Abstract
Studies focused on elderly acute promyelocytic leukemia (APL) are relatively limited. To evaluate prognostic impact in elderly APL, we compared the long‐term outcome of elderly APL patients (60–70 years) with younger patients (15–59 years) treated with all‐trans retinoic acid combined with anthracycline and cytarabine in the Japan Adult Leukemia Study Group (JALSG) APL97 study. Of 283 evaluable patients, 46 (16.3%) were elderly who had more frequent lower platelet (P = 0.04), lower albumin (P = 0.006) and performance status 3 (P = 0.02), higher induction death rate due to differentiation syndrome (P = 0.03), and non‐relapse mortality (NRM) during consolidation therapy (P = 0.001). Overall survival was significantly inferior in elderly patients (P = 0.005), but disease‐free survival and cumulative incidence of relapse were not. Better therapeutic approaches should be considered to reduce NRM during induction and consolidation therapy in elderly APL. This study was registered at http://www.umin.ac.jp/ctrj/ under C000000206.
All‐trans retinoic acid (ATRA) and arsenic trioxide (ATO) has dramatically improved the clinical outcome of acute promyelocytic leukemia (APL).1, 2, 3, 4, 5, 6 However, studies focused on elderly APL are relatively limited, and optimal therapeutic approaches for these patients remain to be explored.7 While the European APL Group (EAG) and Gruppo Italiano Malattie Ematologiche dell' Adulto (GIMEMA), by using ATRA combined with anthracycline ± cytarabine, demonstrated that survival rate of elderly APL was lower than that of younger patients,8, 9 Programa de Estudio y Tratamiento de las Hemopatı′as Malignas (PETHEMA) reported there was no significant difference between them.10 However, only the EAG report compared directly the clinical characteristics and outcomes between two age groups,8 and the clinical characteristics and outcomes between them have not been well elucidated in the treatment of APL.
Here we report the long‐term outcome and prognostic factors of elderly APL patients who were treated in the Japan Adult Leukemia Study Group (JALSG) APL97 study,11 by comparing data between the elderly and the younger APL.
Materials and Methods
Patients
Adult patients with previously untreated APL were registered into the JALSG APL97 study between May 1997 and June 2002.11 Eligibility criteria were: (i) diagnosis of APL with t(15;17) and/or the PML‐RARA fusion gene amplified by RT‐PCR; (ii) age between 15 and 70 years; (iii) Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–3; and (iv) sufficient functioning of the heart, liver and kidney. This study was approved by the institutional review boards of each participating institution. Informed consent was obtained from patients before registration in the study in accordance with the Declaration of Helsinki. All data were updated in January 2009.
Study design and treatments
The detail of treatment schedule has been described previously.11 Briefly, remission induction therapy consisted of ATRA and chemotherapy with idarubicin and cytarabine, with dose and duration determined by initial leukocyte count. After obtaining complete remission (CR) and receiving three courses of intensive consolidation chemotherapy, patients negative for PML‐RARA were randomly allocated either to receive six courses of intensified maintenance chemotherapy or to observation. Patients, who were positive for the PML‐RARA fusion transcript, received late ATRA therapy followed by maintenance therapy, and were scheduled to receive allogeneic hematopoietic stem cell transplantation (HSCT) if they had a human leukocyte antigen‐identical donor. Elderly patients were treated with the same schedule to younger patients. For prevention of hemorrhage, platelets were transfused to maintain the platelet count above 30 × 109/L, and fresh frozen plasma was transfused to maintain the plasma fibrinogen level above 1.5 g/L. Anticoaglants were used according to the discretion of institutions. Differentiation syndrome (DS) was treated with methylprednisolone and transient discontinuation of ATRA.
Definition and evaluation of patients
In this analysis, we set the cut‐off age of 60 years to compare our data with previous reports on elderly APL over 60 years old.5, 6, 8, 9, 10 Hematological response was evaluated by standard criteria generally used for chemotherapy.12 Molecular relapse detected by RT‐PCR analysis of PML‐RARA was also considered as a relapse. The primary end point of the JALSG APL97 study was overall survival (OS) and disease‐free survival (DFS) of patients achieved CR. Overall survival was calculated from the first day of therapy to death or last visit. Disease‐free survival was measured from the date of CR to relapse, death from any cause or last visit. OS and DFS in patients who were randomized to either observation or maintenance chemotherapy groups were also measured from the date of randomization to the same end points mentioned above.
Statistical analysis
Categorical data were compared using χ2 test or Fisher's exact test. Continuous data were compared using Wilcoxon rank‐sum test. Overall survival and DFS were estimated by Kaplan–Meier methods and compared by the log‐rank test. Cumulative incidence of relapse (CIR) was measured from the date of CR to the first relapse, while non‐relapse mortality (NRM) was censored as a competing risk event. Gray's test was used to compare the cumulative incidence curves. Univariate and multivariate Cox proportional hazard analyses were performed to determine prognostic indicators of OS. Prognostic variables of univariate significance were selected for inclusion in the multivariate model. Statistical analyses were performed using spss 11.0 (SPSS Inc, Chicago, IL, USA) and R 2.12.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/). All hypothesis testing was two‐tailed with a significance level of 0.05.
Results
Patient characteristics
Of 302 patients registered, 283 (median age, 48 years; range, 15–70) were evaluated. Nineteen patients were excluded: four misdiagnosis, two inconsistent eligibility, seven negative for t(15;17) and six no test for t(15;17) or PML‐RARA. The median follow‐up period was 7.7 years. Forty‐six patients (16.3%) were elderly (median, 63 years; range, 60–70), and 237 were younger (median, 44 years; range, 15–59). Lower platelet count (<10 × 109/L), lower serum albumin level (<3.5 g/dL) and PS 3 were significantly more frequent in elderly patients than in younger (P = 0.04, P = 0.006 and P = 0.02, respectively). The distribution of Sanz's relapse risk score14 was not different between the two groups (P = 0.88) (Table 1).
Table 1.
Clinical features of acute promyelocytic leukemia (APL) patients according to age
| Parameters | Total | Age (15–59 years) | Age (60–70 years) | P | |||
|---|---|---|---|---|---|---|---|
| n (%) | Median (range) | n (%) | Median (range) | n (%) | Median (range) | ||
| No. patients | 283 | 237 | 46 | ||||
| Gender | |||||||
| Male | 158 (56) | 128 (54) | 30 (65) | 0.2 | |||
| Female | 125 (44) | 109 (46) | 16 (35) | ||||
| Leukocyte count, ×109/L | 0.83 | ||||||
| <3.0 | 174 (61) | 1.7 (0.03–257) | 143 (60) | 1.8 (0.03–152) | 31 (67) | 1.4 (0.5–257) | 0.39 |
| 3.0–10.0 | 58 (21) | 52 (22) | 6 (13) | ||||
| 10.0 or higher | 51 (18) | 42 (18) | 9 (20) | ||||
| APL cell count, ×109/L | 0.91 | ||||||
| <1.0 | 238 (84) | 0.6 (0–253) | 201 (85) | 0.6 (0–143) | 37 (80) | 0.5 (0–253) | 0.51 |
| 1.0–3.0 | 23 (8) | 20 (8) | 3 (7) | ||||
| 3.0 or higher | 19 (7) | 14 (6) | 5 (11) | ||||
| Platelet count, ×109/L | 0.28 | ||||||
| <10 | 39 (14) | 30 (2–238) | 28 (12) | 30 (2–238) | 11 (24) | 30 (5–139) | 0.04 |
| 10–40 | 140 (49) | 119 (50) | 21 (46) | ||||
| 40 or higher | 104 (37) | 90 (38) | 14 (30) | ||||
| ECOG performance status score | |||||||
| 0–2 | 262 (92) | 223 (94) | 39 (85) | 0.02 | |||
| 3 | 19 (7) | 12 (5) | 7 (15) | ||||
| Albumin level, g/dL | 0.89 | ||||||
| <3.5 | 23 (8) | 4.2 (2.0–6.1) | 14 (6) | 4.2 (2.0–6.1) | 9 (20) | 4.2 (2.3–5.0) | 0.006 |
| 3.5 or higher | 247 (87) | 211 (89) | 36 (78) | ||||
| DIC scorea | |||||||
| 0–2 | 23 (8) | 19 (8) | 4 (9) | 0.97 | |||
| 3–9 | 215 (76) | 182 (77) | 34 (74) | ||||
| 10 or higher | 27 (10) | 21 (9) | 5 (11) | ||||
| FAB subtype | |||||||
| Typical | 265 (94) | 220 (93) | 45 (98) | 0.32 | |||
| Variant | 18 (6) | 17 (7) | 1 (2) | ||||
| CD56 expression | |||||||
| Positive | 23 (8) | 20 (8) | 3 (7) | 0.69 | |||
| Negative | 216 (76) | 181 (84) | 35 (76) | ||||
| CD34 expression | |||||||
| Positive | 40 (14) | 30 (13) | 10 (22) | 0.09 | |||
| Negative | 217 (77) | 187 (79) | 30 (65) | ||||
| Past history of malignant disease | 11 (4) | 10 (4) | 1 (2) | 0.51 | |||
| Infectious complications at diagnosis | 72 (24) | 58 (24) | 14 (30) | 0.46 | |||
| Relapse risk (Sanz risk score) | |||||||
| Low | 88 (31) | 75 (32) | 13 (28) | 0.88 | |||
| Intermediate | 145 (51) | 121 (51) | 24 (52) | ||||
| High | 50 (18) | 41 (17) | 9 (20) | ||||
DIC score13; score 3 indicates suspected DIC; score 4–10, definitive DIC; 10 or more, severe DIC. ECOG, Eastern Cooperative Oncology Group; FAB, French‐American‐British.
Treatment outcome
Elderly patients tended to have lower CR rate and higher incidence of early death during induction therapy (89% vs 96%, P = 0.06; 11% vs 4%, P = 0.08; Table 2).
Table 2.
Cause of death between the elderly and younger acute promyelocytic leukemia (APL) patients
| Age (15–59 years) | Age (60–70 years) | P value | ||
|---|---|---|---|---|
| n = 237 | n = 46 | |||
| During induction therapy (n = 283) | n | n (%) | n (%) | |
| Death during induction therapy | 15 | 10 (4) | 5 (11) | 0.08 |
| Hemorrhage | 9 | 8 (3) | 1 (2) | 1 |
| Infection | 1 | 0 | 1 (2) | 0.16 |
| Differentiation syndrome | 2 | 0 | 2 (4) | 0.03 |
| Others | 3 | 2 (0.8) | 1 (2) | |
| During consolidation therapy (n = 258) | n = 222 | n = 36 | ||
| Death during consolidation therapy | 10 | 5 (2) | 5 (13) | 0.001 |
| Death during C1 therapy | 0 | 0 | 0 | |
| Death during C2 therapy | 4 | 2 | 2 | |
| Infection | 4 | 2 (1) | 2 (5) | 0.1 |
| Death during C3 therapy | 6 | 3 | 3 | |
| Infection | 6 | 3 (1) | 3 (9) | 0.04 |
| Post‐consolidation therapy (n = 258) | n = 222 | n = 36 | ||
| Death post‐consolidation therapy | 35 | 28 (13) | 7 (19) | 0.27 |
| After relapse | 25 | 21 | 4 | |
| Death related to salvage therapy (including HSCT) | 11 (5) | 1 (3) | ||
| Death related to APL | 7 (3) | 3 (8) | ||
| Hemorrhage | 1 (0.5) | 0 | ||
| Acute myocardial infarction | 1 (0.5) | 0 | ||
| Secondary leukemia | 1 (0.5) | 0 | ||
| APL in CR | 10 | 7 | 3 | |
| Secondary leukemia | 2 (1) | 0 | ||
| Pneumonia | 1 (0.5) | 0 | ||
| Unknown | 4 (2) | 3 (8) |
CR, complete remission; HSCT indicates hematopoietic stem cell transplantation.
Primary resistant to induction therapy was observed in one patient in the younger group, which was not observed in the elderly patients. Differentiation syndrome was the most frequent cause of early death (4%) in elderly patients, followed by hemorrhage (2%) and infection (2%). Early death rate due to DS was higher in elderly patients (4% vs 0%, P = 0.03), while DS incidence was similar between two groups (P = 0.76). Non‐relapse mortality during consolidation therapies was significantly more frequent in elderly patients (13% vs 2%, P = 0.001), and all were associated with infection (Table 2). Median duration for the recovery of leukocyte over 1.0 × 109/L was significantly longer in elderly patients compared with younger patients in the second consolidation cycle (25 vs 22.5 days, P = 0.03), and granulocyte colony stimulating factor (G‐CSF) was more frequently administered to elderly patients during the first and second cycles (39.5% vs 19.6%, P = 0.007 and 43.2% vs 25.2%, P = 0.02, respectively).
Ten‐year OS was significantly inferior in elderly patients (63% vs 82%, P = 0.005) (Fig. 1a). In the previous analyses of the JALSG APL97 study, the predicted 6‐year OS in patients with or without the intensified maintenance therapy was 86.2% and 98.8%, which was significantly different (P = 0.014).11 Therefore, we additionally analyzed the influence of age on the long‐term outcome between patients with or without the intensified maintenance therapy. In younger patients, 10‐year OS in the intensified maintenance therapy group was significantly inferior compared to the observation group (81% vs 97%, P < 0.001), while OS in the elderly group was not significantly different between patients with and without the intensified maintenance therapy. (86% vs 92%, P = 0.72). 10‐year DFS and CIR were similar between the elderly and younger patients (65% vs 67%, P = 0.70; 15% vs 28%, P = 0.15, respectively) (Fig. 1b,c). Ten‐year NRM was significantly higher in elderly patients (20% vs 7%, P = 0.007) (Fig. 1d). Of 17 deaths among elderly patients, 10 (59%) occurred during induction and consolidation therapies (Table 2). In the multivariate analysis for OS, age (more than 60 years) and PS (score 3) were the independent adverse prognostic variable factors (hazard ratio [HR]: 1.95; 95% confidence interval [CI]: 1.10–3.50; P = 0.02; HR: 2.48; 95% CI: 1.16–5.29; P = 0.02, respectively) (Table 3).
Figure 1.
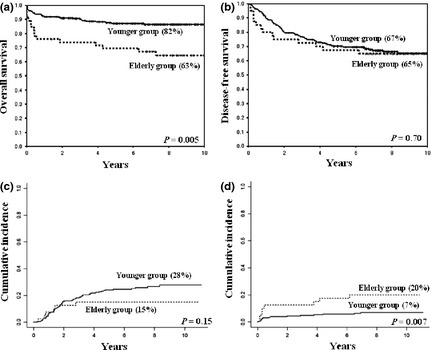
Overall survival, disease‐free survival, cumulative incidence of relapse and non‐relapse mortality at 10 years between the elderly and younger acute promyelocytic leukemia (APL) patients. (a) Overall survival (63% vs 82%, P = 0.005). (b) Disease‐free survival (65% vs 67%, P = 0.70). (c) Cumulative incidence of relapse (15% vs 28%, P = 0.15). (d) Non‐relapse mortality (20% vs 7%, P = 0.007).
Table 3.
Prognostic factors affecting overall survival of acute promyelocytic leukemia (APL) patients
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Initial leukocyte count (more than 10 × 109/L) | 1.56 (0.86–2.84) | 0.14 | 1.48 (0.81–2.69) | 0.21 |
| Initial platelet count (<10 × 109/L) | 1.65 (0.88–3.11) | 0.12 | 1.45 (0.71–2.57) | 0.37 |
| Sex (Male) | 1.73 (1.01–2.96) | 0.05 | 1.69 (1.01–3.00) | 0.05 |
| Albumin (<3.5 g/dL) | 1.07 (0.43–2.68) | 0.88 | ||
| Performance status (score 3) | 2.43 (1.15–5.11) | 0.02 | 2.48 (1.16–5.29) | 0.02 |
| Age (more than 60 years) | 2.20 (1.25–3.85) | 0.006 | 1.95 (1.10–3.50) | 0.02 |
Discussion
We compared the long‐term outcome between the elderly and the younger APL patients who were treated with ATRA combined with anthracycline and cytarabine in the JALSG APL97 study, and extracted prognostic factors in elderly APL patients.
Complete remission rates among elderly APL are reportedly lower than younger patients, due to higher early death.2, 8, 9, 10 In our study, early deaths by DS were more frequent in elderly patients. Programa de Estudio y Tratamiento de las Hemopatı′as Malignas reported that increased death due to DS was associated with higher PS score and lower serum albumin level.15 Our study supported their observation, demonstrating significantly more PS 3 and lower albumin level in elderly patients. While number of cases was limited, we should treat them more carefully in future.
Cumulative incidence of relapse in our study was similar between the two groups, as reported by GIMEMA.9 However, EAG and PETHEMA demonstrated lower CIR in elderly patients compared to younger patients.8, 10 In their studies, Sanz's low relapse‐risk features were more frequent in elderly patients,8, 10 which might explain their lower CIR.
European APL Group also reported that 10 year‐OS in elderly patients was lower than that of whole population (58.1% vs 77%). The major cause of death in their elderly patients was sepsis during myelosuppression.16 Gruppo Italiano Malattie Ematologiche dell' Adulto reported that AIDA0493 amended protocol in which the intensity of consolidation therapy was reduced for elderly patients improved 5‐year OS (76.1%) and 5‐year NRM (7.7%) compared with the original protocol (56% and 13%, respectively).17 Programa de Estudio y Tratamiento de las Hemopatı′as Malignas, using ATRA combined with anthracycline monochemotherapy, showed 6‐year DFS in elderly patients to be 79%, which was similar to younger patients.10 They showed that NRM in elderly patients (60–83 years) was 9.2%, but only 3.2% in patients aged between 60 and 70 years. In our study, NRM (13%) in elderly APL was higher than those of PETHEMA10 or GIMEMA17 studies, and NRM during consolidation therapies was the main reason for inferior long‐term outcome in elderly patients. On the other hand, our study showed that the difference between OS and DFS in the elderly group was subtle compared to those in the younger group. One of the reasons might be that patients who relapsed in the elderly group died due to APL because no effective salvage therapy was available at that time. In contrast, patients who relapsed in the younger group underwent several effective salvage therapies with or without HSCT.
While the disadvantage of intensified maintenance therapy was shown in the total analysis of JALSG APL97 study,11 it was not in the analysis focused on elderly patients. We demonstrated that OS in the elderly group was not significantly different between patients with or without the intensified maintenance therapy in this analysis. The frequency of dose reduction in maintenance therapy was not significantly different between elderly and younger groups. (14.2% vs 4.9%, P = 0.44), and NRM during maintenance therapy was not in the elderly group. Cumulative incidence of relapse in the elderly group was not different between cases with or without the intensified maintenance therapy (0% vs 16.7%, P = 0.27). These results might be affected by the small number of elderly cases analyzed, because only seven patients (7.9%) of the 89 patients in the maintenance arm were elderly. Further investigation will be required in the issue.
Taking into consideration the factors mentioned above, further refinement will be needed to reduce NRM in elderly patients. Recently, several new agents have been introduced in APL. Arsenic trioxide and gemtuzumab ozogamicin (GO) are reportedly effective and well tolerated by elderly APL.6, 18, 19, 20 These agents may be used for elderly patients to reduce the risk of NRM.
In conclusion, elderly APL were more vulnerable to complications such as DS and infection, which resulted in lower OS. Reduction of intensity of induction and post‐remission chemotherapy without increasing relapse should be considered. Early intervention against DS and infection may result in lower induction mortality and NRM. ATO and reduced dosage of GO may be incorporated into the therapy for elderly APL.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
We thank the patients for entering this study and participating physicians from 92 institutions who registered their patients and provided necessary data. We also thank Dr Ryuzo Ohno for his advice and help during the entire study as well as the preparation of manuscript. Drs M. Nishimura and T. Kobayashi are thanked for their assistance in designing the protocol. This work was supported in part by the Grant for Cancer from the Ministry of Health, Welfare and Labour (004) and by the Grant for Cancer Translational Research Project from the Ministry of Education, Culture, Sports, Science and Technology.
(Cancer Sci 2012; 103: 1974–1978)
References
- 1. Fenaux P, Le Deley MC, Castaigne S et al Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood 1993; 82: 3241–9. [PubMed] [Google Scholar]
- 2. Kanamaru A, Takemoto Y, Tanimoto M et al All‐trans retinoic acid for the treatment of newly diagnosed acute promyelocytic leukemia. Japan Adult Leukemia Study Group. Blood 1995; 85: 1202–6. [PubMed] [Google Scholar]
- 3. Mandelli F, Diverio D, Avvisati G et al Molecular remission in PML/RAR alpha‐positive acute promyelocytic leukemia by combined all‐trans retinoic acid and idarubicin (AIDA) therapy. Gruppo Italiano‐Malattie Ematologiche Maligne dell'Adulto and Associazione Italiana di Ematologia ed Oncologia Pediatrica Cooperative Groups. Blood 1997; 90: 1014–21. [PubMed] [Google Scholar]
- 4. Sanz MA, Martin G, Rayon C et al A modified AIDA protocol with anthracycline‐based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha‐positive acute promyelocytic leukemia. PETHEMA group. Blood 1999; 94: 3015–21. [PubMed] [Google Scholar]
- 5. Lo‐Coco F, Avvisati G, Vignetti M et al Front‐line treatment of acute promyelocytic leukemia with AIDA induction followed by risk‐adapted consolidation for adults younger than 61 years: results of the AIDA‐2000 trial of the GIMEMA Group. Blood 2010; 116: 3171–9. [DOI] [PubMed] [Google Scholar]
- 6. Powell BL, Moser B, Stock W et al Arsenic trioxide improves event‐free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 2010; 116: 3751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fenaux P, Chevret S, de Botton S. Treatment of older adults with acute promyelocytic leukaemia. Best Pract Res Clin Haematol 2003; 16: 495–501. [DOI] [PubMed] [Google Scholar]
- 8. Ades L, Chevret S, De Botton S et al Outcome of acute promyelocytic leukemia treated with all trans retinoic acid and chemotherapy in elderly patients: the European group experience. Leukemia 2005; 19: 230–3. [DOI] [PubMed] [Google Scholar]
- 9. Mandelli F, Latagliata R, Avvisati G et al Treatment of elderly patients (> or = 60 years) with newly diagnosed acute promyelocytic leukemia. Results of the Italian multicenter group GIMEMA with ATRA and idarubicin (AIDA) protocols. Leukemia 2003; 17: 1085–90. [DOI] [PubMed] [Google Scholar]
- 10. Sanz MA, Vellenga E, Rayon C et al All‐trans retinoic acid and anthracycline monochemotherapy for the treatment of elderly patients with acute promyelocytic leukemia. Blood 2004; 104: 3490–3. [DOI] [PubMed] [Google Scholar]
- 11. Asou N, Kishimoto Y, Kiyoi H et al A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML‐RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood 2007; 110: 59–66. [DOI] [PubMed] [Google Scholar]
- 12. Cheson BD, Bennett JM, Kopecky KJ et al Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–9. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol 1983; 49: 265–75. [DOI] [PubMed] [Google Scholar]
- 14. Sanz MA, Lo Coco F, Martin G et al Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood 2000; 96: 1247–53. [PubMed] [Google Scholar]
- 15. de la Serna J, Montesinos P, Vellenga E et al Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all‐trans retinoic acid and idarubicin. Blood 2008; 111: 3395–402. [DOI] [PubMed] [Google Scholar]
- 16. Ades L, Guerci A, Raffoux E et al Very long‐term outcome of acute promyelocytic leukemia after treatment with all‐trans retinoic acid and chemotherapy: the European APL Group experience. Blood 2010; 115: 1690–6. [DOI] [PubMed] [Google Scholar]
- 17. Latagliata R, Breccia M, Fazi P et al GIMEMA AIDA 0493 amended protocol for elderly patients with acute promyelocytic leukaemia. Long‐term results and prognostic factors. Br J Haematol 2011; 154: 564–8. [DOI] [PubMed] [Google Scholar]
- 18. Estey E, Garcia‐Manero G, Ferrajoli A et al Use of all‐trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 2006; 107: 3469–73. [DOI] [PubMed] [Google Scholar]
- 19. Ravandi F, Estey E, Jones D et al Effective treatment of acute promyelocytic leukemia with all‐trans‐retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol 2009; 27: 504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takeshita A, Shinjo K, Naito K et al Efficacy of gemtuzumab ozogamicin on ATRA‐ and arsenic‐resistant acute promyelocytic leukemia (APL) cells. Leukemia 2005; 19: 1306–11. [DOI] [PubMed] [Google Scholar]


