Abstract
The aim of this prospective study was to evaluate the changes in stereopsis in patients who underwent vitrectomy for macular hole (MH) and assess the relationship between stereopsis and retinal microstructures. Fifty-two patients who underwent successful vitrectomy for unilateral MH and 20 control participants were recruited. We examined stereopsis using the Titmus Stereo Test (TST) and TNO stereotest (TNO), optical coherence tomography, and best-corrected visual acuity measurements, preoperatively, and at 3, 6, and 12 months postoperatively. As a result, preoperative and postoperative 3, 6, and 12-month values of stereopsis assessed by TST (log) were 2.7, 2.2, 2.2, and 2.2, respectively. TNO (log) were 2.8, 2.5, 2.4, and 2.4, respectively. Stereopsis in MH after surgery was significantly worse than that in normal participants (p < 0.001). Preoperative TST significantly correlated with MH size and defect length of external limiting membrane (ELM). Postoperative TST demonstrated significant correlation with the preoperative ELM defect length, and postoperative TNO was associated with the preoperative interdigitation zone defect length. Vitrectomy for MH significantly improved stereopsis, although not to normal levels. The ELM defect lengths, which approximately correspond to TST circles, are prognostic factors for postoperative stereopsis by TST. The interdigitation zone defect length, similar in size to the TNO index, is a prognostic factor for postoperative stereopsis by TNO.
Subject terms: Retinal diseases, Vision disorders
Introduction
In many patients, visual acuity improves after the successful surgical closure of the idiopathic macular hole (MH)1,2; however, some patients still experience disturbances in visual function after surgery. MH results in reduced visual acuity, metamorphopsia3–9, and aniseikonia10,11.
Stereopsis is the ability to perceive the depth of the field based on the disparity in the images formed in each eye—it is the most advanced visual function. Several studies have reported the relationship between stereopsis and retinal disorders, such as MH12–14, epiretinal membrane (ERM)14,15, and retinal detachment (RD)16, and found that vitrectomy improved stereopsis in patients with MH12,13.
With the introduction of optical coherence tomography (OCT), clinicians can evaluate the microstructural changes in the macular region qualitatively and quantitatively17,18. Several studies have examined the relationship between OCT findings and visual acuity19–25, metamorphopsia4–9, and aniseikonia10,11, in patients with MH. However, to our best knowledge, no study has prospectively investigated the temporal changes in stereopsis or the relationship between stereopsis and OCT findings in patients with MH.
The purpose of the present study was to evaluate stereopsis in patients who underwent vitrectomy for MH and to investigate the relationship between stereopsis and retinal microstructures.
Methods
We analysed 52 patients (18 men and 34 women) who underwent successful vitrectomy surgery for treating unilateral MH, and 20 age-matched normal participants (8 men and 12 women) at the Tsukuba University Hospital. The average age of the patients and controls was 66.7 ± 6.7 and 65.5 ± 6.0 years (mean ± standard deviation), respectively. We conducted this prospective, comparative study in accordance with the Declaration of Helsinki and received approval from the institutional review committees of the University of Tsukuba Hospital. All the patients provided informed consent after the nature of the study was explained to them before inclusion in the study. The exclusion criteria included eyes with MH secondary to uveitis or proliferative diabetic retinopathy, traumatic MH, ophthalmic diseases except mild cataract and refractive errors, and any systemic disease that influenced ocular motility.
The examinations included measurements of stereopsis, best-corrected visual acuity (BCVA), and retinal microstructure before surgery and at 3, 6, and 12 months after surgery. Stereopsis was measured using the Titmus Stereo Test (TST) and TNO stereotest (TNO). These tests were performed at a distance of 40 cm with appropriate spectacle correction. The results of stereopsis tests were expressed as ‘seconds of arc’. We converted these values to logarithms for statistical evaluation.
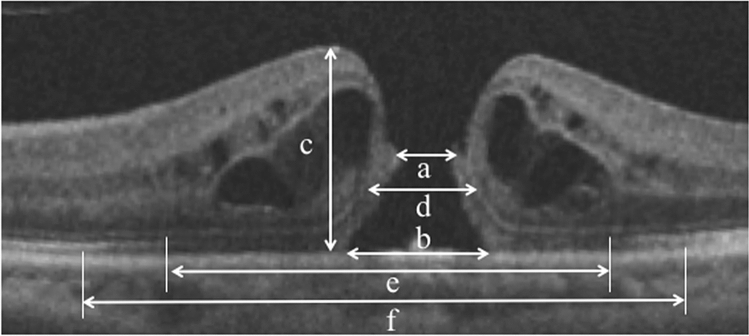
The retinal microstructure was measured with spectral-domain OCT (Cirrus high-definition OCT; Carl Zeiss, Dublin, CA, USA). We used 5-line raster scans for each eye using an analytical software package (Cirrus analysis software, version 3.0; Carl Zeiss) with a signal strength of more than 7/10. We quantified the following parameters before surgery based on OCT images: minimum diameters of MH, base diameters of MH, macular thickness, and external limiting membrane (ELM), ellipsoid zone (EZ), and interdigitation zone (IZ) defect lengths (Fig. 1)8. Based on the images of the 5-line raster scans, we quantified the parameters with an image processing software (ImageJ bundled with Java 1.8.0_172, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available from https://rsbweb.nih.gov/ij/index.html)8. The defect length of each line was determined by agreement between two blinded, well-trained observers (Y.S. and Y.M.), and the mean value of the length of each line was used for further analysis.
Figure 1.
Based on preoperative optical coherence tomography images in patients with macular hole (MH), we measured the minimum MH diameter (a), base MH diameter (b), macular thickness (c), defect lengths of the external limiting membrane (d), ellipsoid zone (e), and interdigitation zone (f). We quantified the parameters with an image processing software (ImageJ bundled with Java 1.8.0_172, developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available from https://rsbweb.nih.gov/ij/index.html).
All surgeries were performed by two surgeons (F.O. and Y.O.) under sub-Tenon’s local anaesthesia. Clinically significant cataracts were simultaneously operated. We induced posterior vitreous detachment and performed core vitrectomy. Subsequently, we injected 0.2 mL of 0.025% brilliant blue G solution over the macula for 10–20 s and thereafter, washed it with an irrigation solution. Internal limiting membrane (ILM) peeling and fluid/gas exchange were performed for all cases. The patients were instructed to be in a face-down position for 1–3 days postoperatively.
The mean scores were compared, and standard deviations were calculated for each parameter of visual function and OCT measurement. BCVA was measured with the Landolt Chart and expressed as a logarithm of the minimum angle of resolution. The Mann–Whitney U test was used to compare stereopsis between patients with MH and normal participants. The Wilcoxon signed rank test was used to compare differences in stereopsis before and after surgery in patients with MH. Repeated-measures analysis of variance was used to clarify the changes in visual acuity and stereopsis. When a significant difference was detected, we conducted the Dunnett post hoc test for multiple comparisons to reveal the time point that demonstrated a significant difference from the baseline value. The associations between stereopsis and OCT parameters and visual acuity were examined by the Spearman rank correlation test. All tests for evaluating associations between parameters were considered statistically significant if P was < 0.05. All analyses were performed using StatView (version 5.0, SAS Inc., Cary, NC, USA).
Results
Table 1 reveals the visual functions and OCT parameters of patients with MH before surgery. Preoperatively, 40 eyes were phakic and 12 eyes were pseudophakic with posterior chamber intraocular lenses. All phakic eyes were performed with cataract surgery combined with vitrectomy.
Table 1.
Clinical Features of visual functions and OCT parameters in patients with macular hole (MH) before surgery.
| Age (years) | 66.7 ± 6.7 |
| Sex (men / women) | 18 / 34 |
| Preoperative best-corrected visual acuity (logMAR) | 0.72 ± 0.34 |
| Stage of MH (2 / 3 / 4) | (15 / 25 / 12) |
| Titmus Stereo Test (TST) (log) | 2.7 ± 0.5 |
| TNO stereotest (log) | 2.8 ± 0.6 |
| Minimum diameters of MH (µm) | 394 ± 174 |
| Base diameter of MH (µm) | 796 ± 266 |
| Macular thickness (µm) | 445 ± 123 |
| Defect length of external limiting membrane (ELM) (µm) | 755 ± 322 |
| Defect length of ellipsoid zone (EZ) (µm) | 1320 ± 504 |
| Defect length of interdigitation zone (IZ) (µm) | 2126 ± 421 |
Values are presented as the mean ± standard deviation.
LogMAR = logarithm of the minimum angle of resolution.
OCT: optical coherence tomography.
Pre- and postoperative stereopsis in patients with MH and normal participants
The preoperative and postoperative 3, 6, and 12-month values of stereopsis assessed by TST (log) were 2.7 ± 0.5, 2.2 ± 0.3, 2.2 ± 0.3, and 2.2 ± 0.4, respectively. The preoperative and postoperative 3, 6, and 12-month values of stereopsis assessed by TNO (log) were 2.8 ± 0.6, 2.5 ± 0.5, 2.4 ± 0.5, and 2.4 ± 0.4, respectively. TST and TNO values (log) in normal participants were 1.6 ± 0.1 and 1.9 ± 0.2, respectively. Stereopsis values in patients with MH before surgery and 12 months after surgery were significantly worse than those of normal participants (p < 0.001).
Temporal changes in visual function in patients with MH
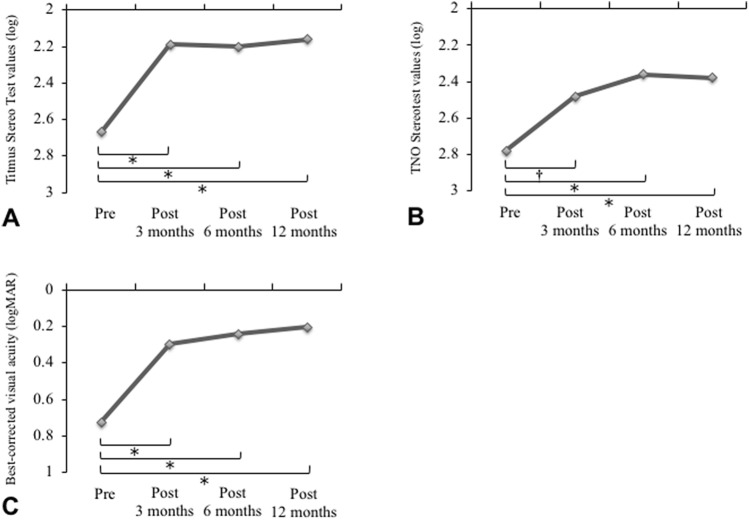
Vitrectomy improved visual acuity and stereopsis. The preoperative TST values were significantly higher than those observed at 3, 6, and 12 months postoperatively. No significant differences were observed between the TST values at 3 and 6 months or at 6 and 12 months postoperatively (Fig. 2A). Similar results were found for TNO (Fig. 2B). The preoperative and postoperative 3, 6, and 12-month values of BCVA were 0.72 ± 0.34, 0.30 ± 0.27, 0.24 ± 0.25, and 0.20 ± 0.19, respectively. Preoperative BCVA was significantly higher than that measured 3, 6, and 12 months postoperatively (Fig. 2C). Postoperative TST at 12 months was significantly correlated with preoperative TST (r = 0.53, P < 0.001), while postoperative TNO at 12 months did not show correlation with preoperative TNO (r = 0.22, P = 0.12).
Figure 2.
Temporal changes in visual function in patients with macular hole before and after surgery. (A) Titmus Stereo Test values. (B) TNO stereotest values. (C) Best-corrected visual acuity. Visual functions significantly improved after surgery. *P < 0.0001; †P < 0.001.
Relationship between stereopsis and visual acuity in patients with MH
Preoperative stereopsis assessed by TST showed a significant correlation with preoperative BCVA (r = 0.32, P < 0.05), but stereopsis assessed by TNO was not associated with preoperative BCVA (r = 0.21, P = 0.13). At 3 months postoperatively, stereopsis was not associated with BCVA (TST: r = 0.17, P = 0.23; TNO: r = 0.15, P = 0.28). At 6 months postoperatively, TST and TNO was not also correlated with BCVA (TST: r = 0.22, P = 0.11; TNO: r = 0.21, P = 0.14). Stereopsis was significantly correlated with BCVA (TST: r = 0.53, P < 0.0001; TNO: r = 0.34, P < 0.05) at 12 months postoperatively. Postoperative TST values significantly correlated with preoperative BCVA (r = 0.30, P < 0.05), while postoperative TNO values showed no association with preoperative BCVA (r = 0.19, P = 0.18).
Relationship between stereopsis and OCT parameters before surgery
The preoperative TST values showed significant correlation with the minimum and base diameters of MH (P < 0.01 and P < 0.01, respectively) and with the ELM defect length (P < 0.01). TNO values were significantly associated with the base diameter of MH (P < 0.05; Table 2).
Table 2.
Correlation between preoperative stereopsis and OCT parameters in patients with macular hole (MH).
| Preoperative parameters | Preoperative TST | Preoperative TNO | ||
|---|---|---|---|---|
| r | p | r | p | |
| Minimum diameter of MH | 0.41 | 0.006* | 0.27 | 0.07 |
| Base diameter of MH | 0.40 | 0.005* | 0.30 | 0.04* |
| Macular thickness | 0.20 | 0.20 | 0.07 | 0.74 |
| Defect length of ELM | 0.39 | 0.007* | 0.24 | 0.12 |
| Defect length of EZ | 0.16 | 0.33 | 0.05 | 0.89 |
| Defect length of IZ | 0.22 | 0.16 | 0.21 | 0.18 |
*Significant correlation between parameters (Spearman rank correlation test).
TST = Titmus Stereo Test, TNO = TNO stereotest, ELM = external limiting membrane, EZ = ellipsoid zone, IZ = interdigitation zone.
OCT: optical coherence tomography, MH: macular hole.
Preoperative OCT parameters affecting postoperative stereopsis
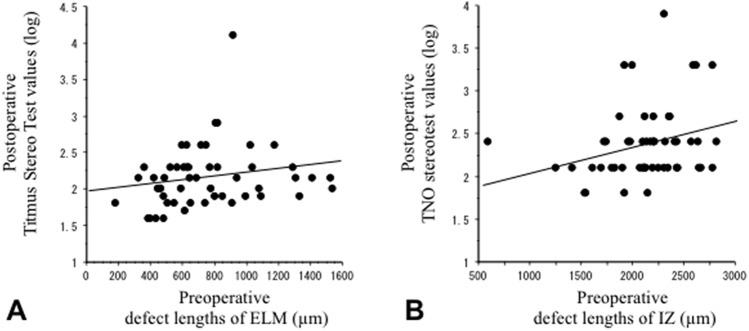
Table 3 shows the relationship between the postoperative 12-month values of stereopsis and preoperative OCT parameters in patients with MH. The postoperative 12-month TST values significantly correlated with preoperative ELM defect length (P < 0.05, Fig. 3A). Postoperative TNO values showed a significant correlation with preoperative IZ defect length (P < 0.05, Fig. 3B).
Table 3.
Correlation between 12-month postoperative stereopsis and preoperative OCT parameters in patients with macular hole (MH).
| Preoperative parameters | Postoperative TST | Postoperative TNO | ||
|---|---|---|---|---|
| r | p | r | p | |
| Minimum diameter of MH | 0.19 | 0.19 | 0.07 | 0.85 |
| Base diameter of MH | 0.25 | 0.09 | 0.08 | 0.81 |
| Macular thickness | 0.01 | 0.99 | 0.06 | 0.98 |
| Defect length of ELM | 0.29 | 0.04* | 0.17 | 0.35 |
| Defect length of EZ | 0.21 | 0.16 | 0.17 | 0.35 |
| Defect length of IZ | 0.23 | 0.12 | 0.32 | 0.04* |
*Significant correlation between parameters (Spearman rank correlation test).
TST = itmus Stereo Test, TNO = NO stereotest, ELM = external limiting membrane, EZ = ellipsoid zone, IZ = nterdigitation zone.
OCT: optical coherence tomography.
Figure 3.
Correlation between postoperative stereopsis and preoperative optical coherence tomography parameters. (A) Postoperative Titmus Stereo Test values versus preoperative defect lengths of the external limiting membrane in patients with macular hole. (B) Postoperative TNO stereotest values versus preoperative defect lengths of the interdigitation zone. ELM, external limiting membrane; IZ, interdigitation zone.
Discussion
The postoperative visual acuity in patients with MH tended to improve gradually over 1 year, while stereopsis improved immediately within 3 months after surgery, after which it remained unchanged. Stereopsis is a more advanced visual function than visual acuity, and there may be a limit to the extent of surgical improvement of stereopsis. It has already been reported that stereopsis improves after surgery for treating ERM and MH;12–15; however, these studies did not report detailed temporal changes in stereopsis. This is the first study to report temporal changes in stereopsis in patients with MH.
Stereopsis was compromised in patients with MH and was worse in patients with MH than that in normal controls in this study. Previous studies have investigated disturbances in stereopsis in retinal disorders, including unilateral ERM and RD, and compared them with stereopsis assessed in normal controls15,16. Stereopsis was affected in patients with MH similar to that in patients with other unilateral retinal diseases14. Moreover, stereopsis was impaired in patients with MH after surgery, compared with that in controls. Stereopsis improved following surgical treatment for ERM and RD15,16, although not to the values found in normal individuals. Despite treatment, patients with MH presented with poor stereopsis similar to that observed in patients with other retinal diseases.
Stereopsis assessed by TST was associated with visual acuity in patients with MH. The relationship between visual acuity and stereopsis has been investigated in normal participants26,27 and in patients who underwent surgery for MH12,13 and RD28. Lam et al.29 reported a difference in visual acuity between the eyes as a cause of disturbance of stereopsis, while Burian30 reported that stereopsis deteriorated with worsening visual acuity in one eye. Judging from these findings, stereopsis, which is binocular vision, is impaired even if visual acuity in one eye is impaired. We included MH patients with one eye and normal vision in the other eye in this study. It is consistent with the results of these articles that worsening visual acuity in one eye impairs stereopsis.
In contrast, preoperative stereopsis assessed by TNO was not significantly associated with visual acuity in patients with MH. This difference may be attributed to the different index sizes used in the two stereotests. The stimulus used for assessing fine stereopsis in TNO was much larger than that used in the TST circles. The TST circles subtend a visual angle of 0.7°, while those in TNO subtend an angle of 8.5°. The diameters of the fovea and foveola were 1500 and 350 μm, respectively, with visual angles of approximately 5° and 1.2°, respectively. Therefore, TST circles are more relevant to visual acuity than TNO. Generally, MH patients do not complain of stereoscopic vision. This may be because the central stereopsis deficit indicated by TST is supplemented by paracentral stereopsis indicated by TNO in the visual field pathway in the brain.
Preoperative stereopsis had a significant correlation with various OCT parameters, including the minimum diameter of MH, base diameter of MH, and ELM defect length. Several studies have investigated the relationship between visual functions and OCT findings in patients with MH. Visual acuity was significantly associated with the minimum and base diameters of MH19, and the EZ20–23, and IZ defect lengths24,25. Moreover, metamorphopsia was associated with the minimum and base diameters of MH6,7, asymmetric elongation of foveal tissue9, and intraretinal cysts within the fluid cuff8. Stereopsis was related to the size of the MH, outer retinal layers, visual acuity, and metamorphopsia.
The prognostic factors for the assessment of postoperative stereopsis in patients with MH were ELM defect length by TST and IZ defect length by TNO. The difference in the prognostic factors between TST and TNO is probably due to the different index sizes used in the two tests as mentioned previously. Generally, the defect length of IZ is longer than that of ELM because of the shape of the MH. In this study, the mean defect length of ELM was 755 µm and that of IZ was 2126 µm. Interestingly, when the indices and results of defect lengths of the two tests are superimposed on the fundus photograph, the indices and defect lengths of the two tests coincide (Fig. 4). Therefore, the extent of impairment of retinal structures was reflected in the results of stereopsis.
Figure 4.
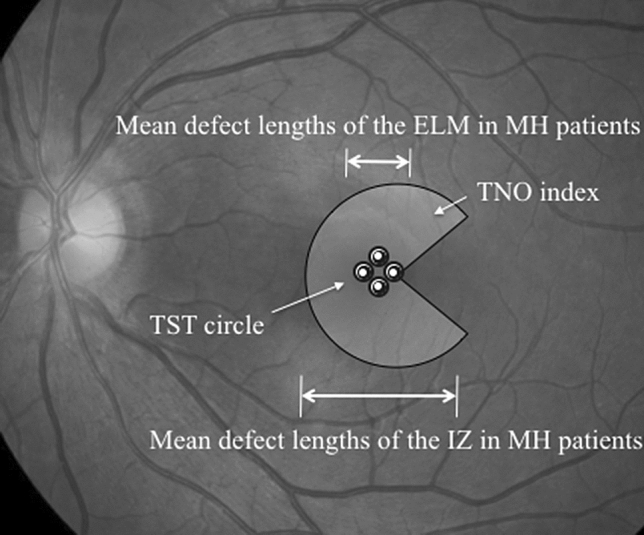
Approximate projection of Titmus Stereo Test circles and TNO stereotest index of the macula in relation to the mean defect lengths of the external limiting membrane and interdigitation zone in patients with macular hole. ELM, external limiting membrane; IZ, interdigitation zone; MH, macular hole; TST, Titmus Stereo Test; TNO, TNO stereotest.
Our study has several limitations, especially the small sample size and short follow-up duration. Visual acuity and stereopsis in patients with MH may improve over the course of a longer follow-up period. Although factors such as eye dominance31,32, pupil size33,34, and accommodation26,35, are known to affect stereopsis, we did not evaluate these factors in this study. In addition, our study population was relatively small MH patients (Minimum diameters of MH was 394 µm in average). The present results may not be totally suitable for larger macular holes. Future studies that include a larger sample size, longer follow-up duration, and evaluate other factors will further improve our understanding of stereopsis and other visual functions in patients with MH.
Author contributions
F.O. and T.O. conduct this study. F.O. and S.M. wrote the main manuscript text. Y.M., Y.S., and T.M. had data collection. S.H. and T.H. had data analysis. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aaberg TM, Blair CJ, Gass JD. Macular holes. Am. J. Ophthalmol. 1970;69:555–562. doi: 10.1016/0002-9394(70)91620-X. [DOI] [PubMed] [Google Scholar]
- 2.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch. Ophthalmol. 1991;109:654–659. doi: 10.1001/archopht.1991.01080050068031. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda S, et al. Vision-related quality of life and visual function in patients undergoing vitrectomy, gas tamponade and cataract surgery for macular hole. Br. J. Ophthalmol. 2009;93:1595–1599. doi: 10.1136/bjo.2008.155440. [DOI] [PubMed] [Google Scholar]
- 4.Arimura E, et al. Quantification of metamorphopsia in a macular hole patient using M-CHARTS. Acta. Ophthalmol. Scand. 2007;85:55–59. doi: 10.1111/j.1600-0420.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 5.Saito Y, et al. The visual performance and metamorphopsia of patients with macular holes. Arch. Ophthalmol. 2000;118:41–46. doi: 10.1001/archopht.118.1.41. [DOI] [PubMed] [Google Scholar]
- 6.Krøyer K, Christensen U, Larsen M, la Cour M. Quantification of metamorphopsia in patients with macular hole. Invest. Ophthalmol. Vis. Sci. 2008;49:3741–3746. doi: 10.1167/iovs.07-1452. [DOI] [PubMed] [Google Scholar]
- 7.Krøyer K, Christensen U, la Cour M, Larsen M. Metamorphopsia assessment before and after vitrectomy for macular hole. Invest. Ophthalmol. Vis. Sci. 2009;50:5511–5515. doi: 10.1167/iovs.09-3530. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura Y, Okamoto F, Okamoto Y, Hiraoka T, Oshika T. Relationship between metamorphopsia and intraretinal cysts within the fluid cuff after surgery for idiopathic macular hole. Retina. 2017;37:70–75. doi: 10.1097/IAE.0000000000001136. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kang SW, Park DY, Kim SJ, Ha HS. Asymmetric elongation of foveal tissue after macular hole surgery and its impact on metamorphopsia. Ophthalmology. 2012;119:2133–2140. doi: 10.1016/j.ophtha.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto F, et al. Aniseikonia and foveal microstructure in patients with idiopathic macular hole. Ophthalmology. 2016;123:1926–1932. doi: 10.1016/j.ophtha.2016.05.051. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Aniseikonia in various retinal disorders. Graefes Arch. Clin. Exp. Ophthalmol. 2017;255:1063–1071. doi: 10.1007/s00417-017-3597-x. [DOI] [PubMed] [Google Scholar]
- 12.Hikichi T, et al. Stereo acuity in patients with unilateral macular hole and after unilateral macular hole surgery. Graefes Arch. Clin. Exp. Ophthalmol. 2001;239:128–132. doi: 10.1007/s004170100257. [DOI] [PubMed] [Google Scholar]
- 13.Mireskandari K, et al. A prospective study of the effect of a unilateral macular hole on sensory and motor binocular function and recovery following successful surgery. Br. J. Ophthalmol. 2004;88:1320–1324. doi: 10.1136/bjo.2004.042093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asaria R, Garnham L, Gregor ZJ, Sloper JJ. A prospective study of binocular visual function before and after successful surgery to remove a unilateral epiretinal membrane. Ophthalmology. 2008;115:1930–1937. doi: 10.1016/j.ophtha.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Stereopsis and optical coherence tomography findings after epiretinal membrane surgery. Retina. 2015;35:1415–1421. doi: 10.1097/IAE.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, et al. Stereopsis after successful surgery for rhegmatogenous retinal detachment. Graefes Arch. Clin. Exp. Ophthalmol. 2014;252:1207–1212. doi: 10.1007/s00417-014-2566-x. [DOI] [PubMed] [Google Scholar]
- 17.Puliafito CA, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–229. doi: 10.1016/S0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins JR, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103:2142–2151. doi: 10.1016/S0161-6420(96)30377-1. [DOI] [PubMed] [Google Scholar]
- 19.Ullrich S, et al. Macular hole size as a prognostic factor in macular hole surgery. Br. J. Ophthalmol. 2002;86:390–393. doi: 10.1136/bjo.86.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh J, Smiddy WE, Flynn HW, Jr, Gregori G, Lujan B. Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Invest. Ophthalmol. Vis. Sci. 2010;51:1651–1658. doi: 10.1167/iovs.09-4420. [DOI] [PubMed] [Google Scholar]
- 21.Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: Spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117:1815–1824. doi: 10.1016/j.ophtha.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 22.Shimoda Y, Sano M, Hashimoto H, Yokota Y, Kishi S. Restoration of photoreceptor outer segment after vitrectomy for retinal detachment. Am. J. Ophthalmol. 2010;149:284–290. doi: 10.1016/j.ajo.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Inoue M, et al. Spectral-domain optical coherence tomography images of inner/outer segment junctions and macular hole surgery outcomes. Graefes Arch. Clin. Exp. Ophthalmol. 2009;247:325–330. doi: 10.1007/s00417-008-0999-9. [DOI] [PubMed] [Google Scholar]
- 24.Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A. Significant correlation between visual acuity and recovery of foveal cone microstructures after macular hole surgery. Am. J. Ophthalmol. 2012;153:111–119. doi: 10.1016/j.ajo.2011.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Itoh Y, Inoue M, Rii T, Hiraoka T, Hirakata A. Correlation between length of foveal cone outer segment tips line defect and visual acuity after macular hole closure. Ophthalmology. 2012;119:1438–1446. doi: 10.1016/j.ophtha.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin RT, Romano PE. Stereoacuity degradation by experimental and real monocular and binocular amblyopia. Invest. Ophthalmol. Vis. Sci. 1985;26:917–923. [PubMed] [Google Scholar]
- 27.Levy NS, Glick EB. Stereoscopic perception and Snellen visual acuity. Am. J. Ophthalmol. 1974;78:722–724. doi: 10.1016/S0002-9394(14)76312-3. [DOI] [PubMed] [Google Scholar]
- 28.Amemiya T, Takami H, Yoshida H, Harayama K. Binocular visual function after surgery for detached retina. Graefes Arch. Clin. Exp. Ophthalmol. 1982;218:304–306. doi: 10.1007/BF02150444. [DOI] [PubMed] [Google Scholar]
- 29.Lam AK, Chau AS, Lam WY, Leung GY, Man BS. Effect of naturally occurring visual acuity differences between two eyes in stereoacuity. Ophthalmic Physiol. Opt. 1996;16:189–195. doi: 10.1046/j.1475-1313.1996.95000348.x. [DOI] [PubMed] [Google Scholar]
- 30.Burian HM. Anomalous retinal correspondence. Its essence and its significance in diagnosis and treatment. Am. J. Ophthalmol. 1951;34:237–253. doi: 10.1016/0002-9394(51)90574-0. [DOI] [PubMed] [Google Scholar]
- 31.Weinman J, Cooke V. Eye dominance and stereopsis. Perception. 1982;11:207–210. doi: 10.1068/p110207. [DOI] [PubMed] [Google Scholar]
- 32.Erickson P, McGill EC. Role of visual acuity, stereoacuity, and ocular dominance in monovision patient success. Optom. Vis. Sci. 1992;69:761–764. doi: 10.1097/00006324-199210000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Pierce DM. Comparability of two methods of estimating real-depth acuity. Ophthalmologica. 1975;171:224–235. doi: 10.1159/000307512. [DOI] [PubMed] [Google Scholar]
- 34.Lovasik JV, Szymkiw M. Effects of aniseikonia, anisometropia, accommodation, retinal illuminance, and pupil size on stereopsis. Invest. Ophthalmol. Vis. Sci. 1985;26:741–750. [PubMed] [Google Scholar]
- 35.Kani W. Stereopsis and spatial perception in amblyopes and uncorrected ametropes. Br. J. Ophthalmol. 1978;62:756–762. doi: 10.1136/bjo.62.11.756. [DOI] [PMC free article] [PubMed] [Google Scholar]