Abstract
Targeting of tumor angiogenesis with vaccines is a potentially valuable approach to cancer treatment. Elpamotide is an immunogenic peptide derived from vascular endothelial growth factor receptor 2, which is expressed at a high level in vascular endothelial cells. We have now carried out a phase I study to evaluate safety, the maximum tolerated dose, and potential pharmacodynamic biomarkers for this vaccine. Ten HLA‐A*24:02‐positive patients with advanced refractory solid tumors received elpamotide s.c. at dose levels of 0.5, 1.0, or 2.0 mg once a week on a 28‐day cycle. Five patients experienced an injection site reaction of grade 1 and 2, which was the most frequent adverse event. In the 1.0 mg cohort, one patient experienced proteinuria of grade 1 and another patient developed both hypertension and proteinuria of grade 1. No adverse events of grade 3 or higher were observed, and the maximum tolerated dose was therefore not achieved. The serum concentration of soluble vascular endothelial growth factor receptor 2 decreased significantly after elpamotide vaccination. Microarray analysis of gene expression in PBMCs indicated that several pathways related to T cell function and angiogenesis were affected by elpamotide vaccination, supporting the notion that this peptide induces an immune response that targets angiogenesis in the clinical setting. In conclusion, elpamotide is well tolerated and our biomarker analysis indicates that this anti‐angiogenic vaccine is biologically active. Clinical trial registration no. UMIN000008336.
Angiogenesis, defined as the formation of new blood vessels from pre‐existing vasculature, is essential for tumor growth and the spread of metastases.1, 2 Vascular endothelial growth factor (VEGF) is a pro‐angiogenic molecule that plays a central role in angiogenesis, primarily through activation of VEGF receptor 2 (VEGFR2). Several approaches to the targeting of VEGF–VEGFR pathways, including those based on neutralizing antibodies to VEGF, small‐molecule VEGFR tyrosine kinase inhibitors, and soluble VEGFR constructs (VEGF‐Trap), are emerging as promising therapeutic options in clinical oncology.3
Vascular endothelial growth factor 2 has been a major target for anti‐angiogenic therapy to date. Studies in mice have shown that tumor angiogenesis is inhibited as a result of cellular immune responses induced by vaccination with cDNA encoding mouse VEGFR2 or with a soluble fragment of the receptor.4, 5 On the basis of these findings, we have examined the possibility of developing a novel anti‐angiogenic immunotherapy for cancer in the clinical setting. We previously identified peptide epitopes of human VEGFR2 and showed that CTLs induced by these peptides manifest potent and specific HLA class I‐restricted cytotoxicity toward not only peptide‐pulsed target cells but also endothelial cells expressing endogenous VEGFR2.6 Furthermore, vaccination with peptides corresponding to these epitopes inhibited angiogenesis induced by tumor xenografts, resulting in marked suppression of tumor growth and prolongation of animal survival without the occurrence of fatal adverse events.6
We have now carried out a phase I clinical trial for treatment of HLA‐A*24:02‐positive patients with advanced refractory solid tumors by vaccination with the VEGFR2‐169 peptide (elpamotide), which was previously shown to be the most effective among human VEGFR2 epitopic peptides tested for the ability to induce CTL precursors among PBMCs from cancer patients.7 We examined the safety of this treatment as a primary endpoint, and the clinical and biological responses as secondary endpoints.
Patients and Methods
Patient eligibility
HLA‐A*24:02‐positive individuals aged ≥20 years with a histologically confirmed diagnosis of an advanced tumor refractory to standard therapy were included in the study if they had an Eastern Cooperative Oncology Group performance status of <2, a life expectancy of ≥3 months, and adequate or acceptable liver (serum bilirubin concentration of ≤2× the upper limit of normal, and both aspartate aminotransferase and alanine aminotransferase levels in serum of ≤2.5× the upper limit of normal) and bone marrow (absolute white blood cell count of ≥3000/mm3 and platelet count of ≥100 000/mm3) function. Patients were excluded if they had symptomatic brain metastases, active bleeding, malignant ascites requiring drainage, or serious medical conditions such as uncontrolled hypertension, arrhythmia, or heart failure, or if they had been treated with an investigational drug within 4 weeks prior to study enrolment. Individuals were excluded if they had serious illness or concomitant non‐oncological disease that was difficult to control by medication. All subjects received information about the nature and purpose of the study, and they provided written informed consent in accordance with institutional guidelines.
Study design
The study was designed as a single‐center, open‐label, dose‐escalation phase I trial. The primary objective was to evaluate the tolerability‐safety and dose‐limiting toxicity (DLT) of elpamotide. Secondary objectives included determination of the maximum tolerated dose, preliminary assessment of antitumor activity and effects on peripheral blood biomarkers of angiogenesis in this patient population. The study was approved by the appropriate Institutional Review Board. Dose levels of elpamotide were 0.5, 1.0, and 2.0 mg per body injected s.c. once a week on a 28‐day cycle. Intrapatient dose escalation was not permitted. If a patient experienced a drug‐related DLT, the treatment with elpamotide was discontinued. The dose escalation–reduction scheme was based on the occurrence of drug‐related DLTs within the first treatment cycle. If a DLT was not observed in any of the first three patients, the dose was escalated to the next level. If a DLT was observed in one of the first three patients, three additional patients were recruited to that dose level. If a DLT occurred in only one of the first six patients, dose escalation was permitted. If two or more of the six patients experienced a DLT, an independent data monitoring committee determined the dose escalation or reduction decision or stopped the recruitment of additional patients.
Safety and efficacy assessments
The safety and tolerability of elpamotide were assessed according to the Common Toxicity Criteria for Adverse Events version 3.0. A DLT was defined as a hematologic toxicity of grade 4 or a non‐hematologic toxicity of grade 3 or 4. Objective tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors version 1.0.8
Circulating level of soluble VEGFR2
The concentration of soluble VEGFR2 (sVEGFR2) in serum was measured with ELISA (THERMOmax; Molecular Devices, Sunnyvale, CA, USA) before vaccination on day 1 and after OTS102 administration on days 8 and 29.
Microarray analysis
The PBMCs were isolated from 3 mL whole blood with the use of an Accuspin system (Sigma‐Aldrich, St. Louis, MO, USA) and were then immediately suspended in an RNA stabilization solution (Isogen; Nippongene, Tokyo, Japan) and stored at −80°C. Total RNA was subsequently extracted from the cells and its quality checked as described previously.9 The RNA was subjected to microarray analysis (Affymetrix, Santa Clara, CA, USA) as described.10 Analysis of the microarray data was carried out with BRB ArrayTools software version 3.6.1 (http://linus.nci.nih.gov/BRB-ArrayTools.html) developed by R. Simon and A. Peng. In brief, a log2 transformation was applied to the raw data, and global normalization was used to calculate the median expression level over the entire array. Genes were excluded if the proportion of data missing or filtered out was >20%. Genes that passed the filtering criteria were then considered for further analysis. Pathway (gene set) analysis was carried out with the BRB ArrayTools software. The level of statistical significance was set at P = 0.01. A P‐value was first computed for each gene, and the set of P‐values was then summarized by LS and KS statistics. The gene set comparison tool analyzes 285 predefined BioCarta gene sets for differential expression among predefined classes (pre‐ vs. post‐treatment).
Other statistical analysis
Serum sVEGFR2 levels at baseline (pretreatment) were compared with those on days 8 or 29 with Student's paired t‐test. A P‐value of <0.05 was considered statistically significant.
Results
Patient demographics
The characteristics of the 10 HLA‐A*24:02‐positive patients enrolled in the study are shown in Table 1. The patients included four with non‐small‐cell lung cancer, three with gastric cancer, two with colorectal cancer, and one with thyroid cancer, all of whom were refractory to standard therapy. Doses of elpamotide for the escalation protocol included 0.5, 1.0, and 2.0 mg. Nine patients completed the first cycle of four injections with elpamotide, with one patient at the dose level of 2.0 mg being withdrawn from the study after two doses of the vaccine because of disease progression. Five patients were subjected to further cycles of vaccination. The median duration of treatment was 58 days (range, 14–279 days), with a median of 8 (range, 2–33) elpamotide vaccinations.
Table 1.
Characteristics of HLA‐A*24:02‐positive patients with advanced refractory solid tumors (n = 10) vaccinated with elpamotide
| Characteristics | Peptide dose | ||
|---|---|---|---|
| 0.5 mg (n = 3) | 1.0 mg (n = 3) | 2.0 mg (n = 4) | |
| Median age (range), years | 58 (58–65) | 64 (58–70) | 57 (30–84) |
| Male/female | 1/2 | 1/2 | 3/1 |
| Performance status (0/1) | 1/2 | 0/3 | 0/4 |
| Non‐small‐cell lung cancer | 1 | 1 | 2 |
| Gastric cancer | 0 | 1 | 2 |
| Colorectal cancer | 1 | 1 | 0 |
| Thyroid cancer | 1 | 0 | 0 |
Safety
All 10 patients received at least one dose of the study treatment and were evaluated for safety (Table 2). No patient showed a toxicity of grade 3 or higher. Five patients (50%) (two in the 0.5 mg cohort, one in the 1.0 mg cohort, and two in the 2.0 mg cohort) developed immunologic reactions, erythema, or induration of grade 1 or 2 at injection sites. In the 1.0 mg cohort, one patient developed proteinuria of grade 1 and another developed both hypertension and proteinuria of grade 1. No DLT was thus observed in the trial.
Table 2.
Summary of toxicities of grades (G) 1/2 or 3
| Adverse events | Peptide dose | Total | |||||
|---|---|---|---|---|---|---|---|
| 0.5 mg (n = 3) | 1.0 mg (n = 3) | 2.0 mg (n = 4) | |||||
| G1/2 | G3 | G1/2 | G3 | G1/2 | G3 | ||
| Reaction at injection site | 2 | 0 | 1 | 0 | 2 | 0 | 5 |
| Nasopharyngitis | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Anorexia | 1 | 0 | 0 | 0 | 1 | 0 | 2 |
| Nausea | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Vomiting | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Diarrhea | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Fatigue | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Fever | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Hypertension | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Proteinuria | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
Tumor response
Nine patients were evaluated for tumor response. Although no complete or partial response was observed, two patients had stable disease for at least two treatment cycles (56 days). A 58‐year‐old female patient with advanced thyroid cancer who had multiple metastases in her lungs and muscle achieved stable disease that persisted for >5 months after the 15th vaccination with elpamotide at 0.5 mg. A 70‐year‐old male with advanced gastric cancer had been treated with three prior chemotherapy regimens. Given that the tumor continued to grow despite chemotherapy, he was enrolled in the elpamotide 1.0 mg cohort. Tumor size as evaluated by computed tomography remained stable for 2 months after initiation of elpamotide treatment, with stable disease being declared after the eighth vaccination. The patient subsequently received another cycle of four vaccinations. During this third cycle of treatment, ascites was detected by computed tomography and progressive disease was declared.
Pathway analysis
To determine whether elpamotide vaccination induced systemic immunologic effects, we examined the gene expression profiles of PBMCs from all 10 patients before vaccination, and on days 8 and 29 after the onset of vaccination. Pathway analysis of microarray data revealed that 17 pathways were selected from among 285 BioCarta pathways at the nominal significance level of P = 0.01 for the LS or KS permutation tests (Table 3). The pathways with the most differentially expressed genes included those related to angiogenesis and T cell function (Table 3), supporting the notion that elpamotide vaccination indeed affects angiogenesis and T cell activation in the clinical setting.
Table 3.
Pathways with most differentially expressed genes between pre‐ and post‐treatment among 285 BioCarta pathways
| Pathway descriptions | No. of genes | LS P‐value | KS P‐value | Related pathways |
|---|---|---|---|---|
| VEGF, hypoxia, and angiogenesis | 23 | 0.0018 | 0.0270 | Angiogenesis |
| Hypoxia‐inducible factor in the cardiovascular system | 26 | 0.0055 | 0.0023 | Angiogenesis |
| Role of nicotinic acetylcholine | 13 | 0.0075 | 0.1485 | N/A |
| Melanocyte development and pigmentation pathway | 14 | 0.0093 | 0.1461 | N/A |
| Transcription regulation by methyltransferase of CARM1 | 20 | 0.0097 | 0.0378 | N/A |
| Classical complement pathway | 6 | 0.0141 | 0.0018 | N/A |
| Role of Tob in T cell activation | 26 | 0.0146 | 0.0097 | T cell |
| Lectin‐induced complement pathway | 6 | 0.0180 | 0.0018 | N/A |
| Deregulation of CDK5 in Alzheimer's disease | 20 | 0.0193 | 0.0054 | N/A |
| IL‐12‐ and Stat4‐dependent signaling pathway in Th1 development | 37 | 0.0254 | 0.0078 | T cell |
| T cell receptor and CD3 complex | 15 | 0.0283 | 0.0023 | T cell |
| NOS2‐dependent IL‐12 pathway in NK cells | 14 | 0.0350 | 0.0031 | T cell |
| T cytotoxic cell surface molecules | 28 | 0.0354 | 0.0072 | T cell |
| T helper cell surface molecules | 28 | 0.0354 | 0.0072 | T cell |
| Role of MEF2D in T cell apoptosis | 31 | 0.0358 | 0.0097 | T cell |
| HIV‐induced T cell apoptosis | 24 | 0.0442 | 0.0012 | T cell |
| ADP‐ribosylation factor | 38 | 0.0485 | 0.0087 | N/A |
CARM1, coactivator‐associated arginine methyltransferase 1; IL, interleukin; NK, natural killer; Stat4, signal transducer and activator of transcription‐4; N/A, not applicable; VEGF, vascular endothelial growth factor.
Serum level of sVEGFR2
The circulating level of sVEGFR2 was previously found to be reduced by other angiogenesis inhibitors that directly target VEGFR2. We determined the serum concentration of sVEGFR2 as a potential biomarker for elpamotide vaccination. The serum concentration of sVEGFR2 decreased significantly (P = 0.026) over the first 4 weeks of treatment (Fig. 1). The decrease in sVEGFR2 level tended to be larger at the higher dose levels of elpamotide, although this trend was not significant.
Figure 1.
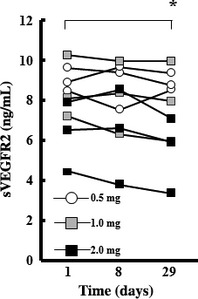
Serum concentrations of soluble vascular endothelial growth factor receptor 2 (sVEGFR2) before and after elpamotide vaccination. Serum samples were collected at baseline (day 1) as well on days 8 and 29 for determination of sVEGFR2 concentration. *P = 0.026 (paired t‐test) for comparison of the mean values for nine patients between days 1 and 29.
Discussion
The targeting of tumor angiogenesis with vaccines has potential advantages over such targeting of tumor cells directly in cancer therapy.4, 5, 6, 11 First, tumor endothelial cells are more accessible to the immune system than are tumor cells located at a distance from the vessels.12 Tumor endothelial cells are thus readily accessed by lymphocytes in the bloodstream, and CTLs can directly damage endothelial cells without penetration into the tumor tissue. In addition, the lysis of even a small number of endothelial cells within the tumor vasculature may result in the disruption of vessel integrity, leading to inhibition of the growth of numerous tumor cells. Endothelial cells are thus a promising target for cancer immunotherapy. Second, the loss or downregulation of HLA molecules on tumor cells is thought to be a major reason for the limited clinical efficacy of vaccines that target tumor cells.13, 14, 15 Given that such HLA loss has not been described for endothelial cells of newly formed tumor vessels, the development of vaccines that target vascular endothelial cells in tumor tissue may overcome the problem of immune‐escape of tumor cells.
Vascular endothelial growth factor receptor 2 is a functional molecule associated with neovascularization and is highly expressed in newly‐induced tumor vessels but not in normal vessels. The VEGFR2‐169 peptide (elpamotide) derived from VEGFR2 has been previously characterized by induction of peptide‐specific CTLs capable of killing VEGFR2‐expressing human endothelial cells.6, 7 The present phase I study was carried out to examine the safety of elpamotide for HLA‐A*24:02‐positive patients with advanced tumors. Injection site reactions of grade 1 or 2 were the most frequent vaccine‐related adverse events. Specific toxicities that have often been associated with anti‐angiogenic treatment with antibodies to VEGF or VEGFR tyrosine kinase inhibitors include hypertension and proteinuria.16, 17 These toxicities occurred only at a low grade in two patients in the present study. No adverse events of grade 3 or higher were observed, indicating that elpamotide vaccination is safe and well tolerated.
Although ex vivo and in vitro studies have provided insight into the specific effects of peptide immunotherapy, they cannot substitute for studies carried out in vivo. To date, however, there has been no valid and widely accepted in vivo analysis to achieve proof of concept during clinical development of cancer vaccines. Microarray technology has allowed the identification of genes related to a given process in a hypothesis‐free approach. The recent introduction of this technology to the field of cancer research has provided insights related to the more accurate classification of cancer, better definition of prognosis, and novel approaches to therapy. Microarray analysis has also proved to be a powerful tool for the identification and characterization of genes related to the ontogeny, differentiation, and activation of immune cells.18 We have now applied such analysis to PBMCs obtained from patients in order to monitor the biological activity of elpamotide. To facilitate the interpretation of the enormous amount of microarray data, we examined gene sets related to biologically relevant pathways rather than individual genes. The results of our analysis indicate that several pathways related to T cell function and angiogenesis were significantly affected by a single treatment with elpamotide, supporting the notion that this peptide induces an immune response that targets angiogenesis. Our present study thus suggests that microarray analysis is a promising approach to achieving proof of concept during early clinical trials of cancer vaccines. We further explored if the changes in gene expression correlated with treatment response; however, definitive differences between responders (stable disease) (n = 2) and non‐responders (n = 7) were not detected, perhaps due to small sample size. Further investigation to validate whether it will be useful for monitoring the treatment response is warranted.
Given that most angiogenesis inhibitors are cytostatic, it has been difficult to assess the biological effects of these agents in the early phase of clinical trials. Therefore, there is a need for validated biomarkers to monitor biological activity. The circulating level of sVEGFR2 was previously found to be reduced by other angiogenesis inhibitors that directly target VEGFR2,19, 20, 21 although the mechanism underlying this consistent effect is not fully understood.16, 17 In the present study, serum sVEGFR2 concentrations showed a time‐dependent decrease at all elpamotide dose levels studied, and this effect tended to be greater at the higher dose levels, suggesting that sVEGFR2 is a potential pharmacodynamic marker of drug exposure.
Inhibition of angiogenesis has provided new treatment avenues for cancer patients; however, there are no reliable biomarkers available to predict therapy response. Although tumor evaluation was not the primary objective of the present study, and the small sample size precludes any conclusions regarding treatment efficacy, the identification of predictive biomarkers to stratify cancer patients is vital to move this anti‐angiogenic vaccine therapy forward. A randomized, controlled clinical trial of elpamotide for advanced cancer patients is being carried out in an effort to find such biomarkers.
In conclusion, elpamotide shows an acceptable safety profile for patients with advanced solid tumors. The preliminary evaluation of the biological activity of elpamotide with the use of microarray analysis as well as our serum marker (sVEGFR2) and disease stabilization data indicate that this agent is indeed biologically active.
Disclosure Statement
Takuya Tsunoda was employed in a leadership position by OncoTherapy Science (Tokyo, Japan). Kazuhiko Nakagawa received research funding for this study from OncoTherapy Science.
Acknowledgments
We thank R. Simon and A. Peng for providing the BRB ArrayTools software.
(Cancer Sci 2012; 103: 2135–2138)
References
- 1. Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2002; 2: 727–39. [DOI] [PubMed] [Google Scholar]
- 2. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011; 473: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tie J, Desai J. Antiangiogenic therapies targeting the vascular endothelial growth factor signaling system. Crit Rev Oncog 2012; 17: 51–67. [DOI] [PubMed] [Google Scholar]
- 4. Li Y, Wang MN, Li H et al Active immunization against the vascular endothelial growth factor receptor flk1 inhibits tumor angiogenesis and metastasis. J Exp Med 2002; 195: 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niethammer AG, Xiang R, Becker JC et al A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med 2002; 8: 1369–75. [DOI] [PubMed] [Google Scholar]
- 6. Wada S, Tsunoda T, Baba T et al Rationale for antiangiogenic cancer therapy with vaccination using epitope peptides derived from human vascular endothelial growth factor receptor 2. Cancer Res 2005; 65: 4939–46. [DOI] [PubMed] [Google Scholar]
- 7. Miyazawa M, Ohsawa R, Tsunoda T et al Phase I clinical trial using peptide vaccine for human vascular endothelial growth factor receptor 2 in combination with gemcitabine for patients with advanced pancreatic cancer. Cancer Sci 2010; 101: 433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Therasse P, Arbuck SG, Eisenhauer EA et al New guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines). J Natl Cancer Inst 2000; 92: 205–16. [DOI] [PubMed] [Google Scholar]
- 9. Yamanaka R, Arao T, Yajima N et al Identification of expressed genes characterizing long‐term survival in malignant glioma patients. Oncogene 2006; 25: 5994–6002. [DOI] [PubMed] [Google Scholar]
- 10. Yamada Y, Arao T, Gotoda T et al Identification of prognostic biomarkers in gastric cancer using endoscopic biopsy samples. Cancer Sci 2008; 99: 2193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ishizaki H, Tsunoda T, Wada S et al Inhibition of tumor growth with antiangiogenic cancer vaccine using epitope peptides derived from human vascular endothelial growth factor receptor 1. Clin Cancer Res 2006; 12: 5841–9. [DOI] [PubMed] [Google Scholar]
- 12. Matejuk A, Leng Q, Chou ST et al Vaccines targeting the neovasculature of tumors. Vasc Cell 2011; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cormier JN, Hijazi YM, Abati A et al Heterogeneous expression of melanoma‐associated antigens and HLA‐A2 in metastatic melanoma in vivo. Int J Cancer 1998; 75: 517–24. [DOI] [PubMed] [Google Scholar]
- 14. Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T‐cell immunotherapy revives an old story. Mol Med Today 1999; 5: 178–86. [DOI] [PubMed] [Google Scholar]
- 15. Paschen A, Mendez RM, Jimenez P et al Complete loss of HLA class I antigen expression on melanoma cells: a result of successive mutational events. Int J Cancer 2003; 103: 759–67. [DOI] [PubMed] [Google Scholar]
- 16. Bertolini F, Shaked Y, Mancuso P et al The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer 2006; 6: 835–45. [DOI] [PubMed] [Google Scholar]
- 17. Brown AP, Citrin DE, Camphausen KA. Clinical biomarkers of angiogenesis inhibition. Cancer Metastasis Rev 2008; 27: 415–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monsurro V, Marincola FM. Microarray analysis for a comprehensive immunological‐status evaluation during cancer vaccine immune monitoring. J Biomed Biotechnol 2011; 2011: 307297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drevs J, Siegert P, Medinger M et al Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol 2007; 25: 3045–54. [DOI] [PubMed] [Google Scholar]
- 20. Norden‐Zfoni A, Desai J, Manola J et al Blood‐based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib‐resistant gastrointestinal stromal tumor. Clin Cancer Res 2007; 13: 2643–50. [DOI] [PubMed] [Google Scholar]
- 21. Okamoto I, Kaneda H, Satoh T et al Phase I safety, pharmacokinetic, and biomarker study of BIBF 1120, an oral triple tyrosine kinase inhibitor in patients with advanced solid tumors. Mol Cancer Ther 2010; 9: 2825–33. [DOI] [PubMed] [Google Scholar]


