Abstract
It is widely accepted that tumors contain cancer stem cells (CSC) possessing self‐renewal potential as well as the ability to generate numerous cancer cells. Cancer stem cells are resistant to conventional cancer therapy and have greater invasive and metastatic behavior. It has been suggested that blood vessels provide a niche that maintains stemness in normal organs. This role also extends to the field of cancer biology. Cancer stem cells have been isolated from leukemias and solid cancers. Identification of these cells and their niche is critical for identifying molecular targets in order to inhibit their growth and to destroy their niche. For this purpose, sorting of living CSC is required to monitor their presence in the presumptive niche to establish whether a CSC candidate actually shows malignant features. Based on and referring to analyses in normal tissues, molecules including nitric oxide, Wnt, neuropilin‐1, hepatocyte growth factor and others involved in the maintenance of CSC have been isolated. Stem cells might affect niche cells and niche cells produce stemness factors on such stimulation. Therefore, the niche might be flexible to support self‐renewal or differentiation of stem cells even in the same niche cells. (Cancer Sci 2012; 103: 1177–1181)
As elucidation of stem cell dynamism is directly related to the development of therapies for cancer as well as tissue regeneration, the mechanisms responsible for self‐renewal and maintenance of immature status in stem cells have been extensively analyzed. It has been asked whether stem cells maintain stemness autonomously or whether the interaction of stem cells with other cell types is required for the maintenance of stemness. It seems that stemness might be sustained by interactions with other cells because most stem cells isolated from tissues cannot be maintained independently in vitro.
Thus far, the location of tissue‐specific stem cells has been analyzed to identify niche cells supporting stemness.1 Among several types of stem cell systems in mammals, research on the stem cell niche in hematopoiesis is better developed because surface phenotypes of hematopoietic stem cells (HSC) have been well defined compared with other stem cell systems. In mice, HSC localize and adhere to endothelial cells (EC) in intraluminal parts of the omphalomesenteric artery at embryonic day (E) 9.5 and form clusters suggesting self‐renewing activity.2 We found that the receptor tyrosine kinase, Tie2, expressed on both HSC and EC, is required for the formation of this HSC vascular niche via activation of integrin.2 Localization of HSC in the vascular area is also observed in the dorsal aorta and placenta at midgestation.3 Moreover, formation clusters of HSC around EC are observed in fetal liver where hematopoiesis is ongoing during embryogenesis. Around the time of birth, HSC home to and migrate into the bone marrow (BM). It has been established that there are two different types of niche for HSC in BM. Osteoblasts in the endosteum constitute the niche for maintenance of dormancy in HSC.4, 5 Here, firm cell–cell adhesion between HSC and osteoblasts via N‐cadherin expressed on both cell types is induced on activation of Tie2 expressed on HSC with angiopoietin‐1. Deficiency of the bone morphogenic protein (BMP) type 1A receptor results in osteoblast proliferation in mouse BM, resulting in increased numbers of HSC.4 Therefore, it seems that osteoblasts control the number of HSC by spatially limiting the pool size. The other niche for HSC in BM is the vascular area termed the sinusoid. Here, in a vascular niche, reticular cells located next to EC release CXCL12/SDF1, a chemokine ligand for CXCR4, and induce recruitment of HSC.6 In the vascular niche, it is not clear whether direct interactions between HSC and EC are required but proliferation (self‐renewal) of HSC in this area has been suggested.6
In this way, when the niche region has been identified, analysis of niche formation and self‐renewal in stem cells can be performed at the molecular level. In the cancer field, the cancer stem cell (CSC) niche is being intensively investigated. In this review, different types of niches for several cancers are overviewed, including the function of the niche.
Perivascular niche for glioblastoma CSC
In the adult brain, neuronal stem cells localize in close proximity to blood vessels in the hippocampus and subventricular zone. During embryonic brain development, the neural ectoderm produces vascular endothelial growth factor (VEGF) for mobilization of neovascularization. Thus, EC and neuronal stem cells localize together in the embryo and this interaction continues after birth. It has been shown that stemness characteristics of neuronal stem cells, such as self‐renewal activity and maintenance of immature status, are induced by the notch signaling pathway.7
Among the cancer cells in glioblastoma tumors, it has been reported that there are CSC having elevated DNA repair capacity and tumor initiating ability. These are nestin+ CSC that localize near CD34+ EC.8 On inoculation of CSC into immunodeficient mice, tumor incidence was higher when EC from the original tumor were injected together with them. In this case, nitric oxide produced from EC seemed to be acting as a factor for self‐renewal of CSC via activation of the notch pathway. In contrast, it has been suggested that CSC prevent EC from undergoing apoptosis by tissue hypoxia, a function that may relate to their resistance to vascular disrupting agents (Fig. 1).
Figure 1.
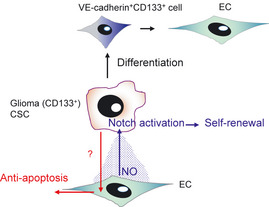
Perivascular niche for glioma stem cells. It has been suggested that cancer stem cells (CSC) in glioma localize near endothelial cells (EC). Nitric oxide (NO) produced from EC seems to induce self‐renewal of CSC mediating activation of the notch pathway. Conversely, CSC prevent EC from undergoing apoptosis when exposed to tissue hypoxia. Consistent with evidence of a higher vascular density in glioblastoma compared with other cancers, it has been reported that CD133+ glioblastoma CSC can differentiate into EC via CD133+ VE‐cadherin+ endothelial progenitors.
It is well known that glioblastoma is a tumor with a high vascular density. Recently, several lines of evidence have suggested that glioblastoma CSC can differentiate into EC and participate in blood vessel formation. Wang et al.9 showed that CD133+VE‐cadherin− CSC differentiate into CD133−VE‐cadherin+ EC via CD133+VE‐cadherin+ endothelial progenitors. Ricci‐Vitiani et al.10 also showed differentiation of CD133+ CSC into EC both in vitro and in vivo (Fig. 1). When EC derived from CSC were killed, tumor growth was greatly suppressed, indicating that such EC directly contribute to blood vessel formation, at least in glioblastoma. Whether EC derived from CSC can function as CSC‐supporting cells in the vascular niche needs to be addressed, so that methods can be developed to disrupt the CSC niche.
Niche for colorectal cancer stem cells
Niches for normal stem cells in the intestinal tract are also being gradually uncovered subsequent to the acquisition of data on HSC and neuronal stem cells. The crypt is the functional unit in the small and large intestine. Intestinal stem cells localize at the bottom of the crypt and self‐renew beside epithelium or mesenchymal cells such as myofibroblasts. In this area, the Wnt signaling pathway is involved in maintaining stemness.11
In the case of colon cancer CSC, the location of the niche has not yet been identified. However, myofibroblasts or mesenchymal stem cells have been suggested to be niche cell components. As observed in the normal intestinal stem cell system, activation of the Wnt signaling pathway is induced in CSC in colon cancer. Such colon cancer cells expressing β catenin in their nuclei form clusters and localize to the invasive front of the tumor together with myofibroblasts.12 Those cancer cells co‐locating with myofibroblasts have not yet been proven to be CSC and therefore it is not clear whether myofibroblasts are niche cells for colon cancer CSC. However, it has been suggested that type I collagen stimulates conversion of cancer cells into CSC in colon cancer cell lines; the main producer of type I collagen is the myofibroblast (Fig. 2). Moreover, killing of myofibroblasts by CD8+ T lymphocytes suppresses the growth and metastasis of cancer.13 Therefore, myofibroblasts have been suggested to be niche cells for induction and maintenance of CSC.
Figure 2.
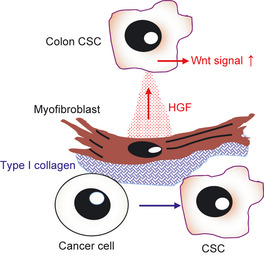
Myofibroblasts are the cell source for the cancer stem cell (CSC) niche in colon cancer. It has been suggested that myofibroblasts promote phenotypic changes from cancer cells to CSC in colon cancer. Type I collagen produced by myofibroblasts is probably involved in this dedifferentiation. Moreover, hepatocyte growth factor (HGF) produced by myofibroblasts enhances the Wnt signaling cascade of CSC in colon cancer. It has been suggested that a bone marrow stem cell population enters the tumor and differentiates into myofibroblasts.
Recently, a system for isolation of CSC utilizing the degree of Wnt signaling activation has been reported.14 Using this method it has been demonstrated that hepatocyte growth factor (HGF) produced by myofibroblasts enhances the Wnt signaling cascade of CSC in colon cancer (Fig. 2). Myofibroblasts have been suggested to come from BM and act as stromal cell components in a mouse tumor xenograft model.15 Myofibroblasts might play roles in the maintenance of CSC as niche cell components not only in colon cancer but various other cancers.
Niche for cancer stem cells in a skin tumor model
Keratinocyte stem cells are located in a line in the basal layer of the epidermis and regularly self‐renew in response to keratinocyte turnover. It has been recently reported that CSC in skin papillomas and EC in a perivascular niche interact in a chemically induced skin carcinoma model.16 In this model, CSC express neuropilin‐1 (Nrp1) that acts as a co‐receptor with the VEGF receptor (VEGFR); VEGF produced by CSC activates Nrp1 by an autocrine and paracrine loop, resulting in their proliferation. In contrast, VEGF from CSC also promotes blood vessel formation via its primary function on EC and increases the area of the vascular niche, providing a site for maintenance of stemness in CSC (Fig. 3). Indeed, a crucial role for CRC‐derived VEGF for tumor growth was proven by knocking down the VEGF gene specifically in CSC.
Figure 3.
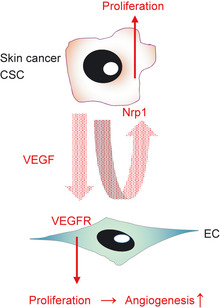
Cancer stem cell (CSC) niche of a skin cancer model. Vascular endothelial growth factor (VEGF) produced by CSC in skin cancer stimulate endothelial cells (EC) and CSC themselves by paracrine and autocrine loops. Vascular endothelial growth factor induces angiogenesis, resulting in expansion of the vascular niche region and also induction of self‐renewal of CSC at the same time. It has been suggested that Nrp1 on CSC is a receptor for VEGF; however, a co‐receptor of Nrp1 has not been identified. VEGFR, VEGF receptor.
Nrp1c expressed on EC forms a heterodimer with VEGFR2 and enhances downstream signaling of the latter compared with the activity of the homodimer of VEGFR2, resulting in excess proliferation and migration of EC for angiogenesis.17 Nrp1 is a cell membrane protein but does not have a kinase domain and therefore another binding partner, that is, VEGFR2 in EC, is required for intracellular signaling. It has been reported that deletion of VEGFR1 (flt1) in epidermal cells delays the appearance of skin papilloma in a model using K5‐Sos transgenic mice.18 This suggests that VEGF signaling in keratinocytes can directly regulate the initiation of skin cancer; however, it has not been shown whether Nrp1 binds to VEGFR1 for enhancement of VEGF signaling in CSC or whether Nrp1 can transduce signals via binding of VEGF to receptors other than VEGFR1. Identification of the VEGF receptor with Nrp1 on CSC might help to clarify whether the direct effect of VEGF produced by CSC can be generalized to other cancer models.
Vascular niche for CSCs in a cancer cell xenograft model
PSF1 is a marker for detection of stem cells in normal tissues
Thus far, the importance of the vascular niche for the maintenance of CSC in various cancer models has been suggested. Xenograft models using mouse cancer cells provide tools to identify niches for CSC and to analyze the precise function of the niche for CSC. To identify the niche, good markers of CSC are required in mice. Several markers to identify CSC in humans have been reported, that is, CD44 for breast cancer and CD133 for lung, brain, liver and other.19 However, in mice, there are no good markers to identify CSC.
It is widely accepted that tissue‐specific stem cells actively self‐renew during embryogenesis for acute expansion of tissue in organs; however, most of these stem cells become dormant in adulthood because the tissue/organ is already established and because of pool (niche) size limitation. In contrast, in tumor tissue, tumor growth is not restricted and there is no limitation of tumor size, so that CSC actively proliferate but do not become dormant. Therefore, molecules expressed in stem cells specifically in the embryo but not the adult might be useful to identify CSC. Following this hypothesis, we tried to identify such molecules by comparing gene expression in HSC cDNA libraries from embryo and adult BM. Among molecules isolated by this method, PSF1 (partner of SLD five 1) was found to be specifically upregulated in HSC in the embryo.20 Thus far, the function of PSF1 has not been identified; however, in yeast, it has been reported that PSF1 forms a complex with PSF2, PSF3 and SLD5 termed GINS (this name is derived from the Japanese words for numbers, i.e., 5: Go; 1: Ichi; 2: Ni; 3: San), which plays a critical role in the generation of the DNA replication fork associated with CDC45.21 To determine whether PSF1 is functional in mice, we generated PSF1‐deficient animals and found that they die at around E 6.5. Lethality was caused by deficient proliferation of epiblasts, a type of totipotent stem cell.20 Moreover, we found that PSF1 is essential for acute proliferation of HSC in experiments following their recovery after BM ablation in PSF1 heterozygous mice.22 Therefore, it was suggested that PSF1 plays an important role in promoting stem cell proliferation.
Visualization of CSC by PSF1 promoter activity
We found that cells strongly positive for PSF1 in human lung and esophageal cancers are localized to the perivascular region of the tumor edge.23 Because we observed that PSF1 is specifically expressed in stem cells such as HSC, spermatogonia, intestinal stem cells, etc. in the normal organ, this suggested that PSF1‐positive cells in cancer are CSC. However, it was difficult to prove whether PSF1‐positive cells in the perivascular region are indeed CSC, because PSF1 is an intracellular protein and therefore we could not sort PSF1‐positive viable cells to evaluate their biological activities in vitro and in vivo. To overcome this problem, we transduced a reporter gene expressing EGFP under the transcriptional control of the PFS1 promoter into several cancer cell lines. Compared with EGFPlow cells, we found that sorted EGFPhigh cells have greater tumor‐initiating abilities with small numbers of cells (100 cells), show a highly invasive ability by digesting extracellular matrices and generate many more metastatic foci on intravenous injection. These findings suggest that promoter activity of PSF1 correlates with tumor cell malignancy. Moreover, gene expression profiles of the EGFPhigh cells were similar to ES cells. Taken together, these data suggest that EGFPhigh cells are in the CSC population. By histology, these EGFPhigh cells are found to be abundant in the perivascular region of the tumor edge, as observed in human cancer.23
Based on our model and together with other models showing localization of CSC,8, 16, 23 it is possible that highly malignant cancer cells like CSC generally utilize vascular regions as their stem cell niche. Here, in our model, it is interesting that EGFPhigh cells cultured in vitro did not show malignant CSC activities on inoculation into mice. This suggests that some molecular cues derived from cells composing the tumor microenvironment might change cancer cells into CSC and that cells in perivascular niches such as EC, mural cells (pericytes and vascular smooth muscle cells), myofibroblasts, fibroblasts and mesenchymal stem cells are possible sources producing such educational factors.
Molecules affecting niche cells alter the function of the niche for stem cells
Niche cells support stemness by definition; however, it is difficult to imagine that niche cells autonomously support stemness independently of stem cells localizing near the niche cells. Most likely, molecular cues derived from stem cells affect niche cells and on receiving these cues, niche cells produce stemness factors or differentiation factors for stem cells (Fig. 4). Here, two lessons from the HSC system showing such abilities can be learned.
Figure 4.
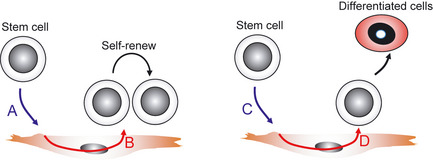
Altered function of niche cells for self‐renewal or differentiation of stem cells. It is hypothesized that niche cells do not autonomously determine the fate of stem cells locating near them, but stem cells might affect the niche and thus influence their behavior. In brief, when stem cells producing factor A come near the niche and factor A then stimulates the niche cells, factor B produced from the niche cells induces self‐renewal of the stem cell. In contrast, when stem cells producing factor C come near the niche and factor C stimulates the niche cells, factor D produced from the niche cells induces differentiation of the stem cell.
As described above, it has been suggested that osteoblasts in the BM osteoblast niche support dormancy of HSC and EC in the vascular niche support self‐renewal.5, 6 However, it is possible that any niche cells, such as EC, osteoblasts or other mesenchymal cells, can support both dormancy, self‐renewal and differentiation of stem cells depending on factors derived from the stem cells. We developed a co‐culture system of HSC with OP9 stromal cells to analyze how the self‐renewal or differentiation of HSC is affected by the stromal cells.24 OP9 cells were derived from calvaria of op/op, M‐CSF‐deficient mice and are defined as osteoblastic cells with adipocyte differentiation ability. When OP9 cells were stimulated with epidermal growth factor (EGF) and co‐cultured with HSC, immature hematopoietic progenitor cells (HPC) including HSC proliferated and the undifferentiated state of such HPC or HSC was maintained long term. In contrast, when fibroblast growth factor‐2 (FGF2) was used instead of EGF, differentiation of HSC into mature hematopoietic cells was induced. When the EGF receptor on OP9 was transactivated by the transfection of a constitutively active erbB2 gene into OP9 to eliminate the direct effect of EGF on HSC, differentiation of HSC was suppressed and proliferation of immature hematopoietic cells was maintained long term.24 Therefore, this suggests that the fate of HSC, that is, maintenance of immature state or differentiation, is altered by factors derived from niche cells (in this case, OP9 cells) and whether niche cells produce stemness or differentiation factors is dependent on exogenous factors stimulating the niche cells.
Recently, Raffi's group also developed a similar culture system using primary EC and drew similar conclusions.25 In brief, they constitutively activated Akt signaling in EC by transduction of the adenoviral region 4 gene.25 When HSC were co‐cultured with these EC, self‐renewal of HSC was induced. In contrast, when MAPK was activated in EC by the transfection of a constitutively active form of c‐raf, differentiation of HSC was induced on co‐culture with these EC. They proposed that production of BMP4, angiopoietin‐1 and other stemness factors for HSC was elevated in the former condition and angiopoietin‐2 and interleukin‐6, which are differentiation factors for HSC, were upregulated in the latter.
Taken together, these data strongly suggest that any niche cells, such as osteoblasts or EC, can alter self‐renewal or differentiation of HSC by extrinsic factors stimulating the niche cells. The CSC must produce several factors and stimulate niche cells such as EC, myofibroblasts and others in the vascular niche of cancer. In this vascular niche, differentiation factors as well as stemness factors might be produced from niche cells; both of these would be useful drug targets to suppress self‐renewal or enhance differentiation of CSC. Therefore, molecular mechanisms regulating stemness or differentiation of CSC in the vascular niche must be identified to develop better strategies to combat cancer.
Conclusions
Based on the knowledge acquired from stem cell research in normal organs, technology to identify CSC niches has been developed. It is suggested that CSC localize to the perivascular region. Blood vessels are generally composed of luminal EC, mural cells such as pericytes and smooth muscle cells, with EC adhering on the basal side, and extracellular matrices such as collagen and fibronectin covering the mural cells. In addition to such components, myofibroblasts expressing α‐smooth muscle actin are abundant near mural cells in tumor blood vessels. To elucidate molecular cues to induce stemness of CSC, it is important to determine which cells are critically involved in the maintenance of CSC. Although it has been reported in colon cancer that myofibroblasts are the candidate niche cells supporting CSC,14 other reports suggest that EC are important to support in vivo tumor initiation from small numbers of CSC.8 Therefore, further analysis is required to determine the roles of cells composing the vascular niche in terms of their effects on survival, proliferation and differentiation of CSC.
Regarding the origin of myofibroblasts in the tumor, it has been reported that a mesenchymal stem cell population recruited from BM differentiates and is involved in supporting tumor growth, as described above.15 Moreover, CSC themselves have the ability to differentiate into EC9, 10 and these can transdifferentiate into mesenchymal stem cells via the endothelial–mesenchymal transition (End‐MT) on stimulation with BMP2 and/or TGFβ.26 This suggests that CSC can differentiate into myofibroblasts and act as niche cells in the tumor.
Molecules associating with stemness of HSC have been identified due to the success of the co‐culture system of HSC with different stromal cells. Therefore, establishment of stromal cell lines that can support stemness of CSC might help to identify the molecular cues inducing self‐renewal and differentiation of CSC.
Disclosure Statement
There are no conflicting financial interests in this work.
Acknowledgments
This work was supported by a grant from the Ministry of Education, Science, Sports, and Culture of Japan.
References
- 1. Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 2004; 116: 769–78. [DOI] [PubMed] [Google Scholar]
- 2. Takakura N, Huang XL, Naruse T et al Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 1998; 9: 677–86. [DOI] [PubMed] [Google Scholar]
- 3. Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell 2005; 8: 377–87. [DOI] [PubMed] [Google Scholar]
- 4. Zhang J, Niu C, Ye L et al Identification of the haematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836–41. [DOI] [PubMed] [Google Scholar]
- 5. Arai F, Hirao A, Ohmura M et al Tie2/angiopoietin‐1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118: 149–61. [DOI] [PubMed] [Google Scholar]
- 6. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12‐CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–88. [DOI] [PubMed] [Google Scholar]
- 7. Shen Q, Goderie SK, Jin L et al Endothelial cells stimulate self‐renewal and expand neurogenesis of neural stem cells. Science 2004; 304: 1338–40. [DOI] [PubMed] [Google Scholar]
- 8. Calabrese C, Poppleton H, Kocak M et al A perivascular niche for brain tumor stem cells. Cancer Cell 2007; 11: 69–82. [DOI] [PubMed] [Google Scholar]
- 9. Wang R, Chadalavada K, Wilshire J et al Glioblastoma stem‐like cells give rise to tumour endothelium. Nature 2010; 468: 829–33. [DOI] [PubMed] [Google Scholar]
- 10. Ricci‐Vitiani L, Pallini R, Biffoni M et al Tumour vascularization via endothelial differentiation of glioblastoma stem‐like cells. Nature 2010; 468: 824–8. [DOI] [PubMed] [Google Scholar]
- 11. Clevers H. Wnt/beta‐catenin signaling in development and disease. Cell 2006; 127: 469–80. [DOI] [PubMed] [Google Scholar]
- 12. Brabletz T, Jung A, Reu S et al Variable beta‐catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA 2001; 98: 10356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor‐associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 2006; 116: 1955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vermeulen L, De Sousa E, Melo F et al Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010; 12: 468–76. [DOI] [PubMed] [Google Scholar]
- 15. Quante M, Tu SP, Tomita H et al Bone marrow‐derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011; 19: 257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beck B, Driessens G, Goossens S et al A vascular niche and a VEGF‐Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 2011; 478: 399–403. [DOI] [PubMed] [Google Scholar]
- 17. Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin‐1 is expressed by endothelial and tumor cells as an isoform‐specific receptor for vascular endothelial growth factor. Cell 1998; 92: 735–45. [DOI] [PubMed] [Google Scholar]
- 18. Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010; 140: 268–79. [DOI] [PubMed] [Google Scholar]
- 19. Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008; 8: 755–68. [DOI] [PubMed] [Google Scholar]
- 20. Ueno M, Itoh M, Kong L, Sugihara K, Asano M, Takakura N. PSF1 is essential for early embryogenesis in mice. Mol Cell Biol 2005; 25: 10528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. MacNeill SA. Structure and function of the GINS complex, a key component of the eukaryotic replisome. Biochem J 2010; 425: 489–500. [DOI] [PubMed] [Google Scholar]
- 22. Ueno M, Itoh M, Sugihara K, Asano M, Takakura N. Both alleles of PSF1 are required for maintenance of pool size of immature hematopoietic cells and acute bone marrow regeneration. Blood 2009; 113: 555–62. [DOI] [PubMed] [Google Scholar]
- 23. Nagahama Y, Ueno M, Miyamoto S et al PSF1, a DNA replication factor expressed widely in stem and progenitor cells, drives tumorigenic and metastatic properties. Cancer Res 2010; 70: 1215–24. [DOI] [PubMed] [Google Scholar]
- 24. Takakura N, Kodama H, Nishikawa S, Nishikawa S. Preferential proliferation of murine colony‐forming units in culture in a chemically defined condition with a macrophage colony‐stimulating factor‐negative stromal cell clone. J Exp Med 1996; 184: 2301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kobayashi H, Butler JM, O'Donnell R et al Angiocrine factors from Akt‐activated endothelial cells balance self‐renewal and differentiation of haematopoietic stem cells. Nat Cell Biol 2010; 12: 1046–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem‐like cells. Nat Med 2010; 16: 1400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


