Abstract
UL16‐binding protein 2 (ULBP2) is one of the ligands for NKG2D (NKG2DL). ULBP2 expression is induced in transformed cells and is recognized by immune effector cells via the activating NKG2D immunoreceptor. Soluble forms of NKG2DL have been reported in the serum of patients with several types of cancer. The present study investigated the diagnostic and prognostic significance of serum‐soluble ULBP2 (sULBP2) in lung cancer patients. We used flow cytometry to evaluate the surface expression of NKG2DL by various lung cancer cells, while sULBP2 was measured using our original ELISA. In addition, the immunological effect of sULBP2 on peripheral blood mononuclear cells (PBMC) was examined by the 51 Cr release assay. We found that ULBP2 was highly expressed and that the sULBP2 level was elevated in supernatants of cultured non‐small‐cell lung cancer (NSCLC) cells as well as in the serum of NSCLC patients. ULBP2 levels were especially high in squamous cell carcinoma (SQ) patients. Clinical stage IIIB and IV NSCLC patients with a sULBP2 level ≥8.7 pg/mL showed significantly shorter survival than patients with sULBP2 <8.7 pg/mL. In multivariate analysis, a sULBP2 level ≥8.7 pg/mL (hazard ratio [HR], 2.13; P = 0.038) and clinical stage IV (HR, 2.65; P = 0.019) were independent determinants of a poor outcome. As a possible mechanism, we demonstrated that sULBP2 directly suppresses the cytolytic activity of PBMC. In conclusion, ULBP2 is the most significant NKG2DL for lung cancer, and sULBP2 is useful in the diagnosis of SQ and as a prognostic indicator for patients with advanced NSCLC. (Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02330.x, 2012)
Lung cancer is the leading cause of cancer death around the world. Despite the recent development of multidisciplinary therapy, the prognosis for lung cancer patients is still very poor, largely due to the advanced stage of their tumors at the time of diagnosis and the unpredictable behavior of the disease. Therefore, effective detection of lung cancer and provision of suitable therapy depending on each patient's prognosis using biomarkers are of vital importance.
The host immune response to tumor cells is mediated by innate and adaptive immune processes that involve lymphocytes such as natural killer (NK) cells, CD8 T cells and γδ+ T cells. These cells have also been reported to play an important role in host immunity against lung cancer.1 Various studies have revealed the mechanisms used by these effector cells to recognize and attack tumor cells.2 NKG2D is an activating receptor that is expressed by NK cells, CD8+ αβ+ T cells, γδ+ T cells and NKT cells.3 The ligands for NKG2D are generally not expressed by normal cells, but their expression is induced in infected4 or transformed cells5 that should be eliminated by the host immune system. NKG2D recognizes its ligands expressed on the surface of target cells and then augments the cytolytic activity and cytokine production of effector cells. Therefore, the NKG2D–NKG2D ligand system is thought to play an essential role in host tumor immunity.6
A diverse range of ligands for NKG2D has recently been identified.7 In humans they consist of two families with structural similarities. One is the MHC class I chain‐related (MIC) family of molecules (MICA and MICB), and the other is a family of cell surface glycoproteins that can bind to human cytomegalovirus UL16 proteins (the ULBP family: ULBP1‐4, RAET1G and RAET1L).8, 9, 10, 11, 12 In vivo expression of NKG2D ligands is very limited in normal tissues,13, 14 whereas the constitutive expression of NKG2D ligands has been reported in many malignant tumors, and such expression is correlated with the NKG2D‐dependent cytotoxicity15, 16, 17, 18 of immune effector cells. Therefore, NKG2D ligands might be a possible marker of transformed cells that have the potential to modify host immune responses.
Recently, it was demonstrated that NKG2D ligands are released in soluble form from the surface of cancer cells,19, 20, 21 and that soluble ligands can be measured in the serum of cancer patients. Indeed, elevated serum levels of soluble MICA,19, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 MICB20, 25, 29, 32 and ULBP221, 27 have been detected in cancer patients. In addition, several clinical studies have suggested a correlation between the level of soluble MICA (sMICA) and a poor prognosis.23, 33 Therefore, soluble NKG2D ligands might have several useful properties as diagnostic/prognostic markers for cancer.
In the present study, we evaluated the pattern of expression of NKG2D ligands by a panel of lung cancer cell lines and focused on the soluble form of ULBP2 in the serum of lung cancer patients, and investigated its usefulness as a diagnostic and prognostic marker for lung cancer.
Materials and Methods
Cells
Details regarding the source of the cells and culture conditions are presented in Data S1.
Reagents
Anti‐MICA (clone 159227), anti‐MICB (clone 236511), anti‐ULBP1 (clone 170818), anti‐ULBP2 (clone 165903), anti‐ULBP3 (clone 166510), polyclonal anti‐ULBP2, recombinant ULBP2‐Fc and recombinant MICA‐Fc were obtained from R&D Systems (Minneapolis, MN, USA). Anti‐MICA (AMO1) and anti‐ULBP2 (BUMO1) were purchased from Bamomab (Munich, Germany), while goat anti‐mouse IgG2a was from SouthernBiotech Associates, Inc. (Birmingham, AL, USA). Isotype controls IgG2a, IgG2b and IgG1 were obtained from Sigma‐Aldrich (St Louis, MO, USA).
Clinical samples
A total of 205 subjects were enrolled, including 24 patients with small‐cell lung cancer (SCLC), 56 patients with non‐small‐cell lung cancer (NSCLC), 25 patients with benign lung diseases and 100 healthy individuals. Samples were collected at Tottori University after obtaining permission from the Tottori University Review Board, and all subjects provided written informed consent. Peripheral blood samples were collected from the patients before they received any treatment. Staging was done according to the American Joint Committee on Cancer Guidelines.
Flow cytometry
Cells were incubated with specific monoclonal antibodies (anti‐MICA, anti‐MICB, anti‐ULBP1, anti‐ULBP2 or anti‐ULBP3) or isotype controls. After washing, goat anti‐mouse IgG labeled with FITC was added and samples were analyzed for their fluorescence intensities on a FACSCalibur (BD Biosciences, La Jolla, CA, USA). Data from 10 000 cells were collected and the geometric mean fluorescence intensity (MFI) was calculated using CELLQuest software (BD Biosciences). The MFI ratios were calculated by dividing the MFI obtained with a specific antibody by that for the isotype control.
ELISA
This procedure is described in detail in Data S1.
Stable transfection of ULBP2
Stable transfection of pCEFL‐ULBP2 or empty vector (pCEFL) into A549 cells was done using the LipofectAMINE method (Life Technologies, Paisley, UK) (Data S1).
Cytotoxicity assay
The cytotoxicity of human immune effector cells for target A549 cells was assessed in a 3.5‐h 51Cr release assay, as described previously34 (Data S1).
Statistical methods
Statistical analyses were performed using spss 16.0 software (SPSS Inc., Chicago, IL, USA) and P < 0.05 was regarded as indicating statistical significance (Data S1).
Results
ULBP2 expression by lung cancer cell lines
We first assessed the cell surface expression of NKG2D ligands by 20 lung cancer cell lines (Fig. 1A). ULBP2 was the most widely expressed of the NKG2D ligands. Analysis using a specific antibody for ULBP2 (clone 165903) showed that ULBP2 was expressed by 7/9 adenocarcinoma (AD) cell lines (78%), 3/4 squamous cell carcinoma (SQ) cell lines (75%), 1/2 large‐cell carcinoma (LA) cell lines (50%) and 2/5 SCLC cell lines (40%). Expression of ULBP2 was relatively high and the MFI ratio was 2.0 to 7.0 for these cell lines. Analysis using a specific antibody for MICA (clone 159227) showed that MICA was strongly expressed by the SQ cell line RERF‐LC‐AI and the LA cell line 86‐2, with MFI ratios of 16 and 14, respectively. However, the overall expression of MICA was less frequent and relatively weak compared with ULBP2. These data were consistent with the results obtained by using another pair of monoclonal antibodies: BUMO1 for ULBP2 and AMO1 for MICA. ULBP3 expression was also relatively widespread, but the expression levels were low and MFI ratios were <4.0. MICB and ULBP1 showed little or no expression by most of these lung cancer cell lines.
Figure 1.
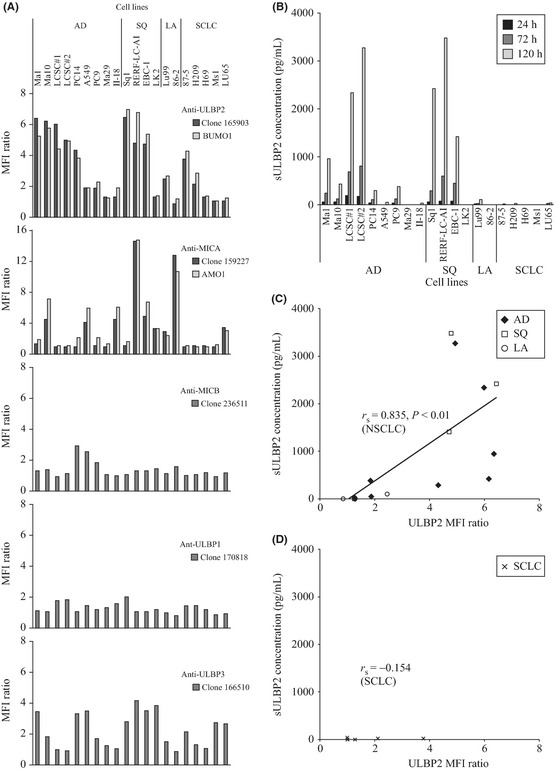
Surface expression of NKG2D ligands by lung cancer cell lines and sULBP2 levels in culture supernatants. (A) Flow cytometric analysis of 20 lung cancer cell lines including nine adenocarcinoma (AD), four squamous cell carcinoma (SQ), two large‐cell carcinoma (LA), and five small‐cell lung cancer (SCLC) cell lines stained with specific mAb. Columns show representative data of three independent experiments. (B) Measurement of sULBP2 in culture supernatants of lung cancer cell lines using our original ELISA. Columns show representative data of three independent experiments. (C,D). Relation between cell surface ULBP2 expression (X‐axis) and sULBP level in culture supernatants (Y‐axis) of non‐small‐cell lung cancer (NSCLC) cell lines (C) and small‐cell lung cancer (SCLC) cell lines (D). MFI, mean fluorescence intensity.
Establishment of a sandwich ELISA
Because ULBP2 was the NKG2D ligand most widely expressed by lung cancer cell lines, we focused on measuring the soluble form of ULBP2 (sULBP2) in culture supernatants. First, we established a sensitive sandwich ELISA for sULBP2. The calibration curve for this ELISA was linear up to 1000 pg/mL on a log–log scale (Fig. S1A) and its detection limit was 7.8 pg/mL (the concentration corresponding to the mean + 2SD absorbance of the calibrator) (Fig. S1B). The standard curve was parallel to data obtained with serial twofold dilutions of serum from a healthy individual (Fig. S1C) and a SQ patient (Fig. S1D), suggesting that other serum proteins were unlikely to interfere with this assay. The intra‐assay coefficient of variation (CV) was between 2.7% and 5.0% (n = 8), while the inter‐assay CV was between 4.0% and 4.6% (n = 4) (Fig. S1E).
Detection of sULBP2 in culture supernatants
Using this new ELISA, we analyzed culture supernatants from 20 lung cancer cell lines at 24, 72 and 120 h after seeding of the cells. The level of sULBP2 increased over time in NSCLC cell lines (AD, SQ and LA), while sULBP2 was not detected in cultures of the SCLC cell lines (Fig. 1B). When the amount of cell surface ULBP2 was compared with that of sULBP2 in these cell lines, a significant correlation was observed among NSCLC cell lines (r s = 0.835, P < 0.01) (Fig. 1C), but not SCLC cell lines (r s = −0.154) (Fig. 1D). These findings suggest that ULBP2 is shed into the culture medium in proportion to the level of its cell surface expression by NSCLC cell lines.
Elevation of serum sULBP2 in NSCLC patients
Our observations suggested that ULBP2 was shed from the cell surface in proportion to its level of expression by NSCLC. Therefore, we investigated serum levels of sULBP2 in 80 lung cancer patients, 25 patients with benign lung diseases and 100 healthy volunteers. The clinical and pathological characteristics of the lung cancer patients and healthy control subjects are presented in Table 1(a). Although the healthy control subjects were significantly younger than the lung cancer patients, their sex ratio and smoking status did not differ. The majority of the control subjects had sULBP2 levels close to the detection limit of the ELISA (7.8 pg/mL). Serum sULBP2 was significantly elevated in SCLC, AD and SQ patients compared with the healthy controls (Mann–Whitney U‐test, P < 0.001). In AD and SQ patients especially, the elevation of serum sULBP2 was marked, with a mean of 21.8 and 263.6 pg/mL, respectively (Fig. 2A). In order to estimate the clinical significance of this elevation of sULBP2 in lung cancer patients, we also measured sULBP2 in the sera of patients with benign lung diseases, which includes pneumonia with focal shadow (n = 8), tuberculosis (n = 5), nontuberculous mycobacteria (n = 2), interstitial pneumonia (n = 5), sarcoidosis (n = 2), pneumosilicosis (n = 1) and granulomatous inflammation (n = 2). The majority of patients with these benign lung diseases showed almost undetectable levels of sULBP2, and these sULBP2 levels did not significantly differ from those of healthy control subjects.
Table 1.
Characteristics and sULBP2 status
| Control group (n = 100) | Lung cancer group (n = 80) | P | ||
|---|---|---|---|---|
| (a) Characteristics of lung cancer patients and controls | ||||
| Sex | ||||
| Female | 25 | 19 | 0.85a | |
| Male | 75 | 61 | ||
| Age (years) | ||||
| Mean ± SD | 44.3 ± 10.9 | 68.6 ± 10.4 | <0.001b | |
| Range | 19–76 | 36–87 | ||
| Smoking status | ||||
| Yes | 60 | 50 | 0.73a | |
| Noc | 40 | 30 | ||
| sULBP2 | ||||
| n | Positive (≥8.7 pg/mL) | Negative (<8.7 pg/mL) | P | |
| (b) Association between sULBP2 positivity and characteristics in lung cancer patients and controls | ||||
| Control group | ||||
| All | 100 | 6 (6.0) | 94 (94.0) | |
| Sex | ||||
| Female | 25 | 2 (8.0) | 23 (92.0) | 0.47d |
| Male | 75 | 4 (5.3) | 71 (94.7) | |
| Age (years) | ||||
| <40 | 39 | 2 (5.1) | 37 (94.9) | 1.00d |
| 40–59 | 54 | 4 (7.4) | 50 (92.6) | |
| 60–79 | 7 | 0 (0.0) | 7 (100.0) | |
| ≥80 | 0 | 0 (0.0) | 0 (0.0) | |
| Smoking status | ||||
| Yes | 60 | 4 (6.7) | 56 (93.3) | 0.54d |
| Noc | 40 | 2 (5.0) | 38 (95.0) | |
| Lung cancer group | ||||
| All | 80 | 39 (48.8) | 41 (52.3) | |
| Sex | ||||
| Female | 19 | 6 (31.6) | 13 (68.4) | 0.086a |
| Male | 61 | 33 (54.1) | 28 (45.9) | |
| Age (years) | ||||
| <40 | 3 | 1 (33.3) | 2 (66.7) | 0.77d |
| 40–59 | 8 | 5 (62.5) | 3 (37.5) | |
| 60–79 | 60 | 28 (46.7) | 32 (53.3) | |
| ≥80 | 9 | 5 (55.6) | 4 (44.4) | |
| Smoking status | ||||
| Yes | 50 | 26 (52.0) | 24 (48.0) | 0.45a |
| Noc | 30 | 13 (43.3) | 17 (56.7) | |
| Histology | ||||
| SCLC | 24 | 11 (45.8) | 13 (54.2) | 0.12d |
| AD | 34 | 13 (38.2) | 21 (61.8) | |
| SQ | 18 | 13 (72.2) | 5 (27.8) | |
| Other NSCLC | 4 | 2 (50.0) | 2 (50.0) | |
| SCLC | ||||
| Stage | ||||
| I–III | 15 | 7 (46.7) | 8 (53.3) | 0.63d |
| IV | 9 | 4 (44.4) | 5 (55.6) | |
| AD | ||||
| Stage | ||||
| I–III | 12 | 4 (33.3) | 8 (66.7) | 0.48d |
| IV | 22 | 9 (40.9) | 13 (59.1) | |
| SQ | ||||
| Stage | ||||
| I–III | 12 | 8 (66.7) | 4 (33.3) | 0.44d |
| IV | 6 | 5 (83.3) | 1 (16.7) | |
| Other NSCLC | ||||
| Stage | ||||
| I–III | 2 | 1 (50.0) | 1 (50.0) | 1.00d |
| IV | 2 | 1 (50.0) | 1 (50.0) | |
χ2 test.
Mann–Whitney U‐test.
<100 cigarettes/lifetime.
Fisher's exact test.
AD, adenocarcinoma; NSCLC, non‐small‐cell lung cancer; SCLC, small‐cell lung cancer; SQ, squamous cell carcinoma; sULBP2, soluble UL16‐binding protein 2.
Figure 2.
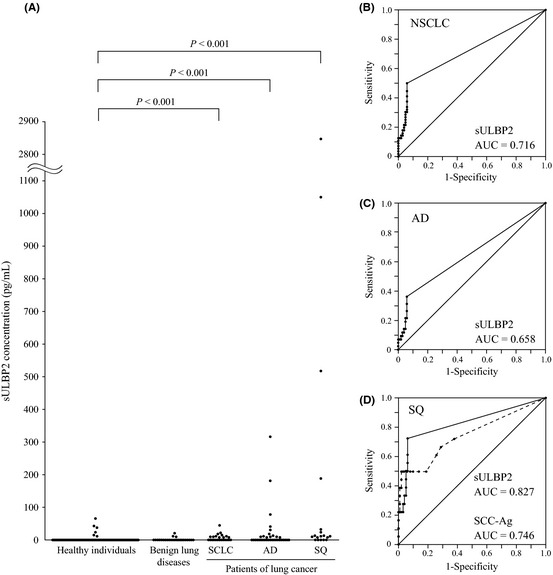
Serum levels of soluble UL16‐binding protein 2 (sULBP2) in healthy controls, patients with benign lung diseases and patients with lung cancer. (A) Scatter plots of the sULBP2 concentration (pg/mL) in healthy individuals, patients with benign lung diseases and patients with lung cancer. (B,C) Receiver operating characteristic (ROC) analysis of sULBP2 for differentiating non‐small‐cell lung cancer (NSCLC) (B) and adenocarcinoma (AD) (C) patients from healthy individuals. Area under the curve (AUC) values are indicated. (D) ROC analysis of sULBP2 and squamous cell carcinoma antigen (SCC‐Ag) for differentiating squamous cell carcinoma (SQ) patients from healthy individuals. Each AUC value is indicated. Solid line, sULBP2; dashed line, SCC‐Ag.
To assess the diagnostic performance of measuring sULBP2, receiver operator characteristic (ROC) curves were generated for NSCLC, AD and SQ patients using healthy individuals as the controls (Fig. 2B–D). The area under the curve (AUC) of serum sULBP2 for distinguishing NSCLC, AD and SQ from healthy controls was 0.716 (95% confidence interval [CI], 0.625–0.806), 0.658 (95% CI, 0.542–0.775) and 0.827 (95% CI, 0.701–0.954), respectively, so the diagnostic potential was greatest in SQ patients. Using a cut‐off value of 8.7 pg/mL to distinguish NSCLC patients from healthy controls, the sensitivity and specificity were 50% and 94%, respectively. Using the same cut‐off value to separate SQ patients from healthy controls, the sensitivity and specificity were 72.2% and 94%, respectively. As squamous cell carcinoma antigen (SCC‐Ag) is the best established serum marker for SQ, we next measured SCC‐Ag in the same serum samples. As shown in Figure 2(D), the AUC for SCC‐Ag was 0.746 (95% CI, 0.600–0.891), which was slightly lower than that for sULBP2, although the difference was not statistically significant. These findings suggested that sULBP2 is comparable with SCC‐Ag as a serum marker for the diagnosis of SQ.
Association of sULBP2 with clinicopathological features of lung cancer patients
The associations between sULBP2 and various clinicopathological characteristics of lung cancer patients and controls are presented in Table 1(b). Based on ROC analysis of NSCLC patients, the cut‐off value of 8.7 pg/mL was used to divide the serum samples into groups that were negative or positive for sULBP2. There was no association between sULBP2 status and gender, age or smoking in both the control and lung cancer groups (P > 0.05). In addition, sULBP2 status did not show any association with tumor histology or disease stage (P > 0.05). These data suggest that the difference of age between the control group and the lung cancer patients in the present study was acceptable, and that occurrence of lung cancer was the only significant factor related to elevation of serum sULBP2.
Serum sULBP2 and survival of patients with advanced NSCLC
Next we evaluated the relationship between serum sULBP2 and overall survival of NSCLC patients. In this analysis, we used patients with advanced NSCLC (clinical stages IIIB and IV) to avoid the influence of surgical resection of tumors. As shown in Figure 3, patients with elevated serum levels of sULBP2 demonstrated significantly shorter survival than patients without sULBP2 elevation (median survival time [MST]: 10.8 vs 16.8 months, P = 0.017).
Figure 3.
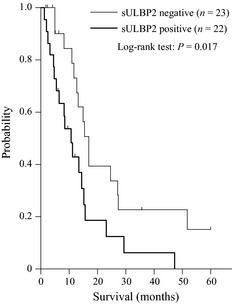
Kaplan–Meier analysis of survival. Soluble UL16‐binding protein 2 (sULBP2) negative = sULBP2 <8.7 pg/mL. sULBP2 positive = sULBP2 ≥8.7 pg/mL. The P‐value was determined using the log‐rank test.
To explore factors contributing to overall survival, six demographic and clinicopathological factors listed in Table 2(a) were subjected to univariate analysis with the log‐rank test. It was found that MST decreased significantly along with advancing clinical stage (P = 0.032) and with elevation of the serum sULBP2 level (P = 0.017). Multivariate analysis was done with Cox's proportional hazards model for the three variables, which reached P ≤ 0.2 (clinical stage, histology and sULBP2 positivity) in the univariate analysis (Table 2b). In addition to clinical stage (HR 2.65, P = 0.019), sULBP2 positivity (HR, 2.13, P = 0.038) was a significant independent determinant of a poor prognosis for lung cancer patients.
Table 2.
Univariate and multivariate analysis for survival time in clinical stage IIIB and IV NSCLC patients
| Variables | n | MST (months) | P (log‐lank) |
|---|---|---|---|
| (a) Univariate analysisa | |||
| Sex | |||
| Female | 12 | 15.7 | 0.35 |
| Male | 33 | 12.6 | |
| Age (years) | |||
| <68 | 20 | 14.8 | 0.88 |
| ≥68 | 25 | 12.6 | |
| Smoking status | |||
| Yes | 22 | 12.6 | 0.72 |
| No | 23 | 14.8 | |
| Clinical stage | |||
| IIIB | 15 | 14.3 | 0.032 |
| IV | 30 | 13.2 | |
| Histology | |||
| Non‐SQ | 32 | 15.1 | 0.19 |
| SQ | 13 | 11.0 | |
| sULBP2 | |||
| <8.7 pg/mL | 23 | 16.8 | 0.017 |
| ≥8.7 pg/mL | 22 | 10.8 | |
| Variables | Hazard ratio | 95% CI | P |
| (b) Multivariate analysisb | |||
| Clinical stage | |||
| IIIB | 1.00 | 0.019 | |
| IV | 2.65 | 1.17–5.99 | |
| Histology | |||
| Non‐SQ | 1.00 | 0.057 | |
| SQ | 2.24 | 0.98–5.15 | |
| sULBP2 | |||
| <8.7 pg/mL | 1.00 | 0.038 | |
| ≥8.7 pg/mL | 2.13 | 1.04–4.37 | |
Log‐lank test.
Cox proportional hazard regression model.
CI, confidence interval; MST, median survival time; SQ, squamous cell carcinoma; sULBP2, soluble UL16‐binding protein 2.
Influence of sULBP2 on cytotoxic activity of effector cells
To explore possible mechanisms leading to the worse prognosis of patients with elevated serum sULBP2 levels, we then tested the influence of sULBP2 on immune effector cells. First, we performed cytotoxicity assays using normal PBMC pretreated with recombinant sULBP2 and PBMC pretreated with sMICA as a positive control. As shown in Figure 4(A), ULBP2‐Fc as well as MICA‐Fc significantly reduced the cytotoxicity of human PBMC. To further examine this effect, we established an A549 adenocarcinoma cell line that stably overexpressed ULBP2 (ALCAN). Flow cytometric analysis showed that ALCAN had 100 times more ULBP2 expression than the mock‐transfected A549 cells (Fig. 4B). Measurement of sULBP2 in the concentrated culture supernatants obtained from these cell lines showed that it increased in proportion to the level of cell surface expression of ULBP2 (Fig. 4C). When a cytotoxic assay of pretreated healthy human PBMC was done with the culture supernatants, it revealed that cytotoxic activity was significantly reduced by sULBP2 (Fig. 4D). These findings suggest that sULBP2 has a substantial immunosuppressive effect on immune effector cells.
Figure 4.

Soluble UL16‐binding protein 2 (sULBP2) inhibits cytotoxic activity of human peripheral blood mononuclear cells (PBMC). (A) Human PBMC were incubated with recombinant soluble NKG2D ligands (ULBP2‐Fc or MICA‐Fc) for 24 h at a concentration of 1 μg/mL, and their cytotoxic activity against A549 cells was examined in a 51 Cr‐release assay at an E:T ratio of 60:1. Data are representative of three independent experiments performed in triplicate. (B) Surface expression of ULBP2 by stable ULBP2‐expressing cell line (ALCAN) as shown using flow cytometry. Mock is a cell line stably transfected with the empty vector as a negative control. (C) The sULBP2 level in concentrated culture supernatant from each cell line measured using ELISA. (D) Human PBMC were incubated with culture supernatants for 22 h and harvested to examine their cytotoxic activity against A549 cells in a 51 Cr‐release assay at an E:T ratio of 60:1. Data are representative of three independent experiments performed in triplicate.
Discussion
In the present study, we found that cell surface ULBP2 is the NKG2D ligand most widely and strongly expressed by lung cancer cells, and that sULBP2 increased in lung cancer cell line culture supernatants in proportion to the level of cell surface expression of ULBP2, especially with NSCLC cells. Also, serum sULBP2 was detectable in lung cancer patients. The diagnostic performance of sULBP2 was substantially superior for NSCLC, and was comparable to the well‐known tumor marker SCC‐Ag for the diagnosis of SQ patients. Finally, we demonstrated that sULBP2 is an excellent prognostic indicator for NSCLC. As a possible explanation, we showed that sULBP2 directly suppresses the activity of immune effector cells. These findings indicate that sULBP2 is a promising diagnostic and prognostic marker for NSCLC.
In the present study, we observed that ULBP2 was the NKG2D ligand most frequently and strongly expressed by lung cancer cells. However, the only other study on the pattern of NKG2D ligand expression by lung cancer cells yielded different results. Le Maux Chansac et al.18 studied 12 human NSCLC cell lines and reported that MICA or MICB and ULBP3 were expressed by most cell lines, while ULBP1 and ULBP2 were rarely expressed. We suspect that the low expression of ULBP2 in their study might have been related to the fixation methods used before examination using flow cytometry. In their study, cells were fixed with formaldehyde before analysis, but it has been suggested that fixation of membrane lipids by formaldehyde is unreliable.35 Our observations are supported by a study on the expression of NKG2D ligands by normal human bronchial epithelial (NHBE) cells.36 Borchers et al.36 reported that ULBP2 mRNA was constitutively expressed at the highest level among NKG2D ligands by NHBE cells from four separate donors. They also reported that surface expression of ULBP2 was clearly increased after hydrogen peroxide treatment of these cells. Their data suggest that bronchial cells might show increased ULBP2 expression after exposure to stress, and might support our observations that ULBP2 is most broadly expressed by transformed cells originating in the lungs. Therefore, we believe that ULBP2 might be most abundantly expressed among NKG2D ligands in lung cancer and play a pivotal role in tumor immunity.
Although reports describing the clinical significance of soluble NKG2D ligands in the serum of cancer patients have been increasing recently, information about lung cancer is still rare. The soluble form of MICA was reported to be elevated in the serum of patients with leukemia,22 multiple myeloma,23 prostate cancer,24 ovarian cancer,25 osteosarcoma,26 melanoma,27 gastrointestinal tumors,19 neuroblastoma,28 pancreatic cancer,29 cervical cancer30 and hepatocellular carcinoma.31, 32 Elevation of soluble MICB (sMICB) has also been reported in patients with ovarian cancer,25 gastrointestinal malignancies,20 pancreatic cancer29 and hepatocellular carcinoma.32 Regarding lung cancer, to our knowledge, only one such study has been reported by Holdenrieder et al.37 They measured sMICA and sMICB38 in 19 lung cancer patients, and reported elevation of both MICA and MICB. However, detailed clinical information on their lung cancer patients was not provided. These observations suggest the possible usefulness of soluble NKG2D ligands as serum markers for various cancers, and the current lack of data about lung cancer suggests that it should be more thoroughly studied.
Compared with studies about soluble MIC family ligands, studies on serum sULBP2 in cancer patients are uncommon and the results are controversial. Waldhauer et al.21 first reported elevated serum levels of sULBP2 in patients with hematopoietic malignancies, but they could not find sULBP2 in patients with gastrointestinal malignancies. Li et al.25 studied eight ovarian cancer patients and reported that serum sULBP2 was not detectable. After failing to detect sULBP2 in the serum of patients with solid tumors, Paschen et al.27 recently found elevated serum levels of sULBP2 in melanoma patients. There are several possible explanations for the discrepancies among these reports. First, the presence of soluble NKG2D ligands is possibly dependent on tumor histology and such ligands might be detectable in a more limited range of solid malignancies compared with hematopoietic malignancies. Second, the sensitivity of the ELISA used in each study would have a significant influence on the detectability of sULBP2. Waldhauer et al.21 used an ELISA that detected sULBP2 at levels as low as 20 pg/mL, but the detection limit was not described for the ELISA in the other two studies. We established an original ELISA that could detect sULBP2 at a concentration of 7.8 pg/mL (Fig. S1). Further studies are needed to clarify whether sULBP2 can be detected in the serum of patients with various solid cancers after the sensitivity of the assay has been thoroughly quantified.
In the present study, we also showed that sULBP2 was a significant prognostic marker for NSCLC. Several previous studies have shown a correlation between elevated levels of sMICA and a poor prognosis for patients with multiple myeloma23 and oral squamous cell carcinoma.33 Regarding sULBP2, the former report for melanoma patients is consistent with the present study, which found elevated levels of sULBP2 were correlated with a poor prognosis.27 However, the reason for this correlation is not discussed in their report and remains unknown because of the lack of information regarding the function of sULBP2. Although multiple studies have shown an inhibitory effect of sMICA on host effector cell function,28, 29, 39 to our knowledge, only one study has addressed this issue for sULBP proteins.40 In that study, a possible inhibitory effect of sULBP proteins was indirectly suggested by the decreased surface expression of NKG2D in NK cells incubated with the culture supernatants of gastric cancer cells expressing ULBP1‐3 protein. Therefore, the role of sULBP1‐3 protein has not been demonstrated separately and the function of sULBP2 remains unknown. In the present study, we clearly showed that sULBP2 itself suppresses the cytotoxic activity of host effector cells. This finding might help us to understand the mechanism leading to deterioration of the prognosis in patients with high serum levels of sULBP2.
There are several possible mechanisms by which sULBP2 suppress the activities of immune effector cells. First, soluble NKG2D ligands might downregulate the surface expression of NKG2D on the effector cells. This mechanism has been proposed in the case of sMICA in several reports.22, 31, 39 Second, soluble NKG2D ligands might competitively bind to NKG2D on the effector cells, and thereby inhibit the binding of the cell surface NKG2D ligands to this receptor and the following activation of the effector cells. Last, soluble NKG2D ligands might activate immunosuppressive T cells, thereby inhibiting effector cell function of PBMC. For example, several studies have reported that sMICA can activate NKG2D+CD4+ T cells that possess immunosuppressive function.41, 42 Compared with these mechanistic studies of sMICA, little is known about sULBP2. Therefore, further study of this mechanism is needed to overcome the immunosuppressive effect of sULBP2.
It is noteworthy that there was a discrepancy between the level of cell surface expression of ULBP2 and the level of sULBP2 in SCLC cell line supernatants in the present study, while a clear correlation was seen for NSCLC cell lines. This discrepancy might have been caused by loss of the shedding mechanism for ULBP2 in this type of cancer. Recently, several mechanisms have been proposed for the shedding of NKG2D ligands from the cell surface. These involve metalloproteinases19 and ERp543 for MICA, phosphatidylinositol‐specific phospholipase C for GPI‐anchored ULBP proteins,40 a disintegrin and metalloproteinase family proteases for ULBP2,21, 44 and release as part of membrane vesicles for ULBP3.44 Although it is still unknown whether one or more of these mechanisms operates for ULBP2 in lung cancer, some might be inhibited in SCLC cells. Another possibility is that the shedding‐resistant form of ULBP2 might be expressed in SCLC cells by genomic mutation or alternative splicing. In any case, understanding the mechanism of both shedding seen in NSCLC and its inhibition in SCLC could be important, because it might help us to manipulate this process in order to modify host antitumor immunity.
Unlike the results of an undetectable level of sULBP2 in the culture supernatant of SCLC cell lines in vitro, the serum level of sULBP2 was significantly elevated in SCLC patients. Although the exact reason for this discrepancy is unclear, the same discrepancies were reported previously in other tumor markers. For example, CYFRA 21‐1, a potential tumor marker for NSCLC, is rarely detectable in the supernatant of cultured SCLC cells,45 whereas it is elevated in nearly half of the sera of SCLC patients.46, 47 One possible mechanism for these discrepancies is the excessive leakage from cancer cells or from involved normal tissues by coexisting inflammation of the cancerous lesion. Another possibility is secretion from the concomitant NSCLC component in the histological heterogenous SCLC lesion, known as combined SCLC.48 In any case, we believe that the clinical significance of serum sULBP2 in SCLC patients is limited, because the level of elevation is very low compared with those seen in NSCLC patients.
In conclusion, we detected sULBP2 in the serum of lung cancer patients and found that it was useful in the diagnosis of NSCLC, especially SQ. In addition, sULBP2 was an independent prognostic indicator for advanced NSCLC. We also observed that sULBP2 has significantly inhibited the cytolytic activity of host effector cells. These findings indicate that sULBP2 is a useful diagnostic and prognostic marker for lung cancer patients. In addition, further study on the shedding process of sULBP2 might be useful for modulating the immune response in cancer patients.
Disclosure Statement
All authors declare that they have no conflict of interest.
Supporting information
Fig. S1. Development of a sensitive ELISA for sULBP2.
Data S1. Includes: cells; reagents; clinical samples; flow cytometry; ELISA; stable transfection of ULBP2; cytotoxic assay; and statistical methods.
Acknowledgments
The authors thank Dr Silvio Gutkind (National Institutes of Health/NIDCR) for providing the pCEFL plasmid and for his advice and discussion. The present study was supported by Grants‐in‐Aid for Scientific Research (C) 21590994 (to H. C. and E. S.) and 22590863 (to E. S. and H. C.) from the Ministry of Education, Science, and Culture, Sports, Science and Technology, Japan.
References
- 1. Baba T, Hanagiri T, Ichiki Y et al Lack and restoration of sensitivity of lung cancer cells to cellular attack with special reference to expression of human leukocyte antigen class I and/or major histocompatibility complex class I chain related molecules A/B. Cancer Sci 2007; 98: 1795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural‐killer‐cell surveillance and therapy of cancer. Nat Rev Cancer 2002; 2: 850–61. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez S, Lopez‐Soto A, Suarez‐Alvarez B, Lopez‐Vazquez A, Lopez‐Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol 2008; 29: 397–403. [DOI] [PubMed] [Google Scholar]
- 4. Borchers MT, Harris NL, Wesselkamper SC et al The NKG2D‐activating receptor mediates pulmonary clearance of Pseudomonas aeruginosa . Infect Immun 2006; 74: 2578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauer S, Groh V, Wu J et al Activation of NK cells and T cells by NKG2D, a receptor for stress‐inducible MICA. Science 1999; 285: 727–9. [DOI] [PubMed] [Google Scholar]
- 6. Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene 2008; 27: 5944–58. [DOI] [PubMed] [Google Scholar]
- 7. Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev 2010; 235: 267–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci USA 1994; 91: 6259–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cosman D, Mullberg J, Sutherland CL et al ULBPs, novel MHC class I‐related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 2001; 14: 123–33. [DOI] [PubMed] [Google Scholar]
- 10. Dunn C, Chalupny NJ, Sutherland CL et al Human cytomegalovirus glycoprotein UL16 causes intracellular sequestration of NKG2D ligands, protecting against natural killer cell cytotoxicity. J Exp Med 2003; 197: 1427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bacon L, Eagle RA, Meyer M, Easom N, Young NT, Trowsdale J. Two human ULBP/RAET1 molecules with transmembrane regions are ligands for NKG2D. J Immunol 2004; 173: 1078–84. [DOI] [PubMed] [Google Scholar]
- 12. Salih HR, Holdenrieder S, Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front Biosci 2008; 13: 3448–56. [DOI] [PubMed] [Google Scholar]
- 13. Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress‐regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci USA 1996; 93: 12445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kraetzel K, Stoelcker B, Eissner G et al NKG2D‐dependent effector function of bronchial epithelium‐activated alloreactive T‐cells. Eur Respir J 2008; 32: 563–70. [DOI] [PubMed] [Google Scholar]
- 15. Pende D, Rivera P, Marcenaro S et al Major histocompatibility complex class I‐related chain A and UL16‐binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D‐dependent natural killer cell cytotoxicity. Cancer Res 2002; 62: 6178–86. [PubMed] [Google Scholar]
- 16. Maccalli C, Pende D, Castelli C, Mingari MC, Robbins PF, Parmiani G. NKG2D engagement of colorectal cancer‐specific T cells strengthens TCR‐mediated antigen stimulation and elicits TCR independent anti‐tumor activity. Eur J Immunol 2003; 33: 2033–43. [DOI] [PubMed] [Google Scholar]
- 17. Chen XM, Xu XQ, Sun K, Hallett WH, Zhao JD, Zhang DL. NKG2D ligands expression and NKG2D‐mediated cytotoxicity in human laryngeal squamous carcinoma cells. Scand J Immunol 2008; 67: 441–7. [DOI] [PubMed] [Google Scholar]
- 18. Le Maux Chansac B, Misse D, Richon C et al Potentiation of NK cell‐mediated cytotoxicity in human lung adenocarcinoma: role of NKG2D‐dependent pathway. Int Immunol 2008; 20: 801–10. [DOI] [PubMed] [Google Scholar]
- 19. Salih HR, Rammensee HG, Steinle A. Cutting edge: down‐regulation of MICA on human tumors by proteolytic shedding. J Immunol 2002; 169: 4098–102. [DOI] [PubMed] [Google Scholar]
- 20. Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: mechanism and soluble MICB in sera of cancer patients. Hum Immunol 2006; 67: 188–95. [DOI] [PubMed] [Google Scholar]
- 21. Waldhauer I, Steinle A. Proteolytic release of soluble UL16‐binding protein 2 from tumor cells. Cancer Res 2006; 66: 2520–6. [DOI] [PubMed] [Google Scholar]
- 22. Salih HR, Antropius H, Gieseke F et al Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood 2003; 102: 1389–96. [DOI] [PubMed] [Google Scholar]
- 23. Rebmann V, Schutt P, Brandhorst D et al Soluble MICA as an independent prognostic factor for the overall survival and progression‐free survival of multiple myeloma patients. Clin Immunol 2007; 123: 114–20. [DOI] [PubMed] [Google Scholar]
- 24. Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain‐related molecule is counteracted by shedding in prostate cancer. J Clin Invest 2004; 114: 560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li K, Mandai M, Hamanishi J et al Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: high expression of ULBP2 is an indicator of poor prognosis. Cancer Immunol Immunother 2009; 58: 641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu SM, Xiao P, Xue L et al Prevalent expression of MHC class I chain‐related molecule A in human osteosarcoma. Neoplasma 2008; 55: 266–72. [PubMed] [Google Scholar]
- 27. Paschen A, Sucker A, Hill B et al Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res 2009; 15: 5208–15. [DOI] [PubMed] [Google Scholar]
- 28. Raffaghello L, Prigione I, Airoldi I et al Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 2004; 6: 558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marten A, von Lilienfeld‐Toal M, Buchler MW, Schmidt J. Soluble MIC is elevated in the serum of patients with pancreatic carcinoma diminishing gammadelta T cell cytotoxicity. Int J Cancer 2006; 119: 2359–65. [DOI] [PubMed] [Google Scholar]
- 30. Arreygue‐Garcia NA, Daneri‐Navarro A, del Toro‐Arreola A et al Augmented serum level of major histocompatibility complex class I‐related chain A (MICA) protein and reduced NKG2D expression on NK and T cells in patients with cervical cancer and precursor lesions. BMC Cancer 2008; 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jinushi M, Takehara T, Tatsumi T et al Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I‐related chain A in advanced human hepatocellular carcinomas. J Hepatol 2005; 43: 1013–20. [DOI] [PubMed] [Google Scholar]
- 32. Kohga K, Takehara T, Tatsumi T et al Serum levels of soluble major histocompatibility complex (MHC) class I‐related chain A in patients with chronic liver diseases and changes during transcatheter arterial embolization for hepatocellular carcinoma. Cancer Sci 2008; 99: 1643–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tamaki S, Sanefuzi N, Kawakami M et al Association between soluble MICA levels and disease stage IV oral squamous cell carcinoma in Japanese patients. Hum Immunol 2008; 69: 88–93. [DOI] [PubMed] [Google Scholar]
- 34. Kurai J, Chikumi H, Hashimoto K et al Antibody‐dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin Cancer Res 2007; 13: 1552–61. [DOI] [PubMed] [Google Scholar]
- 35. Mayor S. Analysis of the cell‐surface distribution of GPI‐anchored proteins. Methods Mol Biol 1999; 116: 23–36. [DOI] [PubMed] [Google Scholar]
- 36. Borchers MT, Harris NL, Wesselkamper SC, Vitucci M, Cosman D. NKG2D ligands are expressed on stressed human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2006; 291: L222–31. [DOI] [PubMed] [Google Scholar]
- 37. Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer 2006; 118: 684–7. [DOI] [PubMed] [Google Scholar]
- 38. Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICB in malignant diseases: analysis of diagnostic significance and correlation with soluble MICA. Cancer Immunol Immunother 2006; 55: 1584–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Groh V, Wu J, Yee C, Spies T. Tumour‐derived soluble MIC ligands impair expression of NKG2D and T‐cell activation. Nature 2002; 419: 734–8. [DOI] [PubMed] [Google Scholar]
- 40. Song H, Kim J, Cosman D, Choi I. Soluble ULBP suppresses natural killer cell activity via down‐regulating NKG2D expression. Cell Immunol 2006; 239: 22–30. [DOI] [PubMed] [Google Scholar]
- 41. Groh V, Smythe K, Dai Z, Spies T. Fas‐ligand‐mediated paracrine T cell regulation by the receptor NKG2D in tumor immunity. Nat Immunol 2006; 7: 755–62. [DOI] [PubMed] [Google Scholar]
- 42. Dai Z, Turtle CJ, Booth GC et al Normally occurring NKG2D+CD4+ T cells are immunosuppressive and inversely correlated with disease activity in juvenile‐onset lupus. J Exp Med 2009; 206: 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaiser BK, Yim D, Chow IT et al Disulphide‐isomerase‐enabled shedding of tumour‐associated NKG2D ligands. Nature 2007; 447: 482–6. [DOI] [PubMed] [Google Scholar]
- 44. Fernandez‐Messina L, Ashiru O, Boutet P et al Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)‐anchored NKG2D ligands. J Biol Chem 2010; 285: 8543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dohmoto K, Hojo S, Fujita J et al Mechanisms of the release of CYFRA21‐1 in human lung cancer cell lines. Lung Cancer 2000; 30: 55–63. [DOI] [PubMed] [Google Scholar]
- 46. Takada M, Masuda N, Matsuura E et al Measurement of cytokeratin 19 fragments as a marker of lung cancer by CYFRA 21‐1 enzyme immunoassay. Br J Cancer 1995; 71: 160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nisman B, Biran H, Ramu N, Heching N, Barak V, Peretz T. The diagnostic and prognostic value of ProGRP in lung cancer. Anticancer Res 2009; 29: 4827–32. [PubMed] [Google Scholar]
- 48. Travis WD. Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non‐small cell carcinomas. Mod Pathol 2012; 25(Suppl 1): S18–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Development of a sensitive ELISA for sULBP2.
Data S1. Includes: cells; reagents; clinical samples; flow cytometry; ELISA; stable transfection of ULBP2; cytotoxic assay; and statistical methods.


