signaling. Indeed, the induction of fibronectin and α‐SMA was completely blocked by the Smad signal inhibitor SB431542 (Fig. 6c). Interestingly, the induction of fibronectin and α‐SMA by AGM was also blocked by the Smad inhibitor. This suggested that endogenous TGF‐β in fibroblasts might be involved in the induction of fibronectin and α‐SMA by AGM.
When morphological changes of HDFs were examined, TGF‐β1‐treated fibroblasts showed markedly contracted cell morphology with tight cell–cell junction as compared with the control cells (Fig. 7). These morphological characteristics resembled those of myofibroblasts with high contractility. Although AGM‐treated cells also showed contracted cell morphology, they exhibited a more elongated and fibrous structure than the control cells and the TGF‐β1‐treated cells. The difference in the cell morphology seems to depend on the difference in the induction levels of α‐SMA, fibronectin and some other factors and to be related with cellular activities such as cell contractility and proliferation. The Smad inhibitor completely blocked the TGF‐β1‐induced morphological change but it partially did the AGM‐treated ones (data not shown).
Figure 7.
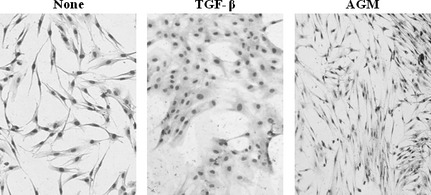
Effects of angiomodulin (AGM) and transforming growth factor‐β1 (TGF‐β1) on morphology of human fibroblasts. Human natal dermal fibroblasts (HDFs) were incubated without (None) or with 5 ng/mL TGF‐β1 or 5 μg/mL AGM in DMEM/F12+1% FCS medium for 2 days. The cells were fixed in 10% formalin, stained with Giemsa, and photographed under a phase‐contrast microscope. ×200.
Effect of AGM on growth and migration of fibroblasts
We next examined effect of AGM on growth of HDFs. When HDFs were incubated with purified TGF‐β1, the cell growth was suppressed at very low concentrations (Fig. 8a). In contrast, AGM enhanced the cell growth in a dose‐dependent manner (Fig. 8b). The growth curves in the presence or absence of AGM are also shown in Fig. 8c. When HDFs were treated with both AGM and the Smad inhibitor, the growth‐stimulating activity of AGM was greatly enhanced by the inhibitor (Fig. 8d). It was supposed that the growth enhancement might be due to the inhibition of the activity of endogenous TGF‐β. The opposite effects of AGM and TGF‐β1 on the cell growth were reproduced in the assays with WI38 cells, but less evidently than their effects on HDFs (Fig. 8e,f).
Figure 8.
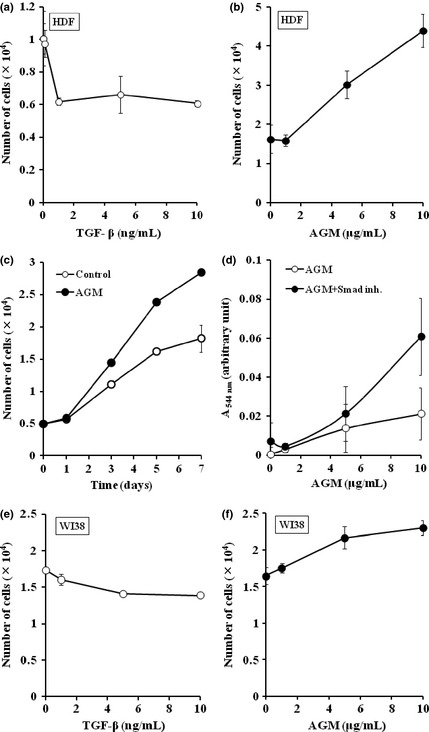
Effects of angiomodulin (AGM) and transforming growth factor‐β1 (TGF‐β1) on growth of human fibroblasts. (a,b) Human natal dermal fibroblasts (HDFs) were incubated with the indicated concentrations of TGF‐β1 for 5 days (a) or AGM for 4 days (β) in DMEM/F12+5% FCS medium on 24‐well plates. Each point represents the mean ± SD of the numbers of cells in triplicate wells. (c) Time course of HDF growth in presence (●) or absence (○) of 10 μg/mL AGM. (d) Effect of varied concentrations of AGM on the growth of HDFs was examined in the presence (●) or absence (○) of 10 μM Smad inhibitor SB431542 (Smad inh.) for 6 days on a 96‐well plate. The cell growth was measured by the crystal violet staining. Each point represents the mean ± SD in triplicate wells. (e,f) Effects of varied concentrations of TGF‐β1 (e) or ΑGΜ (f) on the growth of WI38 cells were examined for 5 days as described in (a) and (b). Other experimental conditions are described in Materials and Methods.
Cell migration activity of AGM was also tested using an electric cell migration assay system. When AGM or TGF‐β1 was added into a lower chamber as a chemoattractant, the migration of HDFs through a membrane filter was weakly but significantly enhanced (Fig. 9a). Although the effective concentrations of AGM and TGF‐β1 greatly differed, their maximum activities appeared to be similar to each other but far lower than that of fibronectin (Fig. 9b). The Smad signal inhibitor SB431542 did not block the chemotactic activity of any factor (data not shown).
Figure 9.
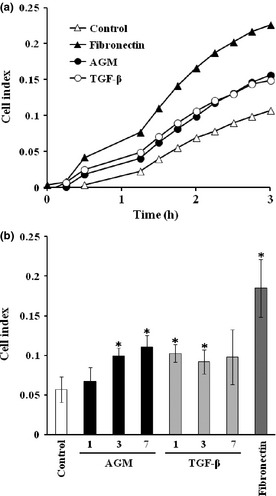
Effects of angiomodulin (AGM) and transforming growth factor‐β1 (TGF‐β1) on chemotactic migration of human fibroblasts. Migration of human natal dermal fibroblasts (HDFs) through a membrane filter with 8 μm pores was analyzed in the presence or absence (△) of 5 μg/mL AGM (●), 5 ng/mL TGF‐β1 (○), or 5 μg/mL fibronectin as a positive control (▲) in the electronic real‐time cell analyzer xCELLigence, as described in Materials and Methods. Each point represents the mean in triplicate wells. (b) Comparison of chemotactic activities of AGM (left) and TGF‐β1 (right) at three different concentrations in a 2‐h assay. Asterisks indicate statistically significant difference from the control (P < 0.05).
Discussion
In the present study, we found that AGM is overexpressed in the stroma as well as the vasculature surrounding tumor cells in human cancer tissues. In vitro analysis indicated that TGF‐β1 might be an important inducer of AGM in human fibroblasts. AGM significantly stimulated the growth of human normal fibroblasts and their fibronectin production in vitro. In addition, AGM weakly stimulated the expression of α‐SMA, a representative marker of myofibroblasts, and promoted the migration of fibroblasts. It is well known that TGF‐β1 plays a critical role in the activation of fibroblasts in the tumor stroma.3 TGF‐β1 strongly stimulates fibroblasts to express α‐SMA and ECM proteins such as fibronectin and type I collagen.27, 28, 29 Indeed, in this study the activities to induce α‐SMA and fibronectin were obviously much higher with TGF‐β1 than AGM. However, their growth activities towards fibroblasts were contrasted: TGF‐β1 significantly suppressed the cell growth, whereas AGM stimulated it. Therefore, it is likely that TGF‐β1 plays a major role in the activation and ECM production of fibroblasts, while AGM plays a specific role in the fibroblast proliferation. Thus, it is expected that AGM and TGF‐β1 cooperatively or complementarily contribute to the stromal activation and connective tissue formation in cancer tissues. It should also be noted that the effective concentration of AGM was about 1000‐times higher than that of TGF‐β1. Our sandwich ELISA analysis has shown that the AGM concentration is approximately 30 ng/mL in normal human serum and exceeded 200 ng/mL in the culture medium of a cancer cell line highly expressing AGM (Kayano Moriyama and Kaoru Miyazaki, unpublished data, 2005). AGM is an extracellular matrix protein rather than a cytokine. Just like fibronectin and laminins, AGM exerted its biological activities at concentrations ranging 1–10 μg/mL. As shown in this study, it is highly deposited on vascular basement membrane and stromal tissues near cancer cells. Therefore, the apparently high concentrations of AGM seem to be pathologically relevant in some tumor microenvironments.
The activity of TGF‐β1 is mainly mediated by the Smad signaling. Unexpectedly, the Smad signal inhibitor SB431542 inhibited the α‐SMA and fibronectin expression induced not only by TGF‐β1 but also by AGM. On the other hand, this inhibitor significantly enhanced the growth‐simulating activity of AGM on fibroblasts. Thus, AGM activities are separated into the TGF‐β‐independent and TGF‐β‐like activities. The latter activity may be mediated by TGF‐β1 or related factors. We could not detect TGF‐β1 in the AGM preparation as analyzed by immunoblotting (data not shown). It is possible that AGM enhances the activity of the endogenous TGF‐β1 produced by fibroblasts, leading to the elevated expression of α‐SMA and fibronectin. The growth‐stimulatory effect of the Smad inhibitor suggests that AGM‐induced endogenous TGF‐β1 has growth‐inhibitory activity on fibroblasts. There are many possible mechanisms for the effect of AGM on TGF‐β action, for example, stimulation of TGF‐β transcription, activation of the latent TGF‐β protein, promotion of the TGF‐β binding to the receptor, and co‐stimulation of TGF‐β signaling. Moreover, we cannot yet exclude the possibility that our AGM preparation contained a trace amount of TGF‐β bound to AGM. Further studies are required for clarifying these possibilities. We have reported that AGM interacts with syndecan‐1 on cell surface.13 In this study, we were unable to identify the receptor and cell signaling that are involved in the growth‐stimulatory activity of AGM.
Our early study on AGM showed that it stimulates the growth of mouse fibroblasts.5 Recent studies by other groups suggested that AGM/IGFBP‐rP1 might be involved in liver fibrosis.30, 31 These studies also show that AGM plays some roles in connective tissue formation. Similarly, there are reports showing that some other IGFBP super families such as IGFBP‐3, IGFBP‐5 and CTGF (IGFBP‐rP2) are expressed in and contribute to pathological fibrosis.30, 32, 33, 34 In addition, CTGF has been shown to promote transdifferentiation of mesenchymal stem cells to fibroblasts.35 Therefore, the connective tissue formation may be a common function of IGFBP‐related proteins. However, no previous studies have reported overexpression of AGM in CAFs. The enhanced proliferation of fibroblasts and accumulation of ECM in tumor stroma, i.e. desmoplasia, is known to be a typical feature of solid tumors.3 The reactive stroma is also a key feature in some pathological conditions such as fibrosis, inflammation and wound healing. In such reactive stroma, activated fibroblasts, i.e. myofibroblasts, secrete various cytokines and acquire the capabilities of migration, proliferation and contraction.3, 36 The mutual interaction between cancer cells and myofibroblasts through cell–cell interaction and secreted proteins is essential for cancer invasion and causes a poor clinical outcome.37, 38 For example, tumor‐derived fibroblasts stimulate tumor cell growth in coculture experiments.39 When colon cancer cells are cocultured with TGF‐β‐treated fibroblasts, the cancer cells acquire invasive potential within collagen gel.40 Similarly, animal experiments demonstrated that CAFs or other types of fibroblast enhance the efficiency of tumor growth when co‐injected with tumor cells.39, 41, 42 However, it is unclear in these studies what factors in the activated fibroblasts are responsible for the enhanced invasive growth of tumor cells. Because the number of cancer specimens analyzed in this study was very low, the relationship between the AGM expression in CAFs and its clinical output in patients is unknown. Based on the facts found in many past studies, it is supposed that AGM expressed in CAFs activates the fibroblasts by an autocrine mechanism and contribute to tumor progression.
The present study also showed the elevated expression of AGM in vasculature in all cases and in tumor cells of some cancer tissues. Past studies have suggested that AGM in blood vessels may be related to elevated vascular permeability10, 43 and angiogenesis.11 On the other hand, a considerable number of studies have suggested the tumor‐suppressive activity of AGM.15, 16, 17, 18, 19, 20, 21, 22, 23 In this regard, it is noted that in the present histochemical analysis, AGM expression was often found in invading carcinoma cells but not normal epithelial cells. It is unknown whether AGM expressed in invading tumor cells has positive or negative activity for tumor growth. AGM is known to have post‐transcriptional modifications such as proteolytic cleavage and glycosylation.13 Such modifications, as well as differences in AGM‐expressing cells, may explain the two opposite effects of AGM on tumor cells in future studies.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgements
We thank Ms Makiko Fujimura, Kanagawa Cancer Research and Information Association (KCRIA), Kanagawa, for preparation of frozen sections and pathological information. We also thank Ms Marii Ise, Mr H. Sato and Mr J. Oyanagi for technical support and discussion. This work was supported by Grants‐in‐Aid (23112517 and 23300351) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Liotta LA, Kohn EC. The microenvironment of the tumour‐host interface. Nature 2001; 411: 375–9. [DOI] [PubMed] [Google Scholar]
- 2. Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer 2007; 7: 139–47. [DOI] [PubMed] [Google Scholar]
- 3. Micke P, Ostman A. Tumour‐stroma interaction: cancer‐associated fibroblasts as novel targets in anti‐cancer therapy? Lung Cancer 2004; 45(Suppl 2): S163–75. [DOI] [PubMed] [Google Scholar]
- 4. Burger AM, Leyland‐Jones B, Banerjee K, Spyropoulos DD, Seth AK. Essential roles of IGFBP‐3 and IGFBP‐rP1 in breast cancer. Eur J Cancer 2005; 41: 1515–27. [DOI] [PubMed] [Google Scholar]
- 5. Akaogi K, Sato J, Okabe Y, Sakamoto Y, Yasumitsu H, Miyazaki K. Synergistic growth stimulation of mouse fibroblasts by tumor‐derived adhesion factor with insulin‐like growth factors and insulin. Cell Growth Differ 1996; 7: 1671–7. [PubMed] [Google Scholar]
- 6. Oh Y, Nagalla SR, Yamanaka Y, Kim HS, Wilson E, Rosenfeld RG. Synthesis and characterization of insulin‐like growth factor‐binding protein (IGFBP)‐7. Recombinant human mac25 protein specifically binds IGF‐I and ‐II. J Biol Chem 1996; 271: 30322–5. [DOI] [PubMed] [Google Scholar]
- 7. Akaogi K, Okabe Y, Funahashi K et al Cell adhesion activity of a 30‐kDa major secreted protein from human bladder carcinoma cells. Biochem Biophys Res Commun 1994; 198: 1046–53. [DOI] [PubMed] [Google Scholar]
- 8. Yamauchi T, Umeda F, Masakado M, Isaji M, Mizushima S, Nawata H. Purification and molecular cloning of prostacyclin‐stimulating factor from serum‐free conditioned medium of human diploid fibroblast cells. Biochem J 1994; 303: 591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murphy M, Pykett MJ, Harnish P, Zang KD, George DL. Identification and characterization of genes differently expressed in meningiomas. Cell Growth Differ 1993; 4: 715–22. [PubMed] [Google Scholar]
- 10. Akaogi K, Okabe Y, Sato J et al Specific accumulation of tumor‐derived adhesion factor in tumor blood vessels and in capillary tube‐like structures of cultured vascular endothelial cells. Proc Natl Acad Sci USA 1996; 93: 8384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hooper AT, Shmelkov SV, Gupta S et al Angiomodulin is a specific marker of vasculature and regulates vascular endothelial growth factor‐A‐dependent neoangiogenesis. Circ Res 2009; 105: 201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Degeorges A, Wang F, Frierson HF Jr, Seth A, Sikes RA. Distribution of IGFBP‐rP1 in normal human tissues. J Histochem Cytochem 2000; 48: 747–54. [DOI] [PubMed] [Google Scholar]
- 13. Ahmed S, Yamamoto K, Sato Y et al Proteolytic processing of IGFBP‐related protein‐1 (TAF/angiomodulin/mac25) modulates its biological activity. Biochem Biophys Res Commun 2003; 310: 612–8. [DOI] [PubMed] [Google Scholar]
- 14. Adachi Y, Itoh F, Yamamoto H et al Expression of angiomodulin (tumor‐derived adhesion factor/mac25) in invading tumor cells correlates with poor prognosis in human colorectal cancer. Int J Cancer 2001; 95: 216–22. [DOI] [PubMed] [Google Scholar]
- 15. Swisshelm K, Ryan K, Tsuchiya K, Sager R. Enhanced expression of an insulin growth factor‐like binding protein (mac25) in senescent human mammary epithelial cells and induced expression with retinoic acid. Proc Natl Acad Sci USA 1995; 92: 4472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson HM, Birnbaum RS, Poot M, Quinn LS, Swisshelm K. Insulin‐like growth factor binding protein‐related protein 1 inhibits proliferation of MCF‐7 breast cancer cells via a senescence‐like mechanism. Cell Growth Differ 2002; 13: 205–13. [PubMed] [Google Scholar]
- 17. Burger AM, Zhang X, Li H et al Down‐regulation of T1A12/mac25, a novel insulin‐like growth factor binding protein related gene, is associated with disease progression in breast carcinomas. Oncogene 1998; 16: 2459–67. [DOI] [PubMed] [Google Scholar]
- 18. Hwa V, Tomasini‐Sprenger C, Bermejo AL, Rosenfeld RG, Plymate SR. Characterization of insulin‐like growth factor‐binding protein‐related protein‐1 in prostate cells. J Clin Endocrinol Metab 1998; 83: 4355–62. [DOI] [PubMed] [Google Scholar]
- 19. Chen Y, Pacyna‐Gengelbach M, Ye F et al Insulin‐like growth factor binding protein‐related protein 1 (IGFBP‐rP1) has potential tumour‐suppressive activity in human lung cancer. J Pathol 2007; 211: 431–8. [DOI] [PubMed] [Google Scholar]
- 20. Mutaguchi K, Yasumoto H, Mita K et al Restoration of insulin‐like growth factor binding protein‐related protein 1 has a tumor‐suppressive activity through induction of apoptosis in human prostate cancer. Cancer Res 2003; 63: 7717–23. [PubMed] [Google Scholar]
- 21. Sprenger CC, Damon SE, Hwa V, Rosenfeld RG, Plymate SR. Insulin‐like growth factor binding protein‐related protein 1 (IGFBP‐rP1) is a potential tumor suppressor protein for prostate cancer. Cancer Res 1999; 59: 2370–5. [PubMed] [Google Scholar]
- 22. Sato Y, Chen Z, Miyazaki K. Strong suppression of tumor growth by insulin‐like growth factor‐binding protein‐related protein 1/tumor‐derived cell adhesion factor/mac25. Cancer Sci 2007; 98: 1055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 2008; 132: 363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wajapeyee N, Kapoor V, Mahalingam M, Green MR. Efficacy of IGFBP7 for treatment of metastatic melanoma and other cancers in mouse models and human cell lines. Mol Cancer Ther 2009; 8: 3009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mori T, Kariya Y, Komiya E et al Downregulation of a newly identified laminin, laminin‐3B11, in vascular basement membranes of invasive human breast cancers. Cancer Sci 2011; 102: 1095–100. [DOI] [PubMed] [Google Scholar]
- 26. Domínguez F, Avila S, Cervero A et al A combined approach for gene discovery identifies insulin‐like growth factor‐binding protein‐related protein 1 as a new gene implicated in human endometrial receptivity. J Clin Endocrinol Metab 2003; 88: 1849–57. [DOI] [PubMed] [Google Scholar]
- 27. Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor‐beta 1 induces alpha‐smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993; 122: 103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ignotz RA, Massagué J. Transforming growth factor‐beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 1986; 261: 4337–45. [PubMed] [Google Scholar]
- 29. Talts JF, Weller A, Timpl R, Ekblom M, Ekblom P. Regulation of mesenchymal extracellular matrix protein synthesis by transforming growth factor‐beta and glucocorticoids in tumor stroma. J Cell Sci 1995; 108: 2153–62. [DOI] [PubMed] [Google Scholar]
- 30. Boers W, Aarrass S, Linthorst C, Pinzani M, Elferink RO, Bosma P. Transcriptional profiling reveals novel markers of liver fibrogenesis: gremlin and insulin‐like growth factor‐binding proteins. J Biol Chem 2006; 281: 16289–95. [DOI] [PubMed] [Google Scholar]
- 31. Liu LX, Huang S, Zhang QQ et al Insulin‐like growth factor binding protein‐7 induces activation and transdifferentiation of hepatic stellate cells in vitro. World J Gastroenterol 2009; 15: 3246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali‐Bostwick CA. Insulin‐like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol 2005; 166: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruan W, Ying K. Abnormal expression of IGF‐binding proteins, an initiating event in idiopathic pulmonary fibrosis? Pathol Res Pract 2010; 206: 537–43. [DOI] [PubMed] [Google Scholar]
- 34. Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal 2010; 4: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 2010; 120: 3340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006; 6: 392–401. [DOI] [PubMed] [Google Scholar]
- 37. De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer 2008; 123: 2229–38. [DOI] [PubMed] [Google Scholar]
- 38. Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res 2001; 264: 169–84. [DOI] [PubMed] [Google Scholar]
- 39. Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma‐associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999; 59: 5002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Denys H, Deryckle L, Hendrix A et al Differential impact of TGF‐β and EGF on fibroblast differentiation and invasion reciprocally promotes colon cancer cell invasion. Cancer Lett 2008; 266: 263–74. [DOI] [PubMed] [Google Scholar]
- 41. Camps JL, Chang SM, Hsu TC et al Fibroblast‐mediated acceleration of human epithelial tumor growth in vivo . Proc Natl Acad Sci USA 1990; 87: 75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Picard O, Rolland Y, Poupon MF. Fibroblast‐dependent tumorigenicity of cells in nude mice: implication for implantation of metastases. Cancer Res 1986; 46: 3290–4. [PubMed] [Google Scholar]
- 43. Pen A, Moreno MJ, Martin J, Stanimirovic DB. Molecular markers of extracellular matrix remodeling in glioblastoma vessels: microarray study of laser‐captured glioblastoma vessels. Glia 2007; 55: 559–72. [DOI] [PubMed] [Google Scholar]


