Abstract
Prosaposin, a secreted protein, is a well‐known pleiotropic growth factor. Although a previous report has indicated that prosaposin is overexpressed in breast cancer cell lines, the role of prosaposin in the development of breast cancer remains to be identified. Here, we first revealed that prosaposin upregulated estrogen receptor alpha expression, nuclear translocation and transcriptional activity by western blot, immunofluorescence assay and dual luciferase reporter gene assay, respectively. Furthermore, we demonstrated prosaposin upregulated estrogen receptor alpha expression through MAPK‐signaling pathway using MAPK inhibitor. Proliferation assay and tumor xenograft experiments in nude mice (n = 6 per group) further confirmed prosaposin could promote breast cancer growth significantly in vitro and in vivo. These findings suggested that prosaposin might enhance estrogen receptor alpha‐mediated signaling axis and play a role in breast cancer development and progression.
Breast cancer is the most common malignancy and the main cause of death from cancers in women.1 The carcinogenesis of breast cancer is frequently hormone‐dependent. The female sex‐steroid hormone estrogen 17β‐estradiol (E2) plays a prominent role in mediating the maturation, proliferation, differentiation, and influences the growth and development of breast cancer.2 Numerous studies have indicated that E2 can induce and promote breast cancer and this process is mediated primarily by estrogen receptor alpha (ERα).3, 4, 5 Estrogen receptor (ER) is a member of the steroid/nuclear receptor family of transcription regulators, while ERα is the predominant receptor isoform expressed in breast cancer. Approximately 70% of breast cancer patients score positive for ERα at diagnosis.6, 7, 8, 9 Estrogen receptor alpha promotes cell growth, metastasis and also mediates resistance to apoptosis or immunosurveillance in breast cancer.10, 11, 12
Prosaposin (PSAP) exists as a secretory protein (70 kDa) as well as a lysosomal protein (65 kDa). The molecular weight difference depends on the post‐translational glycosylation.13 Lysosomal PSAP is the precursor of four sphingolipid activator proteins (saposin A–D) and is involved in hydrolysis of sphingolipids within lysosome.14 Secretory PSAP is a well‐known pleiotropic growth factor found in secretory body fluid, such as seminal plasma, bile, cerebrospinal fluid, human milk,15, 16 as well as neuronal surface membrane.17 The distribution of PSAP suggests it may have some specific extracellular functions. PSAP‐deficiency in human and mice is lethal.18, 19 Prosaposin knock‐out mice also present with a series of developmental abnormalities in the nervous system and male reproductive organs.18, 20 Recent investigations show that PSAP could prevent cell death or apoptosis and promote cell survival.21, 22 Koochekpour et al.23 focus on the function of PSAP in prostate cancer and find PSAP is overexpressed in prostate cancer, and also upregulates androgen receptor (AR) and prostate specific antigen (PSA) expression and activity in prostate cancer cells.24 Serum secreted PSAP levels significantly decrease in primary prostate cancer, but increase in metastatic prostate cancer. So it might be possible to take secreted PSAP in patients' serum as a bio‐marker to differentiate primary and advanced prostate cancer.25 Prosaposin protein purified from human milk has been widely used in experiments.26, 27 The expression and secretion of PSAP is also detected in breast cancer cells,28 while the biological effect of PSAP in breast cancer is not known.
In the present study, we intended to clarify the role of PSAP in breast cancer and uncover the possible molecular mechanism. First of all, we demonstrated that PSAP could upregulate mRNA and protein expression level of ERα in breast cancer cells. It also enhanced the nuclear translocation and transcription activity of ERα. Moreover, PSAP upregulated ERα through MAPKs signaling pathway. These results led us to hypothesize that as a pluripotent growth factor and a regulator of ERα, PSAP may play a role in the progression of breast cancer. Then, we confirmed the role of PSAP in subcutaneous tumor xenografted nude mice. Local injection of recombinant PSAP in tumor promoted the expression of ERα as well as the growth of breast cancer, which may shed new light into the molecular mechanisms of PSAP in breast cancer.
Materials and Methods
Materials
Dulbecco's modified eagle medium (DMEM), phenol red‐free (PR‐free) DMEM, BSA, Triton X‐100 and Hoechst 33258 were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Lipofectamine 2000, Trizol Reagent, FBS were purchased from Invitrogen (Carlsbad, CA, USA). Charcoal‐stripped FBS (CS‐FBS) was purchased from Biowest (Nuaillé, France). Polyvinylidene fluoride (PVDF) membrane, leupeptin, aprotinin and PMSF were purchased from Roche (Indianapolis, IN, USA). Antibodies to p44/42, phospho‐p44/42, p38, phospho‐p38, JNK, and phospho‐JNK were purchased from Cell Signaling Technology (Boston, MA, USA). Mouse monoclonal anti‐ERα, anti‐GAPDH, HRP‐conjugated goat anti‐rabbit and HRP‐conjugated goat anti‐mouse IgG secondary antibodies were from Santa Cruz Biotechnology (Heidelberg, Germany). Rabbit polyclonal anti‐prosaposin and anti‐H2AFX antibody were purchased from Protein Tech Group (Chicago, IL, USA). Fluorescein isothiocyanate‐conjugated donkey anti‐mouse secondary antibody was from Jackson Laboratory (Bar Harbor, ME, USA). Prosaposin recombinant protein was purchased from Abnova (Taipei City, Taiwan). The ECL assay kit was purchased from Tiangen (Beijing, China). E2 was purchased from Fluka (St. Louis, MO, USA).
Experimental animals, cell culture and transfections
Four‐week‐old female ν/ν mice, human breast cancer MCF‐7 cells and T47D cells were obtained from the Institute of Cell Biology, the Chinese Academy Of Sciences (Shanghai, China). MCF‐7 and T47D cells were cultured in DMEM supplemented with 10% FBS, 100 units/mL of penicillin and 50 μg/mL streptomycin. MCF‐7 cells were further supplemented with 0.01 mg/mL bovine insulin. Cells were transfected using the Lipofectamine 2000 with plasmids according to the manufacturer's protocol.
Plasmids construction and RNA interference
Estrogen receptor alpha expression plasmid, Renilla luciferase plasmid (pRL), 3 × estrogen response element (ERE)‐luciferase reporter constructs were kindly provided by Dr. Hongliang Zong. Two complementary oligonudeotides targeted to the PSAP gene were designed to knockdown PSAP expression: 5′‐GATCCATCCCTTCCCTGCGACATATTCAAGGAGATATGTCGCAGGGAAGGGATTTTTTTGGAAA‐3′ and 5′‐AGCTTTTCCAAAAAAATCCCTTCCCTGCGACATATCTCTTGAATATGTCGCAGGGAAGGGATG‐3′. Plasmid psilencer‐PSAP was constructed by inserting the annealed complementary oligonucleotides into the psilencer 2.1‐U6 neo vector (Ambion, New York, NY, USA). PcDNA3.1‐PSAP was constructed from pRC/CMV‐PSAP plasmid kindly provided by Dr. C.R. Morales (Department of Anatomy and Cell Biology, McGill University, Montreal, QC, Canada).
Dual luciferase reporter gene assays
T47D and MCF‐7 cells (3 × 104 cells per well in 24‐well plates) were incubated in 5% CS‐FBS supplemented PR‐free DMEM for 2 days prior to transfection. Cells were cotransfected with 3×ERE‐luciferase reporter construct (200 ng), a control pRL (2 ng). Twenty‐four hours after transfection, the culture medium was replaced with PR‐free DMEM containing 5% CS‐FBS and supplemented with PSAP at indicated concentrations and ethyl alcohol (ETOH) or 100 nM E2. After another 24 h, cells were lysed using Passive Lysis Buffer according to the manufacturer's specifications and assayed immediately for reporter and control gene activities with the Dual‐Luciferase Reporter Gene Assay (Promega, Madison, WI, USA) using a Lumat LB9507 luminometer (EG&G Berthold, Bad Wildbad, Germany).
Western blot analysis
Protein extraction from cultured cells or tumor tissues and western blotting analysis were performed as previously described.29
Total RNA extraction and real‐time PCR
Total mRNA samples of MCF‐7 and T47D breast cancer cells were extracted using Trizol reagent according to the manufacturer's instructions. One microgram of total RNA extracted from the cells was subjected to reverse transcription (RT). The RT and real‐time PCR were performed by using TaKaRa RNA PCR Kit (AMV) Ver. 3.0 and SYBR Premix Ex Taq II according to the manual (TaKaRa, Dalian, China), respectively. Primers used for real‐time PCR were as follows: ERα‐F 5′‐AGGTGGACCTGATCATGGAG‐3′ and ERα‐R 5′‐AAGCTTCGATGATGGGCTTA‐3′; GAPDH‐F 5′‐GGCTGAGAACGGGAAGCTTGTCAT‐3′ and GAPDH‐R 5′‐CAGCCTTCTCCATGGTGGTGAAGA‐3′. Real‐time PCR was performed using 7500 multicolor real‐time PCR detection system (ABI, Carlsbad, CA, USA) with the following cycling conditions: (i) 30 s at 95°C and (ii) 40 cycles, with one cycle consisting of 5 s at 95°C, 34 s at 60°C. Glyceraldehyde 3‐phosphate dehydrogenase was used as an internal reference under the same experimental conditions. Data were analyzed by using 7500 software (ABI). The values were obtained through normalizing to GAPDH copies.
Preparation of nuclear and cytoplasmic extract
To extract the nuclear protein, 1 × 106 MCF‐7 cells were collected for nuclear extraction. Cytoplasmic and nuclear fractions were extracted with nuclear extraction kit (Thermo, Rockford, IL, USA) according to the manufacturer's manual and stored at −70°C for further analysis.
Immunofluorescence assay
The MCF‐7 cells were plated onto cover slips. After 24 h, cells were treated with ETOH, E2 (100 nM) or PSAP (20 ng/mL) and then washed with PBS, fixed in 4% polyformaldehyde, permeabilized in 0.2% Triton X‐100 and blocked in 1% BSA for 1 h at room temperature. Cells were stained with anti‐ERα antibody for 2 h at room temperature followed by incubation with FITC‐conjugated donkey anti‐mouse secondary antibody for 1 h at room temperature. To stain the cell nucleus, the cover slips were washed with PBS three times and incubated in 25 μg/mL Hoechst 33258 solution for 10 min in a dark chamber. Cells were washed three times with PBS, inverted, mounted on slides, and examined in Leica TCS SP5 confocal microscope (Leica, Wetzlar, Germany).
Cell proliferation assay
Cell proliferation was analyzed using the Cell Counting Kit (Dojindo, Kamimashiki‐gun Kumamoto, Japan). Briefly, 2 × 103 MCF‐7 cells (in 100 μL medium) were plated per well in a 96‐well plate, and allowed to adhere overnight. The cells were incubated with ETOH or 100 nM E2 and PSAP protein at indicated concentrations, and then cultured in PR‐free DMEM with 5% CS‐FBS for 24 h. At the end of incubation, 10 μL of CCK‐8 solution was added to each well and the cultures were incubated at 37°C for 40 min. Cell proliferation rate was assessed by measuring the absorbance at 450 nm with the Universal Microplate Reader (Bio‐Tek Instruments, Minneapolis, MN, USA). The results were plotted as means ± SD of three separate experiments having four determinations per experiment for each experimental condition.
Tumor Xenograft experiment
For mouse xenograft experiments, 3 × 106 MCF‐7 cells were injected subcutaneously into the flanks of female nude mice aged 4 weeks. Both groups (six mice per group) were treated with E2 in a dose of 5 mg/kg once a week. The nude mice in PSAP group were additionally injected with 10 μg/kg PSAP protein in the tumor site once a week. The control group was injected with the same volume of saline instead. Tumors were allowed to grow for 5 weeks before being excised and weighed before protein extraction by homogenization.
Results
Prosaposin upregulates ERα level in breast cancer cells
In order to better investigate the effect of PSAP in breast cancer, we treated breast cancer cells with recombinant PSAP protein. Interestingly, we found the ERα protein expression level was positively regulated by PSAP in a dose‐dependent manner in MCF‐7 cells and T47D cells (Figure 1a,b). Similar tendencies were also observed in mRNA level of ERα in MCF‐7 and T47D cells (Figure 1c,d). Meanwhile, ERα protein and mRNA level was decreased when endogenous PSAP was knocked down by RNA interference in MCF‐7 cells (Figure 1e,f). Furthermore, we treated MCF‐7 cells with conditioned media (CM) of PSAP‐transfected MCF‐7 cells, which secreted PSAP in high concentrations (Figure 1h) and then found the CM of PSAP‐transfected cells could upregulate ERα protein expression (Figure 1g), which indicated that the secreted PSAP played a role in regulating ERα expression level in breast cancer cells.
Figure 1.
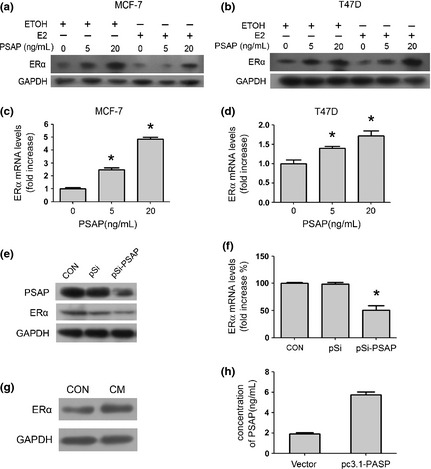
Prosaposin (PSAP) upregulated estrogen receptor alpha (ERα) expression. (a,b) After the initial 48‐h estrogen deprivation, MCF‐7 and T47D cells were treated with increasing concentrations (0, 5, 20 ng/mL) of PSAP protein with or without E2 for 24 h. Estrogen receptor alpha protein level was detected by western blot. (c,d) MCF‐7 and T47D cells were treated with PSAP (0, 5, 20 ng/mL) for 24 h. The total RNA was extracted and subjected to real‐time polymerase chain reaction (PCR) analysis of ERα mRNA level. (e,f) MCF‐7 cells were transfected with pSilencer 2.1 (pSi) or pSilencer‐PSAP (pSi‐PSAP) plasmid. 48 h after transfection, cell lysates were quantified and blotted with anti‐PSAP, anti‐ERα and anti‐glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) antibodies (e), and the total RNA was extracted and subjected to real‐time PCR analysis of ERα mRNA level (f). (g) MCF7 cells were treated with control or conditioned media (CM) from pcDNA3.1‐PSAP transfected MCF7 cells. Estrogen receptor alpha protein level was detected by western blot. The amount of PSAP in CM was detected by enzyme linked immunosorbent assay (ELISA) assay in (h).
Prosaposin enhances ERα transcriptional activity
To further study the effect of PSAP in ERα‐mediated transcriptional activity, we took advantage of a dual luciferase assay system using the 3×ERE‐Luc reporter plasmid containing multiple estrogen‐responsive elements. Figure 2a,b demonstrates that PSAP promoted ERα‐mediated transcription in a dose dependent manner in MCF‐7 and T47D cells. Estrogen treatment could significantly enhance the transcriptional activity of ERα, which was consistent with previous studies. However, we found that the effects of PASP on ERα‐mediated transcription were estrogen‐independent in present study.
Figure 2.
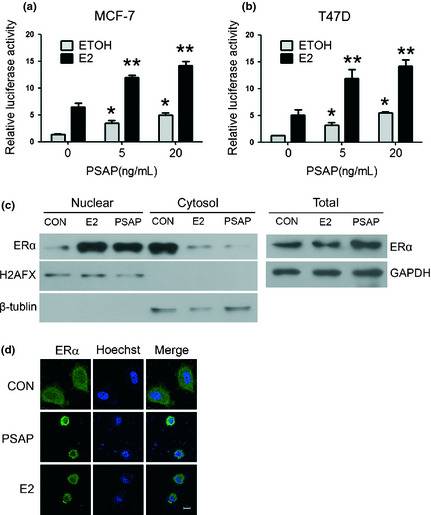
Prosaposin (PSAP) increased the transcriptional activity of estrogen receptor alpha (ERα). (a,b) MCF‐7 and T47D cells were transfected with pRL and 3×ERE‐LUC plasmids. After 24 h, cells were incubated with indicated concentrations of PSAP protein and ethyl alcohol (ETOH) or 100 nM E2, and harvested after another 24 h. Luciferase activity was measured and normalized to Renilla luciferase activity. (*P < 0.05 compared with ETOH control; **P < 0.05 compared with E2 control) (c) MCF‐7 cells were treated in the presence or absence of PSAP or E2 in serum‐ and PR‐free Dulbecco's modified eagle medium (DMEM) for 3 h. Nuclear and cytoplasmic extracts were prepared using a nuclear extraction kit and whole cell lysates were prepared from parallel tissue culture plates. Protein sample was subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE). Immunoblotting was performed using the anti‐ERα antibody. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH), β‐tublin and H2AFX were used as loading controls of whole cell lysates, cytoplasmic and nuclear extracts respectively. (d) Immunofluorescence assay was performed in MCF‐7 cells. The subcellular localization of ERα (green) and nuclear (blue) are shown. Scale bar, 10 μm.
It has been broadly known that steroid receptors translocate into the nuclei, bind to the chromatin, and alter gene expression upon certain stimulation, such as estrogen. We next examined ERα expression in nuclear, cytoplasmic, and whole cell extracts in MCF‐7 cells, and found that PSAP treatment increased nuclear ERα content and reduced cytoplasmic‐ERα in 3 h (Figure 2c). In order to further confirm the nuclear translocation of ERα after PSAP treatment, we conducted immunofluorescence assay in MCF‐7 cells. As shown in Figure 2d, ERα mainly located in plasma in the control group. After PSAP treatment for 3 h, most ERα translocated into nuclear, which was consistent with the positive control: E2 treatment group. Although such a short treatment period was not sufficient to increase ERα expression, the nuclear translocation of ERα was clearly observed. These findings provided an indication that the enhancement of ERα‐mediated transcriptional activity by PSAP was dependent not only upon increasing total ERα protein level but also upon promoting nuclear translocation of ERα. Meanwhile, the nuclear morphometry became loose after E2 or PSAP treatment, suggesting the occurrence of chromatin remodeling, which might provide another clue about the transcription activation.
Regulation of PSAP on ERα is MAPK pathway dependent
Since evidence has suggested that PSAP activates MAPK‐signaling pathway in prostate cancer cells,24 and p44/42 MAPK may be involved in ligand‐independent activation of ERα,30 we detected the role of the MAPK pathway in the regulation of ERα by PSAP in MCF‐7 and T47D cells. As shown in Figure 3a,b, PSAP treatment increased phosphorylated p44/42 (p‐p44/42) rather than p‐p38 and p‐JNK. To further confirm the role of p44/42 in ERα upregulation by PSAP, MCF‐7 and T47D cells were pretreated with U0126 and PD98059. Then expression of p44/42, p‐p44/42 (Figure 3c,d) and ERα (Figure 3e,f) was detected after PSAP treatment. As a result, PSAP increased ERα expression, which was substantially inhibited by U0126 and PD98059 (Figure 3e,f). These findings supported the hypothesis that PSAP could upregulate ERα expression through the MAPK‐signaling pathway.
Figure 3.
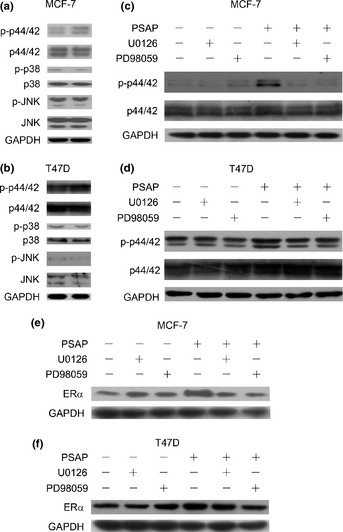
Prosaposin (PSAP) upregulated estrogen receptor alpha (ERα) expression through MAPK‐signaling pathway. (a,b) MCF‐7 and T47D cells starved overnight were treated with PSAP (20 ng/mL) for 15 min. An equal amount of protein (lysates) was subjected to sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE). Immunoblotting was performed using antibody to p44/42, phosphorylated p44/42, p38. phosphorylated p38, JNK and phosphorylated JNK. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as loading controls. (c,d) MCF‐7 and T47D cells starved overnight cells were washed with serum‐ and PP‐free Dulbecco's modified eagle medium (DMEM) and pretreated in this medium with U0126 (20 μM) and PD98059 (20 μM) for 2 h. Cells were then washed and treated with PSAP (20 ng/mL) for 15 min. An equal amount of protein (lysates) was subjected to SDS‐PAGE. Immunoblotting was performed using antibody to p44/42, phosphorylated p44/42. (e,f) MCF‐7 and T47D cells were treated as (c,d) described and treated with PSAP (20 ng/mL) for 24 h rather than 15 min. Estrogen receptor alpha protein level was detected by western blot. GAPDH was used as loading controls.
Prosaposin promotes tumor growth of breast cancer in vitro and in vivo
Based on the role of ERα in breast cancer and the correlation between PSAP and ERα we have proven, we sought to determine the potential role of PSAP in breast cancer. To determine whether PSAP affected cell proliferation in breast cancer, we performed cell proliferation assay using CCK8 in MCF‐7 cells, and found that PSAP stimulated MCF‐7 cells growth in a dose‐dependent manner (Figure 4a).
Figure 4.
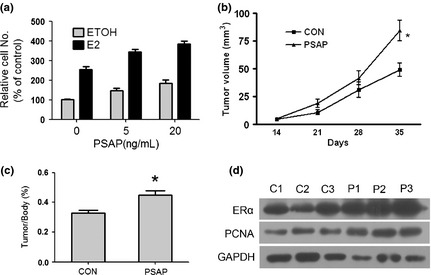
Prosaposin (PSAP) promoted tumor proliferation in vitro and in vivo. (a) After the initial 48 h estrogen deprivation, MCF‐7 cells were incubated in increasing concentrations of PSAP protein with ethyl alcohol (ETOH) or E2. Cell proliferation was measured using a CCK‐8 Counting Kit. (b) In vivo subcutaneous tumor growth curves of control group (n = 6) and PSAP group (n = 6). Each bar represents the mean ± standard deviation (SD). *P < 0.05 (c) Mice were killed after 5 weeks and tumor samples were collected, measured, and photographed. (d) Western blot analysis of estrogen receptor alpha (ERα) and proliferating cell nuclear antigen (PCNA) protein levels in xenografts removed from the nude mice. glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as loading controls.
To further investigate the effects of PSAP on breast cancer in vivo, tumor xenograft experiments were performed in nude mice with MCF‐7 cells. Since MCF‐7 xenograft requires estradiol for growth in nude mice, it is impossible to achieve a nonhormone treated group in vivo. The control group was treated with estradiol only and the PSAP group was treated with both estradiol and PSAP (10 μg/kg per week). Prosaposin treatment significantly promoted tumor growth compared with the control group, as assessed by tumor volume (Figure 4b). Five weeks after tumor xenograft, mice were killed and tumor tissues were examined. Significantly, PSAP enhanced tumor growth compared with control (Figure 4c). Further study also confirmed that ERα and proliferating cell nuclear antigen (PCNA) protein level were increased in the xenografts removed from the nude mice treated with PSAP (Figure 4d). These data identified that PSAP could increase ERα expression, induce proliferation and promote tumor growth in breast cancer.
Discussion
Previous research has shown that PSAP knockout mice displayed some developmental abnormalities in the nervous system and male reproductive organs.31 Meanwhile, PSAP was found in cerebrospinal fluid and seminal plasma.16 The phenomena of PSAP knock‐out mice and distribution of PSAP in the body interested researchers in investigating the role of PSAP in the nervous system and prostate cancer. Koochekpour et al.24 studied the role of PSAP in prostate cancer and found that PSAP played a role in regulating AR and PSA expression and cell activity.32 In addition, PSAP can be secreted by breast cancer cells28 and also exists in human milk.27 But little is currently known about the molecular and cellular function of PSAP in breast cancer. In this study, we investigated a novel function of PSAP in breast cancer.
Estrogen receptor alpha is a steroid hormone receptor indispensable for the development, regulation and maintenance of female phenotype and reproductive physiology. It also plays an important role in the development and progression of breast cancer. Here, we found that PSAP could upregulate ERα expression and ERα‐dependent transcription. As we know, ERα is a nuclear transcription factor, and it should translocate into the nucleus in order to exert its transcriptional role, driving the expression of its target genes. In addition, we still found PSAP enhanced the nuclear ERα‐translocation in breast cancer cells. Thus, our present results indicated a role of PSAP in regulating ERα in breast cancer.
Estrogen signaling and the ER are implicated in breast cancer progress, and the majority of the human breast cancers start out as estrogen dependent. As the endogenous ligand of ERα, estrogen induces receptor transcriptional activity as well as receptor degredation,33 which was also detected in our study (Figures 1 and 2). Meanwhile, our study revealed PSAP could upregulate ERα expression, which partially counteracted ERα degradation induced by estrogen (Figure 1a,b). Prosaposin could also enhance ERα transcriptional activity in the absence or presence of estrogen. Although the transcription enhancement by PSAP was not such effective as estrogen, it was significant and ligand‐independent (Figure 2).
It has been reported that ERα‐positive breast cancer patients can benefit from endocrine therapy (e.g., tamoxifen). Nevertheless, the activation of ERα due to “crosstalk” with growth factors leads to ligand‐independent ERα acivation,34, 35 resulting in failure in endocrine therapy. Taking into consideration that ligand‐independent activation of ERα may define a path to endocrine resistance, enhanced mechanistic insight concerning the function of its ligand‐independent regulator, such as PSAP, could identify novel prognostic markers of endocrine resistance and possible targets for therapeutic intervention in breast cancer. Furthermore, we confirmed PSAP could promote proliferation of breast cancer cells in vitro, and tumor growth in vivo. These results revealed that PSAP might be a novel tumor promoting factor in breast cancer.
On the other hand, endocrine therapy blocks ER‐mediated mitogenic signaling to exert the management of ER‐positive breast cancer. However, therapeutic resistance during treatment remains a significant clinical roadblock to effective disease management. Recent molecular evidence suggested that ‘crosstalk’ pathways originating from activated receptor tyrosine kinases and/or other proliferative and survival signals may be contributing to this endocrine resistance.36 Membrane receptor‐initiated signaling through the MAPK pathway and the phosphatidylinositol‐3‐kinase (PI3K)‐Akt pathway leads to both ligand‐dependent and ligand‐independent ER‐mediated gene activation.37 In the present study, we demonstrated PASP regulated ERα through the p44/42 MAPK pathway, which further confirms the crosstalk between growth factor and ER mediated signaling in breast cancer.
Since ligand‐independent activation of ERα might result in endocrine resistance, investigating the ligand‐independent function (such as PSAP) could identify novel prognostic markers of endocrine resistance and possible targets for therapeutic intervention in breast cancer.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgements
National Basic Research Program of China (973 Program) (2012CB822104, 2010CB912104), State Key Project Specialized for Infectious Diseases of China (2012ZX10002‐008, 2012ZX10002‐012), National High‐tech R&D Program (863 Program) (2012AA020203), the Shanghai Leading Academic Discipline Project (B110), the National Natural Science Fund (31000348), Research Fund for the Doctoral Program of Higher Education of China (20100071120038).
(Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02374.x, 2012)
References
- 1. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin 2000; 50: 7–33. [DOI] [PubMed] [Google Scholar]
- 2. Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol 2005; 67: 335–76. [DOI] [PubMed] [Google Scholar]
- 3. Mangelsdorf DJ, Thummel C, Beato M et al The nuclear receptor superfamily: the second decade. Cell 1995; 83: 835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liao DZ, Pantazis CG, Hou X et al Promotion of estrogen‐induced mammary gland carcinogenesis by androgen in the male Noble rat: probable mediation by steroid receptors. Carcinogenesis 1998; 19: 2173–80. [DOI] [PubMed] [Google Scholar]
- 5. Hilakivi‐Clarke L, Cho E, Cabanes A et al Dietary modulation of pregnancy estrogen levels and breast cancer risk among female rat offspring. Clin Cancer Res 2002; 8: 3601–10. [PubMed] [Google Scholar]
- 6. Ali S, Coombes RC. Estrogen receptor alpha in human breast cancer: occurrence and significance. J Mammary Gland Biol Neoplasia 2000; 5: 271–81. [DOI] [PubMed] [Google Scholar]
- 7. Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol 2001; 2: 133–40. [DOI] [PubMed] [Google Scholar]
- 8. Clarke RB, Anderson E, Howell A. Steroid receptors in human breast cancer. Trends Endocrinol Metab 2004; 15: 316–23. [DOI] [PubMed] [Google Scholar]
- 9. Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst 2004; 96: 218–28. [DOI] [PubMed] [Google Scholar]
- 10. Clarke R, Liu MC, Bouker KB et al Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene 2003; 22: 7316–39. [DOI] [PubMed] [Google Scholar]
- 11. O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol 2004; 18: 1859–75. [DOI] [PubMed] [Google Scholar]
- 12. Jiang X, Ellison SJ, Alarid ET, Shapiro DJ. Interplay between the levels of estrogen and estrogen receptor controls the level of the granzyme inhibitor, proteinase inhibitor 9 and susceptibility to immune surveillance by natural killer cells. Oncogene 2007; 26: 4106–14. [DOI] [PubMed] [Google Scholar]
- 13. Igdoura SA, Rasky A, Morales CR. Trafficking of sulfated glycoprotein‐1 (prosaposin) to lysosomes or to the extracellular space in rat Sertoli cells. Cell Tissue Res 1996; 283: 385–94. [DOI] [PubMed] [Google Scholar]
- 14. O'Brien JS, Kishimoto Y. Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J 1991; 5: 301–8. [DOI] [PubMed] [Google Scholar]
- 15. Kishimoto Y, Hiraiwa M, O'Brien JS. Saposins: structure, function, distribution, and molecular genetics. J Lipid Res 1992; 33: 1255–67. [PubMed] [Google Scholar]
- 16. Hineno T, Sano A, Kondoh K, Ueno S, Kakimoto Y, Yoshida K. Secretion of sphingolipid hydrolase activator precursor, prosaposin. Biochem Biophys Res Commun 1991; 176: 668–74. [DOI] [PubMed] [Google Scholar]
- 17. Fu Q, Carson GS, Hiraiwa M, Grafe M, Kishimoto Y, O'Brien JS. Occurrence of prosaposin as a neuronal surface membrane component. J Mol Neurosci 1994; 5: 59–67. [DOI] [PubMed] [Google Scholar]
- 18. Elleder M, Jerabkova M, Befekadu A et al Prosaposin deficiency – a rarely diagnosed, rapidly progressing, neonatal neurovisceral lipid storage disease. Report of a further patient. Neuropediatrics 2005; 36: 171–80. [DOI] [PubMed] [Google Scholar]
- 19. Fujita N, Suzuki K, Vanier MT et al Targeted disruption of the mouse sphingolipid activator protein gene: A complex phenotype, including severe leukodystrophy and wide‐spread storage of multiple sphingolipids. Hum Mol Genet 1996; 5: 711–25. [DOI] [PubMed] [Google Scholar]
- 20. Morales CR, Zhao Q, El‐Alfy M, Suzuki K. Targeted disruption of the mouse prosaposin gene affects the development of the prostate gland and other male reproductive organs. J Androl 2000; 21: 765–75. [PubMed] [Google Scholar]
- 21. Misasi R, Sorice M, Di Marzio L et al Prosaposin treatment induces PC12 entry in the S phase of the cell cycle and prevents apoptosis: activation of ERKs and sphingosine kinase. FASEB J 2001; 15: 467–74. [DOI] [PubMed] [Google Scholar]
- 22. Misasi R, Garofalo T, Di Marzio L et al Prosaposin: a new player in cell death prevention of U937 monocytic cells. Exp Cell Res 2004; 298: 38–47. [DOI] [PubMed] [Google Scholar]
- 23. Koochekpour S, Zhuang YJ, Beroukhim R et al Amplification and overexpression of prosaposin in prostate cancer. Genes Chromosom Cancer 2005; 44: 351–64. [DOI] [PubMed] [Google Scholar]
- 24. Koochekpour S, Lee TJ, Wang R et al Prosaposin upregulates AR and PSA expression and activity in prostate cancer cells (LNCaP). Prostate 2007; 67: 178–89. [DOI] [PubMed] [Google Scholar]
- 25. Koochekpour S, Hu S, Vellasco‐Gonzalez C et al Serum prosaposin levels are increased in patients with advanced prostate cancer. Prostate 2012; 72: 253–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patton S, Carson GS, Hiraiwa M, O'Brien JS, Sano A. Prosaposin, a neurotrophic factor: presence and properties in milk. J Dairy Sci 1997; 80: 264–72. [DOI] [PubMed] [Google Scholar]
- 27. Kondoh K, Hineno T, Sano A, Kakimoto Y. Isolation and characterization of prosaposin from human milk. Biochem Biophys Res Commun 1991; 181: 286–92. [DOI] [PubMed] [Google Scholar]
- 28. Campana WM, O'Brien JS, Hiraiwa M, Patton S. Secretion of prosaposin, a multifunctional protein, by breast cancer cells. Biochim Biophys Acta 1999; 1427: 392–400. [DOI] [PubMed] [Google Scholar]
- 29. Jiang J, Wei Y, Shen J et al Functional interaction of E1AF and Sp1 in glioma invasion. Mol Cell Biol 2007; 27: 8770–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J 1996; 15: 2174–83. [PMC free article] [PubMed] [Google Scholar]
- 31. Morales CR, Zhao Q, Lefrancois S, Ham D. Role of prosaposin in the male reproductive system: effect of prosaposin inactivation on the testis, epididymis, prostate, and seminal vesicles. Arch Androl 2000; 44: 173–86. [DOI] [PubMed] [Google Scholar]
- 32. Hu S, Delorme N, Liu Z et al Prosaposin down‐modulation decreases metastatic prostate cancer cell adhesion, migration, and invasion. Mol Cancer 2010; 9: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Preisler‐Mashek MT, Solodin N, Stark BL, Tyriver MK, Alarid ET. Ligand‐specific regulation of proteasome‐mediated proteolysis of estrogen receptor‐alpha. Am J Physiol Endocrinol Metab 2002; 282: E891–8. [DOI] [PubMed] [Google Scholar]
- 34. Carascossa S, Dudek P, Cenni B, Briand PA, Picard D. CARM1 mediates the ligand‐independent and tamoxifen‐resistant activation of the estrogen receptor alpha by cAMP. Genes Dev 2010; 24: 708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Atanaskova N, Keshamouni VG, Krueger JS, Schwartz JA, Miller F, Reddy KB. MAP kinase/estrogen receptor cross‐talk enhances estrogen‐mediated signaling and tumor growth but does not confer tamoxifen resistance. Oncogene 2002; 21: 4000–8. [DOI] [PubMed] [Google Scholar]
- 36. Zhou Y, Eppenberger‐Castori S, Eppenberger U, Benz CC. The NFkappaB pathway and endocrine‐resistant breast cancer. Endocr Relat Cancer 2005; 12(Suppl 1): S37–46. [DOI] [PubMed] [Google Scholar]
- 37. Benz CC, Scott GK, Sarup JC et al Estrogen‐dependent, tamoxifen‐resistant tumorigenic growth of MCF‐7 cells transfected with HER2/neu. Breast Cancer Res Treat 1992; 24: 85–95. [DOI] [PubMed] [Google Scholar]


