Abstract
Endometrial cancer is the most common malignancy of the female genital tract and is associated with poor prognosis. It is primarily a hormone‐dependent cancer that is regulated by steroid hormones, including estrogen and progesterone. Forkhead box A1 (FOXA1) is a member of the forkhead box transcription factor family and functions as a pioneer factor in estrogen receptor (ER)‐positive breast cancer. In the present study, we investigated the expression of FOXA1 in endometrial cancers by immunohistochemical analysis. Nuclear immunoreactivity for FOXA1 was detected in 40 of 109 cases (37%), and was found to be negatively associated with lymph node status (P = 0.033). In ER‐positive Ishikawa endometrial cancer cells, small interfering RNA‐mediated downregulation of FOXA1 promoted cell proliferation and migration. Furthermore, exogenously introduced FOXA1 suppressed both proliferation and migration of Ishikawa cells. These results suggest that FOXA1 functions as a tumor suppressor through modulation of proliferation and migration of endometrial cancer cells. (Cancer Sci 2012; 103: 806–812)
Endometrial cancer is the most common malignancy of the female genital tract, and the incidence of endometrial cancer has markedly increased in recent years. Because of the poor prognosis of endometrial cancer, several studies have focused on the development of effective treatment strategies for this cancer.1 A widely accepted notion is that new approaches to the treatment for endometrial cancer are pivotal to further improve the prognosis of this disease.2
Endometrial cancers are divided into two types, based on biology and clinical course. Type 1 is the estrogen‐dependent adenocarcinoma with an endometrial morphology, and type 2 is the non‐estrogen‐dependent endometrial carcinoma with serous papillary or clear cell morphology.3 Approximately 80–90% of sporadic endometrial cancers are distinguished as type 1 carcinomas and are associated with endometrial hyperplasia, hyperestrogenism and expression of the estrogen receptor (ER). The remaining 10–20% constitute type 2 carcinomas, which are generally unrelated to estrogen; type 2 carcinomas show negative or low ER expression.4 Estrogen‐dependent endometrial cancers are thought to arise from prolonged exposure to estrogens in the absence of sufficient progesterone (the “unopposed estrogen hypothesis”).5 Unopposed estrogen can result from various causes, including obesity, ovarian tumors that secrete estrogen, estrogen therapy (in the absence of progestin) and tamoxifen treatment (agonist activity). The more frequent type 1 endometrial cancer is associated with an endocrine milieu of estrogen predominance, involving loss of phosphatase, and tensin homolog (PTEN) tumor suppressor gene expression and dysfunction of DNA‐mismatch repair genes.6 Estrogen‐dependent activation of ERα can upregulate the expression of the insulin‐like growth factor receptor (IGFR), and autocrine IGFR‐mediated growth mechanisms can activate the PI3K/AKT pathway independently of PTEN.6
The forkhead box A1 (FOXA1) is a member of the forkhead family consisting of the winged‐helix DNA‐binding domain, and the N‐terminal and C‐terminal transcriptional domains. FOXA1 is expressed in various organs, including breast, liver, pancreas and prostate, and can bind to the promoters of a large number of genes associated with metabolic processes, regulation of signaling and the cell cycle.7, 8, 9 Expression of FOXA1 has been reported in various tumors, including lung, esophageal, prostate and breast cancers.10, 11, 12, 13
Recent global gene expression studies of breast cancer have shown that high FOXA1 expression was positively correlated with ERα and progesterone receptor (PR), but negatively correlated with histological grade and proliferation markers.13, 14, 15 FOXA1 expression was associated with better cancer‐specific survival,13, 14, 15 thus, FOXA1 expression is considered to be a better predictor of survival in breast cancer. Recently, FOXA1 was identified as a pioneer factor, and was shown to bind to chromatinized DNA and open the chromatin for binding of additional transcription factors, including ER.16, 17 Furthermore, FOXA1‐binding sites were detected in 50% of genes that are regulated by ER, and depletion of FOXA1 partially attenuated the estrogen response in breast cancer cells.12, 18, 19 Previous studies have shown that FOXA1 can act either as a growth stimulator or as a repressor in breast cancer. The effect of FOXA1 on cancer cell growth remains to be fully elucidated. In particular, information about the expression levels, clinical relevance and functional role of FOXA1 in estrogen‐dependent endometrial cancer is not currently available.
The aim of the present study was to determine the clinical relevance of FOXA1 in endometrial cancer, and to examine the role of FOXA1 in the proliferation and migration of endometrial cancer cells. The present results showed that FOXA1 immunoreactivity is negatively correlated with lymph node status. Knockdown of FOXA1 promotes both proliferation and migration of endometrial carcinoma Ishikawa cells. These data suggest that FOXA1 functions as a tumor suppressor through modulation of proliferation and migration of endometrial cancer cells.
Materials and Methods
Tissue selection and patient characteristics
Formalin‐fixed, paraffin‐embedded sections were prepared from samples obtained during surgery from 109 consecutive patients diagnosed with endometrial cancer at Juntendo University Hospital, Tokyo, Japan, between January 1994 and December 2002. The study was approved by the institutional review boards at Juntendo University Hospital, and informed consent was obtained from all the patients. The age of the patients was 27–82 years (mean age 57 years). The clinicopathological characteristics of endometrial cancer patients are presented in Table 1.
Table 1.
Correlation of forkhead box A1 immunoreactivity with clinicopathological parameters in endometrial cancer
| Variable | FOXA1 H‐score | |||
|---|---|---|---|---|
| Positive | Negative | χ2 | P‐value | |
| Age (years) | ||||
| ≤50 | 11 | 15 | 0.463 | 0.496 |
| 50< | 29 | 54 | ||
| FIGO stage | ||||
| Stage1, 2 | 35 | 54 | 0.892 | 0.345 |
| Stage3, 4 | 5 | 15 | ||
| TNM | ||||
| T1 | 32 | 56 | 0.21 | 0.976 |
| T2 | 4 | 5 | ||
| T3 | 3 | 8 | ||
| T4 | 1 | 0 | ||
| N0 | 37 | 50 | 4.562 | 0.033 |
| N1 | 0 | 9 | ||
| M0 | 38 | 69 | 1.287 | 0.257 |
| M1 | 2 | 0 | ||
| Grade | ||||
| Grade 1 | 25 | 41 | 0.747 | 0.582 |
| Grade 2 | 10 | 14 | ||
| Grade 3 | 5 | 14 | ||
| Tumour type | ||||
| Endometrioid | 39 | 65 | 0.316 | 0.957 |
| Serous | 0 | 2 | ||
| Clear | 0 | 2 | ||
| Adenosquamous | 1 | 0 | ||
| Survival (5 years) | ||||
| Alive | 38 | 61 | 0.649 | 0.421 |
| Death | 2 | 8 | ||
| ERα LI6 | ||||
| Average | 49.0 | 46.1 | 0.692 | |
| Ki‐67 LI6 | ||||
| Average | 23.1 | 27.3 | 0.236 | |
H‐scores of <20 and 20≤ were defined as negative and positive immunoreactivity, respectively. Adenosquamous, adenosquamous carcinoma; Clear, clear cell carcinoma; Endometrioid, endometrioid adenocarcinoma; FIGO, International Federation of Obstetrics and Gynecology; LI, labeling index; Serous, papillary serous adenocarcinoma; TNM, tumor‐node‐metastasis system for staging cancer.
Antibodies
Anti‐Flag and anti‐β‐actin antibodies were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Anti‐FOXA1 antibody was purchased from Abcam (Cambridge, UK). Polyclonal antibody for ERα (CONFIRM anti‐ER SP1) and monoclonal antibody for Ki‐67 (MIB‐1) were purchased from Ventana Medical Systems, (Tucson, AZ, USA) and Dako (Carpinteria, CA, USA), respectively.
Immunohistochemistry
Immunohistochemical analysis of FOXA1 was carried out using the CSA2 Biotin‐free Tyramide Signal Amplification System (Dako) according to the manufacturer's protocol. Tissue sections (6 μm) were deparaffinized, rehydrated through a graded ethanol series and rinsed in Tris‐buffered saline with 0.05% Tween‐20 (TBST). For antigen retrieval, the sections were autoclaved at 121°C for 10 min in 10 mM sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked using 3% hydrogen peroxide, and the sections were then incubated in serum‐free blocking reagent for 5 min. The primary antibody, a monoclonal antibody for FOXA1 (1:3000 dilution), was applied for 15 min at room temperature, followed by horseradish peroxidase (HRP)‐conjugated anti‐mouse IgG for 15 min at room temperature. Fluorescyl‐tyramide and antifluorescein antibody conjugated with HRP were used for signal amplification. The antigen‐antibody complex was visualized with 3,3′‐diaminobenzidine in chromogen solution (DAB+ CHROMOGEN). Counterstaining was carried out using Meyer's hematoxylin solution (×2; Wako Pure Chemical Industries, Osaka, Japan). As a positive control, a section of breast cancer tissue was immunostained with the anti‐FOXA1 antibody in the same manner. As a negative control, slides were immunostained as described earlier, except that a primary antibody was not used.
Immunohistochemical assessment
The histochemical score (H‐score) was used to assess the intensity of staining and the percentage of stained cells after immunohistochemistry.20 Staining intensity was scored as 0 (no intensity), 1 (weak intensity) or 2 (strong intensity), and the percentage of positive cells at each intensity was subjectively estimated to produce a final score in the range of 0–200. The cut‐off point for determining positive and negative staining was H‐score ≥ 20, based on the criteria used in some studies for breast cancer.14, 15, 21 Two investigators (Y.A. and H.K.) evaluated the tissue sections, and the average H‐score was considered. Immunoreactivities of ERα and Ki‐67 were scored in >1000 carcinoma cells for each case, and the percentage of immunoreactivity was determined as labeling index (LI).
Plasmid construction and small interfering RNA
Human FOXA1 (hFOXA1) was N‐terminally tagged with Flag and subcloned into a pcDNA3 vector (pcDNA3‐Flag‐hFOXA1). Synthetic siRNA duplexes targeting the human FOXA1 gene (Silencer Select Pre‐designed siRNA; siFOXA1) and the control siRNA duplexes (siCont) were purchased from Applied Biosystems (Carlsbad, CA, USA) and Dharmacon (Lafayette, CO, USA), respectively.
Cell culture and transfection
Ishikawa cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Transfection of expression plasmids or siRNA duplexes was carried out using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol.
Quantitative real‐time reverse transcription polymerase chain reaction
Total RNA from Ishikawa cells treated with estrogen or transfected with FOXA1‐specific siRNA was extracted and then subjected to qRT–PCR analysis. Total RNA extraction, first‐strand cDNA synthesis and quantitative PCR have been described previously.22 The primers for FOXA1 and GAPDH were as follows: FOXA1, 5′‐AGGTGTGTATTCCAGACCCG‐3′ and 5′‐TTGACGGTTTGGTTTGTGTG‐3′; GAPDH, 5′‐GGTGGTCTCCTCTGACTTCAACA‐3′ and 5′‐GTGGTCGTTGAGGGCAATG‐3′. Fold induction of mRNA expression was determined by comparing the mRNA levels of the si FOXA1‐treated samples with those of the siCont‐treated samples.
Western blot analysis
Whole‐cell lysates were prepared using a sodium dodecyl sulfate (SDS) sample buffer, resolved by 12% SDS polyacrylamide gel electrophoresis, and then transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Blotted membranes were probed with anti‐FOXA1 or anti‐Flag antibodies, incubated with horseradish peroxidase‐conjugated anti‐mouse immunoglobulin G (GE Healthcare, Buckinghamshire, UK), and visualized using enhanced chemiluminescence (GE Healthcare). Membranes were stripped and reprobed with a mouse monoclonal anti‐β‐actin antibody to verify equal loading of the protein across the lanes.
Cell proliferation assay
Ishikawa cells were seeded in 96‐well plates at a density of 2000 cells/well in DMEM containing 10% FBS for 24 h. Subsequently, 0.2 μg of pcDNA3‐Flag‐hFOXA1 or 20 pmol of siRNA targeting FOXA1 was transfected for 12 h. Cell proliferation was examined at the indicated time‐points by using the (2‐(2‐methoxy‐4‐nitrophenyl)‐3‐(4‐nitrophenyl)‐5‐(2,4‐disulfophenyl)‐2H‐tetrazolium, monosodium salt; WST‐8) assay kit (Nacalai Tesque, Kyoto, Japan) according to the manufacturer's protocol.
Cell migration assay
For the measurement of the cell migration activity, migration assays were carried out using Cell Culture Inserts for 24‐well plates (8.0 μm pore; BD FALCON, Franklin Lakes, NJ, USA). Before cell culture, the inner side of the insert membranes was coated with purified fibronectin (BD FALCON) in PBS (−) at a concentration of 10 μg/mL for 30 min at room temperature. After transfection of siRNA or expression vector for 48 h, cells were collected and resuspended in DMEM containing 10% FBS. Subsequently, Ishikawa cells were added to the upper chamber at 3 × 105 cells/well. After 24 h of incubation, the cells on the upper surface of the membrane were completely removed using a cotton swab. The cells on the lower surface of the membrane were fixed with 100% methanol, the insert membranes were cut and stained with Giemsa stain solution (Wako), and the permeating cells within five randomly selected areas were counted under an inverted microscope. At least three independent experiments were carried out for all conditions. The data are shown as mean (SD).
Survival curve analysis
Disease‐free and overall survival curves were obtained using the Kaplan–Meier method and verified by the log–rank (Mantel–Cox) test. JMP 9.0 software (SAS Institute, Cary, NC, USA) was used, and P‐values of <0.05 were regarded as statistically significant.
Statistical analyses
The correlation between the H‐score and clinicopathological characteristics was evaluated with the χ2‐test. A P‐value of <0.05 was regarded as statistically significant. Differences between the two groups were analyzed using two‐sample, two‐tailed Student's t‐test. A P‐value of <0.05 was considered significant. All data presented in the text and figures are the mean (SD).
Results
Negative correlation of FOXA1 immunoreactivity with lymph node status in endometrial cancer
To investigate the expression levels of the FOXA1 protein in endometrial cancer, immunohistochemical analysis was carried out using 109 endometrial cancer samples (Fig. 1). These samples were obtained from patients primarily treated by surgery. The clinicopathological characteristics of the patients are summarized in Table 1. The percentage of tumors with histological grade 1, 2 and 3, was 61%, 22% and 17%, respectively. Almost all tumors were endometrioid adenocarcinomas (95%), whereas 1.8% and 1.8% of tumors were papillary serous carcinomas and clear cell carcinomas, respectively. A total of 75% of the tumors were in International Federation of Obstetrics and Gynecology (FIGO) stage 1, based on FIGO 1988 staging system. FOXA1 immunoreactivity in endometrial cancer was assessed using the histochemical score (H‐score),20 and a H‐score ≥ 20 was regarded as positive. Strong immunoreactivity for FOXA1 was diffusely observed in grade 1 endometrial cancer (Fig. 1a), compared with grade 3 endometrial cancer (Fig. 1c). As a positive control, breast cancer samples were immunostained with the FOXA1 antibody, and the nuclear immunoreactivity of FOXA1 was confirmed in breast cancer (Fig. 1e). No immunoreactivity was observed in the corresponding cancer tissues in the absence of a primary antibody (Fig. 1b,d,f). We also examined FOXA1 expression in normal endometrium. We found that FOXA1 immunoreactivity was negative in five specimens of normal endometrium at the proliferative phase. In contrast, intense FOXA1 immunoreactivity was observed in one specimen of endometrial hyperplasia (H‐score = 200).
Figure 1.
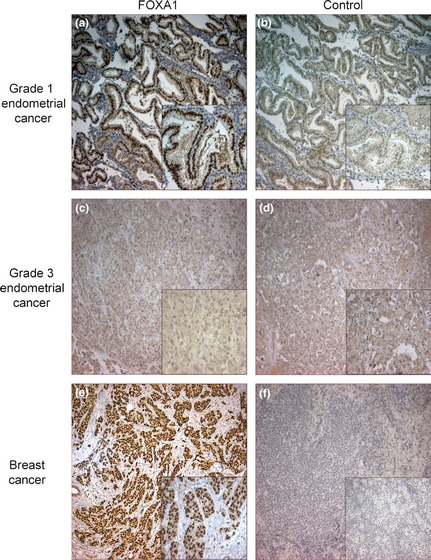
Immunohistochemical analysis of forkhead box A1 (FOXA1) in endometrial cancer. Representative immunohistochemical staining of (a–d) endometrial cancer and (e,f) breast cancer with (a,c,e) anti‐FOXA1 antibody or (b,d,f) without any primary antibody. (a) Strong immunoreactivity for FOXA1 in grade 1 adenosquamous cell carcinoma of the endometrium and (c) weak immunoreactivity in grade 3 endometrioid adenocarcinomas of the endometrium were shown. (b,d,f) No immunoreactivity was observed in the no‐antibody controls in the corresponding specimens. Bar, 100 μm.
Statistical analysis showed that the nuclear immunoreactivity of FOXA1 was negatively associated with lymph node status (P = 0.029, Table 1). We examined the correlation of FOXA1 immunoreactivity with ERα or Ki‐67 labeling index. The mean value of ERα labeling index in FOXA1‐positive cases was slightly higher than that in FOXA1‐negative cases; however, this finding was not statistically significant (P = 0.692) in our endometrial cancer specimens. The mean value of Ki‐67 immunoreactivity in FOXA1‐positive cases was slightly lower than that in FOXA1‐negative cases; however, this finding was not statistically significant (P = 0.236) in our endometrial cancer samples (Table 1). Consistent with these findings, the results of Kaplan–Meier survival curve analyses showed that patients with a positive nuclear immunoreactivity for FOXA1 had longer disease‐free and overall survivals, although the differences were not statistically significant (Fig. 2a,b, respectively). These results suggest that FOXA1 can serve as a prognostic biomarker for endometrial cancer.
Figure 2.
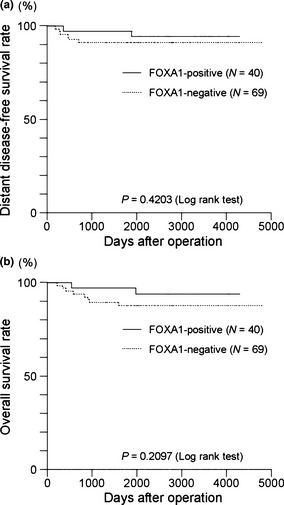
Kaplan–Meier survival analyses according to forkhead box A1 (FOXA1) immunoreactivity in endometrial cancer (n = 109). (a) Distant disease‐free and (b) overall survival curves of patients with endometrial cancer according to FOXA1 immunoreactivity were plotted using JMP 9.0 software (SAS Institute), and statistical significance was analyzed by the log–rank (Mantel–Cox) test.
Knockdown of FOXA1 expression promotes cell proliferation and migration of Ishikawa cells
To assess the effect of FOXA1 on proliferation of endometrial cancer cells, Ishikawa cells were transiently transfected with FOXA1‐specific siRNA. Treatment of cells with siRNA specific for FOXA1 reduced mRNA expression by 40% (Fig. 3a). These cells were then subjected to the WST‐8 cell proliferation assay. Knockdown of FOXA1 expression significantly promoted proliferation of Ishikawa cells at 3 and 5 days after transfection, compared with control siRNA transfected cells (Fig. 3b). Furthermore, knockdown of FOXA1 significantly enhanced the migration activity of Ishikawa cells (Fig. 3c). These results show that FOXA1 has inhibitory effects on both proliferation and migration activity of endometrial cancer cells.
Figure 3.
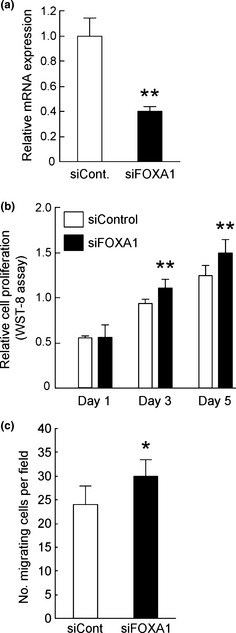
Knockdown of forkhead box A1 (FOXA1) expression promoted proliferation and migration activity of Ishikawa cells. (a) Knockdown efficiency of siRNA against FOXA1 in Ishikawa cells. Total RNA was prepared from Ishikawa cells transfected with control siRNA (siCont) or siRNA specific for FOXA1 (siFOXA1) for 48 h, and relative expression levels of FOXA1 mRNA were examined by qRT–PCR (upper). **P < 0.01 compared with siCont (Student's t‐test). (b) Ishikawa cells were transfected with siCont or siFOXA1 and then cultured for 5 days. Relative cell proliferation at the indicated time‐points was examined using a WST‐8 assay kit. **P < 0.01 compared with siCont (Student's t‐test). (c) Ishikawa cells were transfected with siCont or siFOXA1 for 48 h and then reseeded into Cell Culture Inserts with 8‐μm pores. Cells that showed migration in 24 h were stained with Giemsa staining solution and visualized under a microscope. Bars represent the mean number of cells (SD) counted in five fields. *P < 0.05 compared with siCont (Student's t‐test).
Overexpression of FOXA1 suppresses cell proliferation and migration of endometrial cancer cells
For further verification of the inhibitory effects of FOXA1 on proliferation and migration of endometrial cancer cells, Ishikawa cells were transiently transfected with a FOXA1 expression vector. Overexpression of FOXA1 at the protein level in Ishikawa cells was confirmed by western blotting (Fig. 4a). Cells were subjected to the WST‐8 cell proliferation assay. In contrast to the results of FOXA1 knockdown, overexpression of FOXA1 in Ishikawa cells significantly repressed cell proliferation on days 3 and 5 after transfection (Fig. 4b). In the transwell migration assay, FOXA1‐overexpressing Ishikawa cells also showed significantly reduced migration activity (Fig. 4c). Taken together, these results suggest that FOXA1 suppresses proliferation and migration activity of endometrial cancer Ishikawa cells.
Figure 4.
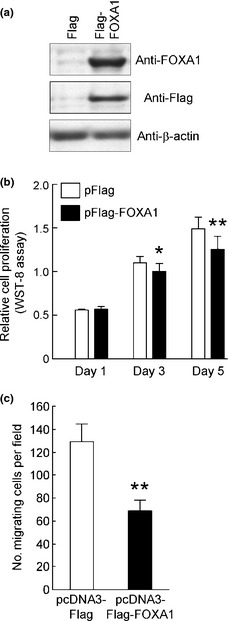
Proliferation and migration activity of Ishikawa cells are suppressed by exogenously transfected forkhead box A1 (FOXA1). (a) Total cell lysates of Ishikawa cells (−) or Ishikawa cells transfected with pcDNA3‐Flag or pcDNA3‐Flag‐FOXA1 for 48 h were immunoblotted with anti‐Flag, anti‐FOXA1 or anti‐β‐actin antibodies. (b) Ishikawa cells were transfected with pcDNA3‐Flag (control) or pcDNA3‐Flag‐FOXA1 and then incubated for 5 days. Relative cell proliferation at the indicated time‐points was examined using a WST‐8 assay kit. *P < 0.05, **P < 0.01 compared with control vector (Student's t‐test). (c) Ishikawa cells were transfected with pcDNA3‐Flag (control) or pcDNA3‐Flag‐FOXA1 for 48 h and then reseeded into Cell Culture Inserts with 8‐μm pores. Cells that showed migration in 24 h were stained with Giemsa staining solution and visualized under a microscope. Bars represent the mean number of cells (SD) counted in five fields. **P < 0.01 compared with control vector (Student's t‐test).
Discussion
The clinical relevance of FOXA1 in breast cancer has been extensively analyzed.23 Recent gene expression profiling studies have classified breast cancers into five intrinsic subtypes with unique molecular characteristics and prognostic significance.24, 25 These include luminal subtypes A and B, HER2 +/ER−, basal‐like and normal‐like subtypes. Luminal subtypes A and B are ERα‐positive breast cancers, and subtype A expresses higher levels of ERα and has a better prognosis than subtype B does.25 FOXA1 expression correlates with luminal subtype A breast cancer and is a significant predictor of cancer‐specific survival in patients with ER‐positive tumors. Similar to breast cancer, endometrial cancer is an estrogen‐dependent cancer, and approximately 80–90% of sporadic endometrial cancers are distinguished as type 1 carcinomas and are associated with the expression of the ER.
In our immunohistochemical analysis, nuclear immunoreactivity for FOXA1 was detected in 40 of 109 cases (37%) in endometrial cancer, whereas FOXA1 immunoreactivity was negative in five specimens of normal endometrium at proliferative phase (H‐score = 0). Intense FOXA1 immunoreactivity was observed in one specimen of endometrial hyperplasia, which is generally considered as precancerous changes of endometrium (H‐score = 200). Although FOXA1 immunoreactivities in normal endometrium at secretory and menstrual phases remain to be elucidated, these data suggest that FOXA1 might play a role in the pathogenesis of endometrial proliferative diseases and cancer rather than in the physiological proliferation phase of normal endometrium.
In the present study, we showed that FOXA1 immunoreactivity was negatively correlated with lymph node status in endometrial cancer, suggesting that FOXA1 is a favorable prognostic factor of endometrial cancer. In addition, patients with a positive nuclear immunoreactivity for FOXA1 had longer disease‐free and overall survivals, although the differences were not statistically significant. No significant difference might be partially as a result of a low recurrence rate of endometrial cancer in the present subjects, therefore, a larger scale cohort study would be necessary to verify the prognostic significance of FOXA1 in endometrial cancer survival. Nevertheless, these results imply that common molecular mechanisms underlie the contribution of FOXA1 to longer survival of patients with estrogen‐dependent tumors, including breast and endometrial cancers. Thus, the prognostic ability of FOXA1 in these tumors might be useful in decisions regarding clinical treatment.14, 15
The effect of FOXA1 on the proliferation of cancer cells has been a subject of controversy. In an in vitro model, downregulation of FOXA1 by RNAi significantly suppressed proliferation of HER2‐negative and FOXA1‐positive breast cancer cell lines.26 Meanwhile, the repressor function of FOXA1 was shown through its overexpression, which blocked metastatic progression by affecting the expression of the BRCA1‐associated cell cycle inhibitor, p27, and promoting E‐cadherin expression.27, 28 In the present study, we showed that overexpression of FOXA1 suppressed, and knockdown of FOXA1 conversely promoted, both proliferation and migration of endometrial cancer Ishikawa cells. These findings could partially account for the favorable prognostic ability of FOXA1 in endometrial cancer. Consistent with the suppressive effect of FOXA1 on the proliferation of endometrial cancer cells, the mean value of Ki‐67 immunoreactivity in FOXA1‐positive cases was slightly lower than that in FOXA1‐negative cases, although this finding was not statistically significant in our endometrial cancer specimens. Elucidation of the mechanism responsible for the inhibitory effects of FOXA1 on proliferation and migration of endometrial cancer cells should be the focus of future studies.
E2 is known to induce cell motility in MCF‐7 cells,29, 30 and in some endometrial cancer cell lines, including Ishikawa cells,31, 32 although the underlying mechanisms of this effect and cell type specificity remain to be elucidated. Recent genome‐wide studies aimed at identifying ER‐ and androgen receptor (AR)‐binding sites have shown that FOXA1 plays a role in the regulation of both nuclear receptor networks.12, 17, 19 FOXA1 can bind to chromatinized DNA and open the chromatin for binding of additional transcription factors, and hence, it has been considered a pioneer factor.16, 17 By binding to specific regions of the chromatin, it creates an epigenetic signature that enables transcription factors, such as ER, to establish a transcriptional program.16 Consistent with this possibility, FOXA1‐binding sites have been detected in 50% of genes that are regulated by ER, and depletion of FOXA1 partially attenuates the estrogen response in breast cancer cells.12, 18 FOXA1 therefore might modulate E2‐induced cell motility through the activation of the expression of ERα‐target genes in endometrial cancer cells. It is also notable that Dr Carroll's group reported that FOXA1 could also mediate ER function in a non‐breast cancer context, including ovarian and osteosarcoma cell lines.19 In our endometrial cancer specimens, there is a tendency for ERα immunoreactivity to be positively associated with FOXA1 immunoreactivity; however, this finding was not statistically significant. Taken together, FOXA1 has been reported to function as a pioneer factor in ERα‐positive breast cancer, whereas the present results suggest that FOXA1 might play a distinct role in endometrial cancer rather than function as a pioneer factor for ERα‐mediated pathways.
More recently, genome‐wide mapping of ERα‐, AR‐ and FOXA1‐binding events in breast and prostate cancer cells by using high‐throughput sequencing has uncovered additional details of transcriptional control mechanisms in nuclear receptor‐mediated transcription. As another pioneer factor of ER, transducin‐like enhancer protein 1 (TLE1) was shown to positively assist some ER‐chromatin interactions independently of and/or cooperatively with FOXA1, and to be essential for effective ER‐mediated cell division of breast cancer cells.33 As a novel collaborative factor in ERα‐mediated transcription, AP‐2γ was found to interact with FOXA1 in the majority of shared binding regions and be essential for the regulation of ER‐mediated long‐range chromatin interactions and gene transcription in breast cancer cells.34 With regard to AR‐mediated transcription programs, Wang et al.35 showed that FOXA1 could simultaneously facilitate and restrict the action of AR on structurally and functionally distinct classes of enhancers, thus showing the ability to reprogram the hormonal response by causing a massive switch in AR binding to a distinct cohort of pre‐established enhancers. Interestingly, FOXA1 was shown to mediate AR binding and regulation of ER cis‐regulatory elements in ER‐negative and AR‐positive molecular apocrine tumors, suggesting that FOXA1 functions in a cell‐lineage specific manner, depending on the contexts of nuclear receptors and/or collaborative factors.36 These findings suggest that uncovering endometrial cancer‐specific transcriptional control mechanisms mediated by factors including FOXA1, nuclear receptors and other factors will be useful to develop more effective treatments for endometrial cancer.
In summary, we showed that FOXA1 exerted repressive effects on proliferation and migration of endometrial cancer cells, and was correlated with the lymph node status of endometrial cancer. These results might provide new insights into the prognosis of endometrial cancer and help design effective antitumor therapies.
Disclosure Statement
The authors declare that they have no conflict of interest.
Acknowledgments
We thank W. Sato, T. Nagai, Y. Maruyama and T. Ujihira for their technical assistance. We also thank T. Shigekawa, Saitama Medical University, for kindly providing human breast cancer tissue samples. We are also grateful to Dr M. Nishida for kindly providing Ishikawa cells (Ishikawa 3H12 No. 74). This work was supported in part by Grants‐in‐Aid for Cancer Research (21‐4) from the Ministry of Health, Labor and Welfare; the Program for Promotion of Fundamental Studies in Health Science of the NIBIO; by grants of the Cell Innovation Program and the Support Project of Strategic Research Center in Private Universities from the MEXT.
References
- 1. Oza AM, Elit L, Tsao MS et al Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC clinical trials group. J Clin Oncol 2011; 29: 3278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsoref D, Oza AM. Recent advances in systemic therapy for advanced endometrial cancer. Curr Opin Oncol 2011; 23: 494–500. [DOI] [PubMed] [Google Scholar]
- 3. Lax SF, Kurman RJ. A dualistic model for endometrial carcinogenesis based on immunohistochemical and molecular genetic analyses. Verh Dtsch Ges Pathol 1997; 81: 228–32. [PubMed] [Google Scholar]
- 4. Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular‐based classification. Virchows Arch 2004; 444: 213–23. [DOI] [PubMed] [Google Scholar]
- 5. Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta‐analysis. Obstet Gynecol 1995; 85: 304–13. [DOI] [PubMed] [Google Scholar]
- 6. Block M, Fister S, Emons G, Seeber S, Gründker C, Günthert AR. Antiproliferative effects of antiestrogens and inhibitors of growth factor receptor signaling on endometrial cancer cells. Anticancer Res 2010; 30: 2025–31. [PubMed] [Google Scholar]
- 7. Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol 2002; 250: 1–23. [DOI] [PubMed] [Google Scholar]
- 8. Wolf I, Bose S, Williamson EA, Miller CW, Karlan BY, Koeffler HP. FOXA1: growth inhibitor and a favorable prognostic factor in human breast cancer. Int J Cancer 2007; 120: 1013–22. [DOI] [PubMed] [Google Scholar]
- 9. Kaestner KH. The FoxA factors in organogenesis and differentiation. Curr Opin Genet Dev 2010; 20: 527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin L, Miller CT, Contreras JI et al The hepatocyte nuclear factor 3 alpha gene, HNF3alpha (FOXA1), on chromosome band 14q13 is amplified and overexpressed in esophageal and lung adenocarcinomas. Cancer Res 2002; 62: 5273–9. [PubMed] [Google Scholar]
- 11. Gao N, Zhang J, Rao MA et al The role of hepatocyte nuclear factor‐3α (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol 2003; 17: 1484–507. [DOI] [PubMed] [Google Scholar]
- 12. Carroll JS, Liu XS, Brodsky AS et al Chromosome‐wide mapping of estrogen receptor binding reveals long‐range regulation requiring the forkhead protein FoxA1. Cell 2005; 122: 33–43. [DOI] [PubMed] [Google Scholar]
- 13. Habashy HO, Powe DG, Rakha EA et al Forkhead‐box A1 (FOXA1) expression in breast cancer and its prognostic significance. Eur J Cancer 2008; 44: 1541–51. [DOI] [PubMed] [Google Scholar]
- 14. Badve S, Turbin D, Thorat MA et al FOXA1 expression in breast cancer–correlation with luminal subtype A and survival. Clin Cancer Res 2007; 13: 4415–21. [DOI] [PubMed] [Google Scholar]
- 15. Thorat MA, Marchio C, Morimiya A et al Forkhead box A1 expression in breast cancer is associated with luminal subtype and good prognosis. J Clin Pathol 2008; 61: 327–32. [DOI] [PubMed] [Google Scholar]
- 16. Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA‐4. Mol Cell 2002; 9: 279–89. [DOI] [PubMed] [Google Scholar]
- 17. Lupien M, Eeckhoute J, Meyer CA et al FoxA1 translates epigenetic signatures into enhancer‐driven lineage‐specific transcription. Cell 2008; 132: 958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laganière J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguère V. From the cover: location analysis of estrogen receptor α target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci USA 2005; 102: 11651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hurtado A, Holmes KA, Ross‐Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 2011; 43: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCarty KS Jr, Miller LS, Cox EB, Konrath J, McCarty KS Sr. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med 1985; 109: 716–21. [PubMed] [Google Scholar]
- 21. Mehta RJ, Jain RK, Leung S et al FOXA1 is an independent prognostic marker for ER‐positive breast cancer. Breast Cancer Res Treat 2011; DOI: 10.1007/s10549-011-1482-6 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22. Ijichi N, Shigekawa T, Ikeda K et al Estrogen‐related receptor γ modulates cell proliferation and estrogen signaling in breast cancer. J Steroid Biochem Mol Biol 2011; 123: 1–7. [DOI] [PubMed] [Google Scholar]
- 23. Nakshatri H, Badve S. FOXA1 as a therapeutic target for breast cancer. Expert Opin Ther Targets 2007; 11: 507–14. [DOI] [PubMed] [Google Scholar]
- 24. Perou CM, Sørlie T, Eisen MB et al Molecular portraits of human breast tumours. Nature 2000; 406: 747–52. [DOI] [PubMed] [Google Scholar]
- 25. Sørlie T, Perou CM, Tibshirani R et al Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamaguchi N, Ito E, Azuma S et al FoxA1 as a lineage‐specific oncogene in luminal type breast cancer. Biochem Biophys Res Commun 2008; 365: 711–7. [DOI] [PubMed] [Google Scholar]
- 27. Williamson EA, Wolf I, O'Kelly J, Bose S, Tanosaki S, Koeffler HP. BRCA1 and FOXA1 proteins coregulate the expression of the cell cycle‐dependent kinase inhibitor p27(Kip1). Oncogene 2006; 25: 1391–9. [DOI] [PubMed] [Google Scholar]
- 28. Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH. Regulatory mechanisms controlling human E‐cadherin gene expression. Oncogene 2005; 24: 8277–90. [DOI] [PubMed] [Google Scholar]
- 29. Albini A, Graf J, Kitten GT et al 17β‐Estradiol regulates and v‐Ha‐ras transfection constitutively enhances MCF7 breast cancer cell interactions with basement membrane. Proc Natl Acad Sci USA 1986; 83: 8182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saji S, Kawakami M, Hayashi S et al Significance of HDAC6 regulation via estrogen signaling for cell motility and prognosis in estrogen receptor‐positive breast cancer. Oncogene 2005; 24: 4531–9. [DOI] [PubMed] [Google Scholar]
- 31. Fujimoto J, Hori M, Ichigo S, Morishita S, Tamaya T. Estrogen activates invasiveness of endometrial cancel cells to the interstitium. Invasion Metastasis 1995; 15: 135–43. [PubMed] [Google Scholar]
- 32. Fujimoto J, Hori M, Ichigo S, Morishita S, Tamaya T. Estrogen activates migration potential of endometrial cancer cells through basement membrane. Tumour Biol 1996; 17: 48–57. [DOI] [PubMed] [Google Scholar]
- 33. Holmes KA, Hurtado A, Brown GD et al Transducin‐like enhancer protein 1 mediates estrogen receptor binding and transcriptional activity in breast cancer cells. Proc Natl Acad Sci USA 2011; DOI: 10.1073/pnas.1018863108 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tan SK, Lin ZH, Chang CW et al AP‐2γ regulates oestrogen receptor‐mediated long‐range chromatin interaction and gene transcription. EMBO J 2011; 30: 2569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang D, Garcia‐Bassets I, Benner C et al Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 2011; 474: 390–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Robinson JL, Macarthur S, Ross‐Innes CS et al Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J 2011; 30: 3019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


