Abstract
Microvascular free flap transfer for post‐oncologic reconstructive surgery in oral cancer is considered to be a challenge due to the complexity of the anatomy and function of the region. We sought to identify possible factors associated with microsurgical complications and to assess the impact of these complications in relation to patient survival. Following the inclusion and exclusion protocol, 142 patients with stage III and IV oral squamous cell carcinoma (OSCC) who underwent immediate free flap reconstruction after tumor ablation were included in the study. Clinical and surgical procedural‐related factors were retrieved from a database and analyzed retrospectively; survival data were evaluated using the Kaplan–Meier method. Major complications that required re‐anastomosis of the flap vessels occurred in 23 patients (16.2%); total necrosis of the flaps, regardless of salvage treatment, occurred in seven cases, with 95.1% of full flap survival. The American Society of Anesthesiologists classification, types of neck dissection, and number of flaps were regarded as strong predictors for surgical complications. Patients with these complications appeared to have a shortened survival (5‐year cancer‐specific survival of approximately 60%, both in stage III and IV OSCC). However, the impact of surgical complications on survival was significant only in stage III OSCC (P = 0.037). Strategies to minimize surgical complications should be used to ensure better prognoses for these patients.
Oral cancer, particularly oral squamous cell carcinoma (OSCC), is one of the most distressing diseases worldwide and is characterized by high locoregional recurrence and poor long‐term survival rates. In Taiwan, OSCC is the fourth leading cause of cancer deaths in the male population. Previous reports of OSCC survival in Taiwan indicated that approximately 50% of new cases at our medical center presented with advanced stages of the disease at their first visit. Comparing the data of the previous (before 1996) and current (after 2004) reports at our institution,1 there was an approximately 20% increase (77.2% vs 56.3%) in the 5‐year overall survival, regardless of tumor stage. We believe that the improved results may be attributed to the increased understanding of the clinicopathological characteristics of the disease, optimized surgical techniques, and standardization of treatment modalities based on the disease characteristics. According to the current evidence, surgery with or without radiation and chemotherapy is the curative treatment for OSCC. Excision with wide margins is important to ensure good local control of OSCC.2, 3 However, large defects created by tumor ablation can cause great morbidity, dysfunction, and disfigurement for the patient.
Head and neck reconstructive procedures were first performed using a pedicled myocutaneous flap in the 1960s, mainly from a pectoralis major myocutaneous flap.4 Over the past few decades, free flap reconstruction has had a major role in the rehabilitation of large defects following oral tumor resections. The accumulated evidence supports that microsurgical free flap reconstruction not only offers good functional and esthetic outcomes for patients,5, 6, 7 but also improves outcomes for cancer of the head and neck compared with the direct closure of the defect.3, 8, 9 A possible explanation for survival improvement is that the free flap transfer enables for a large panel of tissue to be transferred and to cover virtually any defect size, thereby allowing for more aggressive oncologic ablation.10, 11 However, the advantages of this procedure have been debated because the complexity of microvascular surgery and the easily contaminated environment of the upper aero‐digestive tract might lead to the high frequency of compromised vessels and wound infections.3, 12, 13
From the perspective of surgical oncologists, strategies to predict microsurgical complications by preoperative patient assessment and further optimize surgical techniques are essential. Especially for OSCC patients with advanced stages, microsurgical complications that require additional surgeries could also delay the postoperative adjuvant therapy and lead to subsequent treatment failure of OSCCs. To our knowledge, there is little evidence regarding the factors linking microsurgical outcomes and the prognosis of OSCC. Therefore, the current study aims to analyze multiple variables, to determine which are associated with surgical complications. We also sought to examine the relationship of these surgical complications and survival among patients with locally advanced OSCC.
Materials and Methods
Study population and data entry
We performed a retrospective review of the medical records of OSCC patients who underwent primary radical surgery at Taipei Veterans General Hospital from January 2004 to December 2007. Tumor staging was performed in accordance to the 2002 American Joint Committee on Cancer (AJCC), 6th Edition. Patients were eligible for subsequent analysis if they met the following inclusion criteria: (i) previously untreated and newly diagnosed stage III or stage IV OSCC, and (ii) had received ablative surgery and immediate reconstruction with microvascular flaps. The exclusion criteria were as follows: (i) recurrent or metastatic disease, (ii) previously treated with radiation or chemotherapy, and (iii) malignant disease of salivary glands or tumor entities other than OSCC. The medical chart review was performed by two investigators and consisted of a complete examination of all progress and nursing notes, operative dictations, pathological reports, radiographic studies, and laboratory results. According to the predefined criteria, 142 patients were enrolled in the study.
All 142 patients had biopsy‐proven OSCC and received definitive surgery with curative intent of complete surgical resection; this procedure involved primary tumor excision with at least a 15–20 mm safety margin and neck dissections. Surgery of the neck consisted of a supra‐omohyoid neck dissection (SOHND) for clinically negative (N0) cases and either modified or classical radical neck dissection (MRND or RND) for clinically detected neck nodal metastasis (N+). The image criteria for neck nodal metastasis were size (maximum nodal diameter larger than 1.5 cm at level I and II or larger than 1 cm elsewhere in the neck), the presence of rim enhancement, central necrosis, or extranodal invasion. Postoperative adjuvant concurrent chemoradiation (CCRT) was administered to patients with pT4 tumors, positive lymph node metastases, extracapsular nodal spread, or more than two adverse pathological prognosticators, such as perineural invasion, tumor emboli, and lymphovascular permeation. One hundred and sixteen patients (81.7%) received adjuvant postoperative CCRT treatment according to our treatment guidelines. The other 26 patients were subjected to postoperative adjuvant radiotherapy only.
All patients had follow‐ups continued until December 2011. Locoregional recurrence and distant metastases were evaluated by a complete clinical examination, including a regular chest X‐ray and CT/MRI. Local recurrence was defined as tumor growth at a distance of <2 cm away from the primary tumor. Survival time was calculated from the day of surgery to the last day of patient contact or patients' death from disease.
After approval by the institutional review board (IRB), information was collected from medical records linking the patient's name and hospital number. Pre‐existing patient factors included age, gender, cigarette smoking, body mass index (BMI), history of diabetes mellitus and cardiovascular disease, as well as the American Society of Anesthesiologists classification of preoperative status (ASA score). Tumor characteristics included tumor size (T), nodal involvement (N), tumor stage, and locations of tumors. Parameters relating to surgical procedures, including the types and numbers of flaps and the type of neck dissection, were also recorded.
All microsurgical complications that required two or more re‐operative interventions (salvage surgery) during the interval between surgery and radiation therapy were registered and subclassified as major or minor surgical complications. Major complications were defined as complications occurring in the surgical field of the recipient site that required reanastomosis of flap vessels. Minor complications were those unrelated to compromised vessels, including abscesses, hematomas/bleeding, seroma formation, and incisive dehiscence of wounds, which required minor‐scale salvage surgery, such as debridement. Complications only requiring conservative medical therapy were not included in this study.
Statistical analysis
Data were analyzed with the statistical software package SPSS 17.0 (SPSS, Chicago, IL, USA). The means and standard deviations are given to describe the continuous variables, and the frequencies and percentages are given for categorical variables. To evaluate the group difference, the Fisher's exact test or chi‐squared test was applied for categorical variables and the Mann–Whitney U‐test or one‐way anova was used for continuous variables (age and BMI). A logistic regression was performed to adjust relevant covariates for confounding factors to obtain the adjusted odds ratio (OR) and 95% confidence interval (CI). The Kaplan–Meier method was used to calculate the rate of cumulative survival. The unadjusted comparison of survival curves between different groups was made by the log‐rank test. In all analyses, P‐values <0.05 were considered to be statistically significant.
Results
The patients' demographics, medical history of cardiovascular disease and diabetes, ASA classification, and tumor characteristics are summarized in Table 1. The mean age of our study population was 49.39 years (range 27–84 years). For the 142 study patients, 136 (95.8%) were men and six (4.2%) were women. The mean BMI was 24.62 kg/m2. Most of the patients (83.8%) had a history of cigarette smoking, with 37 (26.1%) being habitual users (consumed at least one pack of cigarettes per day). Regarding their past medical history, only 10 patients (7.0%) had co‐existent illnesses of cardiovascular disease, while diabetes was relatively common, found in 26 patients (18.3%).
Table 1.
Demographic and tumor characteristics of patients with microsurgical free flap reconstruction (n = 142)
| Characteristics | No. | % |
|---|---|---|
| Age (years) | ||
| Mean | 49.39 | |
| SD | 10.87 | |
| Range | 27–84 | |
| BMI (kg/m2) | ||
| Mean | 24.62 | |
| SD | 4.29 | |
| Gender | ||
| Male | 136 | 95.8 |
| Female | 6 | 4.2 |
| Cigarette smoking | ||
| ≥1 ppd | 37 | 26.1 |
| <1 ppd | 82 | 57.7 |
| Non‐smoker | 23 | 16.2 |
| Diabetes | ||
| No | 116 | 81.7 |
| Yes | 26 | 18.3 |
| Cardiovascular disease | ||
| No | 134 | 94.4 |
| Yes | 8 | 5.6 |
| ASA score | ||
| 1 | 58 | 40.8 |
| 2 | 64 | 45.1 |
| 3 | 20 | 14.1 |
| Stage | ||
| III | 36 | 25.4 |
| IV | 106 | 74.6 |
| T stage | ||
| T2 | 22 | 15.5 |
| T3 | 36 | 25.4 |
| T4 | 84 | 59.2 |
| N stage | ||
| N0 | 66 | 46.5 |
| N1 | 23 | 16.2 |
| N2 | 52 | 36.6 |
| N3 | 1 | 0.7 |
| Tumor subsites | ||
| Buccal mucosa | 71 | 50.0 |
| Tongue | 34 | 23.9 |
| Gingiva | 28 | 19.7 |
| Mouth floor | 5 | 3.5 |
| Lip | 3 | 2.1 |
| Palate | 1 | 0.7 |
ASA, American Society of Anesthesiologists classification of preoperative status; BMI, body mass index; ppd, pack per day.
Of all 142 patients treated by comprehensive ablative surgery and immediate microsurgical reconstruction, 58 (40.8%) had an ASA score of 1, 64 (45.1%) had an ASA score of 2, and 20 (14.1%) had an ASA score of 3. When the patients presented, the tumor was at stage III in 36 patients (25.4%) and stage IV in 106 patients (74.6%). The majority of OSCC cases were located on the buccal mucosa, which accounted for a total of 50.0% of all of the cases. The other two common sites of OSCC were the tongue (23.9%) and the gingiva (19.7%).
The classification of major and minor surgical complications is shown in Table 2. Major surgical complications included problems related to the anastomosis, such as vein or artery thrombosis, and miscellaneous problems, including infection, hematoma, or others that caused secondary vessel problems. In 142 cases, major surgical complications were encountered in 23 patients (16.2%), with most of them caused by vein thrombosis (n = 10) or infection (n = 6). Complete flap loss after the re‐exploration of the microsurgical anastomoses occurred in seven (4.9%) patients. Alternative regional or free flaps were required for their secondary reconstruction. The success rate of immediate microsurgical reconstruction after composite resection in locally advanced OSCC was therefore 95.1%. Minor surgical complications that did not require reanastomosis of flaps but more minor‐scale salvage surgeries included abscesses, hematomas/bleeding, incisive dehiscence, and seroma formation, either due to chyle or saliva leakage. In our study, 50 patients (35.2%) experienced minor surgical complications, and the majority of them were incisive dehiscence (n = 20).
Table 2.
Classification of microsurgical complications
| n = 142 | No. | % |
| Minor complications | 50 | 35.2 |
| Infection/abscess | 17 | 12.0 |
| Incisive dehiscence | 20 | 14.1 |
| Hematoma/bleeding | 11 | 7.7 |
| Seroma | 3 | 2.1 |
| Major complications | 23 | 15.5 |
| Infection | 6 | 2.8 |
| Vein thrombosis | 10 | 7.0 |
| Artery thrombosis | 3 | 2.1 |
| Miscellaneous | 4 | 2.8 |
Miscellaneous complications include seroma formation, chyle leakage, and saliva leakage.
We divided the factors related to surgical complications into three categories: pre‐existing patient factors, tumor characteristics, and surgical procedure‐related factors. The occurrence of surgical complications in relation to pre‐existing patient factors and tumor characteristics is shown in Table 3. The ASA classification and tumor stage were significantly associated with surgical complications. Although not significant, there was a trend showing an association between cardiovascular disease and surgical complications. Other factors, such as age, level of BMI, history of diabetes, and tumor size, were unrelated to surgical complications.
Table 3.
Correlation of preexisting factors and surgical complications
| Factor | Total number of patients (n = 142) | No. of complications (%)b | Significance(P‐value) |
|---|---|---|---|
| Age | 48.63 ± 9.87 | NSc | |
| BMI | 25.10 ± 4.09 | NSc | |
| Cigarette smoking | |||
| No | 105 | 53 (50.3) | NS (0.35) |
| Yes | 37 | 22 (59.5) | |
| Diabetes | |||
| No | 116 | 59 (50.9) | NS (0.32) |
| Yes | 26 | 16 (61.5) | |
| Cardiovascular disease | |||
| No | 132 | 67 (50.8) | NS (0.10) |
| Yes | 10 | 8 (80.0) | |
| ASA score | |||
| 1 | 58 | 19 (32.8) | <0.001a |
| 2 | 64 | 44 (68.8) | |
| 3 | 20 | 12 (60.0) | |
| T stage | |||
| T2 | 22 | 13 (59.1) | NS (0.15) |
| T3 | 36 | 14 (38.9) | |
| T4 | 84 | 48 (57.1) | |
| Stage | |||
| III | 36 | 13 (36.1) | 0.02a |
| IV | 106 | 62 (58.5) | |
P < 0.05.
Complications refer to either major or minor complications that required re‐exploration operation.
Comparison of means is based on the Mann–Whitney U‐test.
BMI, body mass index; NS, non‐significant.
Table 4 provides an overview of the surgical techniques and the correlation with surgical complications. These surgical procedural factors were classified as neck dissection, sites of donor flaps, osseous or non‐osseous flaps, and single or double/triple flaps. In the different types of neck dissections, RND was found to have the highest rate of complications (66.7%), followed by MRND (54.3%) and SOHND (33.3%). A statistically lower complication rate was found in non‐osseous and single free flaps compared to osseous and double/triple flaps. The result was similar when the complications were stratified into major and minor ones.
Table 4.
Comparison between surgical techniques and complications
| Factor | Total number of patients | No. of complications (%)b | Significance (P‐value) | Major complications (%) | Minor complications (%) |
|---|---|---|---|---|---|
| Neck dissection | |||||
| SOHND | 42 | 14 (33.3) | 0.005a | 4 (9.5) | 10 (23.8) |
| MRND | 46 | 25 (54.3) | 7 (15.2) | 17 (37.0) | |
| RND | 54 | 36 (66.7) | 12 (22.2) | 24 (44.4) | |
| Flap type | |||||
| Non‐osseous flap | 53 | 39 (43.8) | 0.006a | 8 (9.0) | 31 (34.8) |
| Osseous flap | 89 | 36 (67.9) | 15 (28.3) | 20 (37.7) | |
| Flap number | |||||
| Single flap | 118 | 56 (47.5) | 0.008a | 14 (11.9) | 42 (35.6) |
| Double or triple flap | 24 | 19 (82.6) | 9 (37.5) | 9 (37.5) | |
| Sites of flap donor | |||||
| Forearm | 32 | 16 (50.0) | 0.024a c | 3 (9.4) | 13 (40.6) |
| Anterolateral thigh | 51 | 19 (37.3) | 5 (9.8) | 14 (27.5) | |
| Fibular flap | 31 | 18 (58.1) | 6 (19.4) | 12 (38.7) | |
| Rectus abdominis flap | 3 | 2 (66.7) | 0 (0) | 2 (66.7) | |
| Latissimus dorsi | 1 | 1 (100) | 0 (0) | 1 (100) | |
| PMMCF combine one free flap | 16 | 12 (75.0) | 6 (37.5) | 6 (37.5) | |
| Fibular combine ALT | 8 | 7 (87.5) | 3 (37.5) | 3 (37.5) | |
| Sum | 142 | 75 (52.8) | 23 (16.2) | 50 (35.2) | |
P < 0.05.
Complications refer to major and minor complications that required re‐exploration operation.
The statistical analysis result excludes rectus abdominus and latissimus dorsi flaps.
ALT, anterolateral thigh flap; MRND, modified radical neck dissection; NS, non‐significant; PMMCF, pectoralis major myocutaneous flap; RND, classical radical neck dissection; SOHND, supra‐omohyoid neck dissection.
Considering the use of microvascular donor flaps, the majority of cases used anterolateral thigh flaps (ALT, n = 51), followed by forearm flaps (n = 32) and fibular osteocutaneous flaps (n = 31). Less commonly used flaps were the rectus abdominus musculocutaneous (RAM, n = 3) and latissimus dorsi flaps (n = 1). There were 23 double flaps (including eight double free flaps and 15 pectoralis major myocutaneous flaps plus one of the free flaps above). One triple flap was performed by combining the ALT, fibular flap, and RAM. The major complication rate was the highest in combined flaps (double or triple flaps); both were 37.5%. The second highest rate was found in single fibular flap, 19.4%. Forearm and ALT flaps were 9.4% and 9.8%. When Fisher's exact test was performed, RAM and latissimus dorsi flaps were excluded from the statistical analysis because of the small number of cases. It was found that the type of free flap donor site was statistically associated with surgical complications, with P‐values of 0.024.
We performed a multivariate logistic regression analysis to determine the independent predictors of microsurgical complications. The factors that had predictive potential in the univariate analysis, including ASA score, tumor stage, types of neck dissection, and flap numbers, were included in the multivariate analysis (Table 5). The type of flap (osseous or non‐osseous) was not included in the multivariate analysis because it had a strong correlation with the number of flaps (Fisher's exact test, P < 0.001). The result showed that the ASA score was the strongest independent predictor of surgical complications (P = 0.001; odds ratio with respect to ASA score 2 and 3, 3.454; 95% CI, 1.625–7.343). The type of neck dissection (RND only) also significantly increased the risk for surgical complications (P = 0.032, odds ratio, 2.799; 95% CI, 1.094–7.180). Additionally, the number of flaps influenced the rate of surgical complications (P = 0.06; odds ratio, 2.905; 95% CI, 0.958–8.814).
Table 5.
Multivariate analysis of factors associated with surgical complications
| Factor | Adjusted odds ratio | 95% CI | Significance (P‐value) |
|---|---|---|---|
| ASA score | |||
| 1 | 1 | 1.625–7.343 | 0.001a |
| 2 and 3 | 3.454 | ||
| Tumor stage | |||
| III | 1 | 0.686–4.042 | 0.260 |
| IV | 1.665 | ||
| Neck dissection | |||
| SOHND | 1 | ||
| MRND | 1.949 | 0.763–4.981 | 0.163 |
| RND | 2.799 | 1.094–7.180 | 0.032a |
| Number of flaps | |||
| Single flap | 1 | 0.958–8.814 | 0.060 |
| Double and triple flaps | 2.905 | ||
P < 0.05.
ASA, American Society of Anesthesiologists classification of preoperative status; MRND, modified radical neck dissection; RND, classical radical neck dissection; SOHND, supra‐omohyoid neck dissection.
We also analyzed the postoperative onset of surgical complications and when salvage re‐operations were performed (Fig. 1a). All of the arterial and vein thrombosis complications were detected within the first week of the postoperative period. Infections, regardless of flap necrosis, were found almost 1 week postoperatively, with a peak incidence 1–2 weeks postoperatively and the highest occurrence rate within this period. However, minor surgical complications other than infections occurred mostly within 3–4 weeks postoperatively. Furthermore, we analyzed the frequencies of re‐operation in different surgical complications (Fig. 1b). The results showed that most of these surgical complications could be resolved by two re‐operations. Unfortunately, some of the vein problems and infections required more than three re‐operations.
Figure 1.

(a) Postoperative onset of surgical complications and (b) frequencies of re‐exploration operations for surgical complications.
Finally, we aimed to investigate the impact of surgical complications in OSCC patients. In a Kaplan–Meier analysis, patients who had surgical complications were associated with a worse 5‐year cancer‐specific survival (CSS) of 60.1% compared with 73.9% for those who did not have surgical complications (log‐rank P = 0.217). In addition, there was an increased risk of locoregional recurrence and distant metastasis in patients with surgical complications (62.1%) compared with patients without surgical complications (37.9%) (P = 0.066); the actuarial 5‐year recurrence‐free survival (RFS) was 46.0% and 65.0%, respectively (detailed analysis not shown). After stratifying the cases into stage III and stage IV disease, a comparison of survival between the group of patients with and without surgical complications was re‐analyzed. The results demonstrated that surgical complications had a significant negative impact on survival in the stage III group with complications (5‐year CSS 90.9% and 59.8%, respectively) (P = 0.037) (Fig. 2a), while there was no difference in the stage IV group (Fig. 2b).
Figure 2.
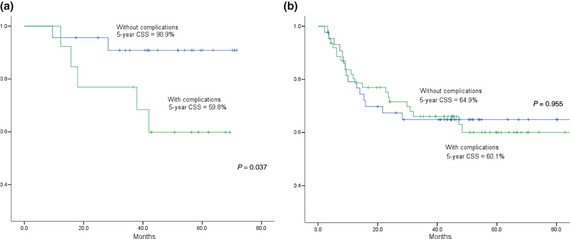
Comparison of cumulative survival curves in stage III (a) and IV (b) oral squamous cell carcinoma (OSCC) patients, with or without reoperation.
Discussion
For patient factors related to head and neck microsurgical complications, previous studies revealed different results.14, 15, 16, 17, 18, 19, 20 Our results were consistent with certain groups regarding diabetes21, 22, 23 and elderly patients.20, 23, 24, 25, 26, 27, 28 Nevertheless, contradicted groups have suggested that diabetes interferes with free flap survival;20, 29 and there was an unsatisfactory wound repair and higher incidence of surgical complications in smokers.20, 30, 31 In this study, the only strongest independent preoperative factor that predicted an increased risk for surgical complications was the ASA score; this finding was compatible with the statements of others.4, 26, 32 It indicated that a detailed preoperative evaluation of general physical status is more important than merely focusing on a single particular medical history.
One of the most interesting findings in this study is that the type of neck dissection (RND) was a predictor for surgical complications. In current practice, both MRND and RND are performed in clinical N+ patients by different surgical oncologists. In our series, 46 patients received MRND and 54 patients received RND. Although the same levels of lymph nodes are resected in RND, a significant correlation to surgical complications was not found for MRND when compared to SOHND. We consider the possible reasons are that transaction of internal jugular vein could devastate anastomosis of vessels and circulation of head and neck region. The surgeons might need to take another vein graft for facilitating anastomosis, subsequently prolong the operation time and complicate the surgical procedures. In the other aspect, sacrifice of sternocleidomastoid muscle could lead to loss of its protective function for the carotid artery, its barrier function when flap necrosis or fistula formation occurs, and also cause distortion of skin flaps which subsequently impedes primary closure of flap, leading to dehiscence of wound. Therefore, although MRND is technically more complicated than RND, we should consider MRND as the first choice for clinical N+ cases unless there is evidence of extracapsular spread on image examination.
Similar to Kroll et al. and Nakatsuka et al.,28, 33 we found that fibula osteocutaneous flaps had a significantly higher surgical complication rate compared to other non‐osseous flaps (especially for major surgical complications, 28.3% vs 9.0%). We believe that this phenomenon is rooted in the large and complex three‐dimensional defects caused by tumor resection in combination with bony structures. The fibular graft and the orientation of its pedicle were not always perfectly fitted to the defect and had to be reshaped, even with compromising tension and pressure on the pedicle, which resulted in poor vascularization and tissue perfusion. We have also shown that the number of flaps had a near‐significant impact on surgical complications (P = 0.06); among the 23 double flaps and one triple flap, 22 (91.7%) were fibular osteocutaneous flaps that had to be combined with other soft tissue flaps due to the insufficient soft tissue paddle of fibular flaps to fully cover the defect. These limitations of fibular osteocutaneous flaps and multiple flaps complicated the reconstructive procedure and led to a higher surgical complicated rate.
In addition to factors relating to surgical complications, we also reported the classification, time interval and frequencies of major and minor surgical complications. Compared to previous literature reports of wound infection rates of 21–45% for reconstructive surgeries of the aero‐digestive tract,34, 35 our infection rate of 16.2% (23 cases) was quite low. However, wound infections generally occurred later in the postoperative period and required more salvage surgeries. For patients who are candidates for adjuvant RT or CCRT treatment, wound infections might become major surgical complications that are devastating to ongoing treatment plans. Because the oral cavity is an environment prone to wound infections, we suggest that not only should prophylactic antibiotics be administered, but they should also be administered during the postoperative period.
The rate of surgical re‐exploration to check microvascular anastomoses was 15.5% in our series. The percentage was quite similar to Dassonveille et al. who evaluated 213 cases of head and neck reconstruction with free flaps.4 The reasons for the major complications in the current study included venous thrombosis, arterial thrombosis, and infection or miscellaneous reasons leading to vascular insufficiency. Venous thromboses occurred more commonly (10 cases) and were detected later than arterial thromboses (three cases). There was a survival rate of 100% after salvage thrombectomy for the three cases of arterial thrombosis in the current study. In contrast, venous thromboses resulted in complete flap loss in three cases (survival rate 70%) even though surgical re‐exploration was performed as soon as vascular insufficiencies were detected. We believed that these findings are related to the fact that arterial insufficiency and thromboses are easier to detect by temperature, capillary refill, bleeding to needle sticks, and external Doppler monitoring compared to venous problems. Similar to our findings, Chen et al.36 also showed that the time when the flap compromise occurs is a significant predictor of flap salvage outcomes; there was an 80–100% salvage rate when the first circulatory compromise occurred during the first to fifth 24‐hour period after surgery. In our series, 23 patients experienced major surgical complications and received salvage reanastomosis operations. Seven out of 23 patients failed regardless of salvage surgery due to total necrosis of the flaps and required new flaps for the secondary reconstruction. Our success rate of immediate microvascular free flap reconstruction was 95.1%, consistent with the reported success rate of 95–97%.37
In this study, we observed an interesting relationship between surgical complications and the OSCC 5‐year CSS. Our study revealed an overall 5‐year CSS of 78.5% in stage III and 61.9% in stage IV cases. However, when further stratifying stage III and stage IV patients into groups with and without surgical complications, we found that surgical complications are associated with lower survival rates (stage III/IV: 5‐year CS = 59.8%/60.1%) than those without surgical complications (stage III/IV: 5‐year CSS = 90.9%/64.9%). Of note, we demonstrated a significant difference (31.1%) in CSS between stage III patients with and without complications. Although there is a limitation of statistical power in the group of stage III OSCC patients due to the relatively small number of cases (36 cases), we believe that there is a trend showing the significant negative prognostic impact of surgical complications in stage III OSCC patients. This might be due to the complications that would delay the timing of adjuvant radiotherapy or CCRT, since there was a significant correlation between the two time intervals of the duration between tumor‐resection/the last salvage surgery and the duration between tumor‐resection/adjuvant therapy (Pearson correlation coefficient = 0.847, P < 0.001). Despite these findings, we continue to support the use of free flap transfer for the reconstruction of defects created by the ablation of oral cancers in advanced tumor stages; Mucke et al.3 advocated that immediate microsurgical reconstruction should be administered in patients with higher tumor stages for better survival. Nevertheless, quality improvement is much more important to reduce surgical complications in stage III than stage IV OSCC patients, thus enhancing their prognosis and outcomes.
In conclusion, we have shown that particular groups of patients (those with an ASA score of 2 or 3) and surgical techniques (radical neck dissection and multiple flaps combining osseous flaps) would have a higher tendency to experience surgical complications. In addition, microsurgical complications have a large effect on survival rates for stage III OSCC patients and strategies to minimize complications should be used to ensure better prognoses for these patients.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
This work was supported by grant VGH‐B‐042 of the Taipei Veterans General Hospital.
(Cancer Sci, 2012; 103: 1672–1678)
References
- 1. Lo WL, Kao SY, Chi LY, Wong YK, Chang RC. Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: factors affecting survival. J Oral Maxillofac Surg 2003; 61: 751–8. [DOI] [PubMed] [Google Scholar]
- 2. Liao CT, Chang JT, Wang HM et al Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann Surg Oncol 2008; 15: 915–22. [DOI] [PubMed] [Google Scholar]
- 3. Mucke T, Wolff KD, Wagenpfeil S, Mitchell DA, Holzle F. Immediate microsurgical reconstruction after tumor ablation predicts survival among patients with head and neck carcinoma. Ann Surg Oncol 2010; 17: 287–95. [DOI] [PubMed] [Google Scholar]
- 4. Dassonville O, Poissonnet G, Chamorey E et al Head and neck reconstruction with free flaps: a report on 213 cases. Eur Arch Otorhinolaryngol 2008; 265: 85–95. [DOI] [PubMed] [Google Scholar]
- 5. Kruse AL, Luebbers HT, Gratz KW, Obwegeser JA. Factors influencing survival of free‐flap in reconstruction for cancer of the head and neck: a literature review. Microsurgery 2010; 30: 242–8. [DOI] [PubMed] [Google Scholar]
- 6. Rinaldo A, Shaha AR, Wei WI, Silver CE, Ferlito A. Microvascular free flaps: a major advance in head and neck reconstruction. Acta Otolaryngol 2002; 122: 779–84. [PubMed] [Google Scholar]
- 7. Thorwarth M, Eulzer C, Bader R, Wolf C, Schmidt M, Schultze‐Mosgau S. Free flap transfer in cranio‐maxillofacial surgery: a review of the current data. Oral Maxillofac Surg 2008; 12: 113–24. [DOI] [PubMed] [Google Scholar]
- 8. Hanasono MM, Friel MT, Klem C et al Impact of reconstructive microsurgery in patients with advanced oral cavity cancers. Head Neck 2009; 31: 1289–96. [DOI] [PubMed] [Google Scholar]
- 9. Lidman D, Niklasson M. Survival and function in patients with tumours of the head and neck operated on and reconstructed with free flaps. Scand J Plast Reconstr Surg Hand Surg 2008; 42: 77–85. [DOI] [PubMed] [Google Scholar]
- 10. Rogers SN, Brown JS, Woolgar JA et al Survival following primary surgery for oral cancer. Oral Oncol 2009; 45: 201–11. [DOI] [PubMed] [Google Scholar]
- 11. Binahmed A, Nason RW, Abdoh AA. The clinical significance of the positive surgical margin in oral cancer. Oral Oncol 2007; 43: 780–4. [DOI] [PubMed] [Google Scholar]
- 12. Hudgins PA. Flap reconstruction in the head and neck: expected appearance, complications, and recurrent disease. Eur J Radiol 2002; 44: 130–8. [DOI] [PubMed] [Google Scholar]
- 13. Marchetti C, Pizzigallo A, Cipriani R, Campobassi A, Badiali G. Does microvascular free flap reconstruction in oral squamous cell carcinoma improve patient survival? Otolaryngol Head Neck Surg 2008; 139: 775–80. [DOI] [PubMed] [Google Scholar]
- 14. Eckardt A, Fokas K. Microsurgical reconstruction in the head and neck region: an 18‐year experience with 500 consecutive cases. J Craniomaxillofac Surg 2003; 31: 197–201. [DOI] [PubMed] [Google Scholar]
- 15. Farwell DG, Reilly DF, Weymuller EA et alPredictors of perioperative complications in head and neck patients. Arch Otolaryngol Head Neck Surg 2002; 128: 505–11. [DOI] [PubMed] [Google Scholar]
- 16. Khouri RK, Cooley BC, Kunselman AR et al A prospective study of microvascular free‐flap surgery and outcome. Plast Reconstr Surg 1998; 102: 711–21. [DOI] [PubMed] [Google Scholar]
- 17. Shaari CM, Buchbinder D, Costantino PD, Lawson W, Biller HF, Urken ML. Complications of microvascular head and neck surgery in the elderly. Arch Otolaryngol Head Neck Surg 1998; 124: 407–11. [DOI] [PubMed] [Google Scholar]
- 18. Singh B, Cordeiro PG, Santamaria E, Shaha AR, Pfister DG, Shah JP. Factors associated with complications in microvascular reconstruction of head and neck defects. Plast Reconstr Surg 1999; 103: 403–11. [DOI] [PubMed] [Google Scholar]
- 19. Suh JD, Sercarz JA, Abemayor E et al Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg 2004; 130: 962–6. [DOI] [PubMed] [Google Scholar]
- 20. Valentini V, Cassoni A, Marianetti TM et al Diabetes as main risk factor in head and neck reconstructive surgery with free flaps. J Craniofac Surg 2008; 19: 1080–4. [DOI] [PubMed] [Google Scholar]
- 21. Nahabedian MY, Momen B, Manson PN. Factors associated with anastomotic failure after microvascular reconstruction of the breast. Plast Reconstr Surg 2004; 114: 74–82. [DOI] [PubMed] [Google Scholar]
- 22. Cooley BC, Hanel DP, Anderson RB, Foster MD, Gould JS. The influence of diabetes on free flap transfer: I. Flap survival and microvascular healing. Ann Plast Surg 1992; 29: 58–64. [DOI] [PubMed] [Google Scholar]
- 23. Lee S, Thiele C. Factors associated with free flap complications after head and neck reconstruction and the molecular basis of fibrotic tissue rearrangement in preirradiated soft tissue. J Oral Maxillofac Surg 2010; 68: 2169–78. [DOI] [PubMed] [Google Scholar]
- 24. Haughey BH, Wilson E, Kluwe L et al Free flap reconstruction of the head and neck: analysis of 241 cases. Otolaryngol Head Neck Surg 2001; 125: 10–7. [DOI] [PubMed] [Google Scholar]
- 25. Jones NF, Jarrahy R, Song JI, Kaufman MR, Markowitz B. Postoperative medical complications–not microsurgical complications–negatively influence the morbidity, mortality, and true costs after microsurgical reconstruction for head and neck cancer. Plast Reconstr Surg 2007; 119: 2053–60. [DOI] [PubMed] [Google Scholar]
- 26. Rosenberg AJ, Van Cann EM, van der Bilt A, Koole R, van Es RJ. A prospective study on prognostic factors for free‐flap reconstructions of head and neck defects. Int J Oral Maxillofac Surg 2009; 38: 666–70. [DOI] [PubMed] [Google Scholar]
- 27. Ozkan O, Ozgentas HE, Islamoglu K, Boztug N, Bigat Z, Dikici MB. Experiences with microsurgical tissue transfers in elderly patients. Microsurgery 2005; 25: 390–5. [DOI] [PubMed] [Google Scholar]
- 28. Kroll SS, Schusterman MA, Reece GP et al Choice of flap and incidence of free flap success. Plast Reconstr Surg 1996; 98: 459–63. [DOI] [PubMed] [Google Scholar]
- 29. Bozikov K, Arnez ZM. Factors predicting free flap complications in head and neck reconstruction. J Plast Reconstr Aesthet Surg 2006; 59: 737–42. [DOI] [PubMed] [Google Scholar]
- 30. Kuri M, Nakagawa M, Tanaka H, Hasuo S, Kishi Y. Determination of the duration of preoperative smoking cessation to improve wound healing after head and neck surgery. Anesthesiology 2005; 102: 892–6. [DOI] [PubMed] [Google Scholar]
- 31. Silverstein P. Smoking and wound healing. Am J Med 1992; 15: 22S–4S. [DOI] [PubMed] [Google Scholar]
- 32. Eckardt A, Meyer A, Laas U, Hausamen JE. Reconstruction of defects in the head and neck with free flaps: 20 years experience. Br J Oral Maxillofac Surg 2007; 45: 11–5. [DOI] [PubMed] [Google Scholar]
- 33. Nakatsuka T, Harii K, Asato H et al Analytic review of 2372 free flap transfers for head and neck reconstruction following cancer resection. J Reconstr Microsurg 2003; 19: 363–8; discussion 9. [DOI] [PubMed] [Google Scholar]
- 34. Penel N, Fournier C, Lefebvre D, Lefebvre JL. Multivariate analysis of risk factors for wound infection in head and neck squamous cell carcinoma surgery with opening of mucosa. Study of 260 surgical procedures. Oral Oncol 2005; 41: 294–303. [DOI] [PubMed] [Google Scholar]
- 35. Cloke DJ, Green JE, Khan AL, Hodgkinson PD, McLean NR. Factors influencing the development of wound infection following free‐flap reconstruction for intra‐oral cancer. Br J Plast Surg 2004; 57: 556–60. [DOI] [PubMed] [Google Scholar]
- 36. Chen KT, Mardini S, Chuang DC et al Timing of presentation of the first signs of vascular compromise dictates the salvage outcome of free flap transfers. Plast Reconstr Surg 2007; 120: 187–95. [DOI] [PubMed] [Google Scholar]
- 37. Genden EM, Rinaldo A, Suarez C, Wei WI, Bradley PJ, Ferlito A. Complications of free flap transfers for head and neck reconstruction following cancer resection. Oral Oncol 2004; 40: 979–84. [DOI] [PubMed] [Google Scholar]


