Abstract
To investigate the clinical significance of granulocytic sarcoma (GS) in adults with acute myeloid leukemia (AML), 434 consecutive patients with AML were analyzed retrospectively. Forty‐five patients (10.4%) with GS at diagnosis were younger (P < 0.001), presented with higher white blood cell counts (P = 0.03) and were more likely to conform to French–American–British M4 (P = 0.001) and M5 (P = 0.045) classifications than those without GS. In contrast, no significant difference in frequency of cytogenetic abnormalities was found between the GS and non‐GS groups. Treatment outcomes in 260 patients (40 with GS) who underwent intensive chemotherapy, excluding patients with acute promyelocytic leukemia, were investigated. Complete remission rates did not differ significantly between the GS and non‐GS groups (75.0% vs 79.1%; P = 0.192, respectively) or the 5‐year overall survival (OS) rates (39.9% vs 38.7%; P = 0.749, respectively). However, the GS group had a significantly higher relapse rate than the non‐GS group (74.2% vs 55.3%; P = 0.048) and a significantly lower 5‐year disease‐free survival rate (8.2% vs 25.7%, respectively; P = 0.005). When considered together with the results of multivariate analysis, which identified the presence of GS as an independent predictor for disease‐free survival time, these findings indicate that GS might identify a high‐risk subset of patients with AML. (Cancer Sci, doi: 10.1111/j.1349‐7006.2012.02324.x, 2012)
Granulocytic sarcoma (GS), a rare extramedullary tumor composed of malignant granulocytic precursor cells that affects 3–9% of patients diagnosed with acute myeloid leukemia (AML),1, 2, 3 occurs concomitantly with or after a diagnosis of AML. Granulocytic sarcoma can occur in any organ and common sites are bones, lymph nodes, soft tissues and skin.4 Other organs, including the brain,5, 6 orbit7, 8 and genitourinary system,9, 10 are infrequently affected by GS. Such variation in its site of occurrence complicates accurate diagnosis of GS, and, moreover, the low incidence of GS has impeded establishment of the clinical characteristics of patients with GS. Several studies have reported that specific factors, including French–American–British (FAB) M4 and M5 classification11, 12 and the expression of CD5613, 14 or T‐cell antigens (CD2, CD4 and CD7)15, 16 on leukemia cells, are associated with GS, while others have identified an association between GS and t(8;21)17, 18 or inv(16).19, 20
Establishment of the clinical characteristics of GS has been further impeded by the mixed results obtained by several studies that investigated the prognosis of GS in limited subpopulations of AML patients. Byrd et al.21 found that GS was associated with unfavorable outcomes and a shorter survival time (median 5.4 months with GS vs 59.5 months without GS) among AML patients carrying t(8;21), whereas Felice et al.22 argued that GS had no adverse prognostic impact on AML patients carrying t(8;21). In light of these discrepant findings, this study aimed to clarify the clinical significance of GS among AML patients by comparing the clinical characteristics and treatment outcomes of AML patients with GS and those without GS. By using a large‐scale retrospective study design to examine 434 AML patients, this study fills an important research need to investigate the clinical significance of GS among a large general population of adult AML patients.
Patients and Methods
The medical records of 434 consecutive patients who had received a diagnosis of AML between January 1990 and December 2008 at our three cooperative hospitals in Gunma, Japan were analyzed retrospectively. All patients gave informed consent in accordance with the Declaration of Helsinki before initiation of treatment. The present study was approved by the Institutional Review Board of Gunma University Hospital. Acute myeloid leukemia had been diagnosed according to the FAB classification23 or the World Health Organization classification.24 Granulocytic sarcoma was defined as the presence of extramedullary tumors that were identified on physical examination and/or by imaging studies (computed tomography scan or magnetic resonance imaging) at the initial diagnosis of AML and responded to chemotherapy. Pathological confirmation was not necessarily required. With regard to lymph nodes, those with a size of more than 1.5 cm were included, whereas those that were more likely to be purulent lymphadenitis with tenderness and redness were excluded. Patients with hepatomegaly, splenomegaly or disseminated infiltration of leukemia cells in the skin and central nervous system that did not form tumors were excluded from the definition of GS.
The clinical data during the period before the induction chemotherapy were collected from the medical records of all 434 patients. Of the 434 patients, 174 (40.1%) were excluded from the survival analysis if they fulfilled one or more of the following criteria: (i) diagnosis of acute promyelocytic leukemia (APL) (54 patients; 31.0%); (ii) over 65 years of age (113 patients; 64.9%); or (iii) comorbidity and poor performance status (seven patients; 4.0%). Thus, the remaining 260 patients who were eligible for intensive chemotherapy were investigated for treatment outcomes.
Analysis of surface markers on the leukemia cells was performed using flow cytometry with monoclonal antibodies against CD7, CD11b, CD13, CD14, CD15, CD19, CD33, CD34, CD56 and HLA‐DR. Expression of each antigen was considered positive if at least 20% of the leukemia cells expressed the respective antigen. Cytogenetic subgroups were classified as favorable, intermediate and adverse risk according to a largely accepted classification system as follows:25 (i) favorable risk included t(8;21), t(15;17) and inv(16); (ii) intermediate risk included normal karyotypes, 11q23 aberrations and others; (iii) adverse risk included ‐5, ‐7, del(5), 3q abnormalities and complex karyotype (five or more abnormalities).
Treatment protocols
Two hundred and sixty patients who were eligible for intensive chemotherapy were treated according to the Japan Adult Leukemia Study Group (JALSG) treatment protocols (JALSG AML92,26 AML95,27 AML9728 or AML20129). All protocols consisted of single induction therapy with anthracycline (daunorubicin or idarubicine) plus cytarabine or enocitabine and were followed by three or four courses of consolidation therapy. Local irradiation to the site of GS, if possible, was planned for patients with large GS (>5 cm) and five patients received it. Patients in a high‐risk group had been considered as candidates for allogeneic stem cell transplantation (allo‐SCT). Of the 260 patients, 94 had undergone an allo‐SCT (19 patients in the GS‐group and 75 patients in the non‐GS group).
Statistical analysis
In patients with GS, a diagnosis of complete remission required the disappearance of GS with imaging studies in addition to the usual bone marrow examination. Overall survival (OS) was defined as the interval from the date of diagnosis to the date of death. Disease‐free survival (DFS) was defined as the interval from the date of the first CR to the date of the first relapse or the date of death during the period of the first CR. Patients who underwent allo‐SCT were censored. The χ2 test was used for comparison of binary variables and the Mann–Whitney U‐test was used for comparison of continuous variables. The OS and DFS were estimated using the Kaplan–Meier method and compared using the log‐rank test. The Cox proportional hazards regression model was used for multivariate analysis of prognostic factors. All calculations were performed using the SAS software package (SAS Institute Inc., Cary, NC, USA) and P < 0.05 was considered statistically significant.
Results
Baseline characteristics
Of the 434 AML patients, 260 patients were men and 174 were women, and the median age was 57 years (range, 15–89 years). The patients' baseline characteristics are shown in Table 1. Of the 434 patients, 45 (10.4%) had GS at the time of diagnosis of AML, and among those with GS only one was diagnosed as isolated GS. The patients in the GS group were significantly younger (GS: median age, 46 years; range, 15–80 years vs non‐GS: median age, 58 years; range, 15–89 years; P < 0.001) and had significantly higher white blood cell (WBC) counts (GS: median, 27.1 × 109/L vs non‐GS: 10.2 × 109/L; P = 0.03) than those for the non‐GS group. In contrast, no significant differences were observed between the GS and non‐GS groups in gender or lactate dehydrogenase levels.
Table 1.
Characteristics and French–American–British (FAB) classifications of 434 consecutive patients diagnosed with acute myeloid leukemia
| GS group | Non‐GS group | P‐value | |
|---|---|---|---|
| Patients, n (%) | 45 (10.4) | 389 (89.6) | |
|
Median age (range) (years) |
46 (15–80) | 58 (15–89) | <0.001 |
|
No. men/no. women |
31/14 | 229/160 | 0.194 |
|
Median WBC (range) (×109/L) |
27.1 (0.8–507.5) | 10.2 (0.3–561.4) | 0.030 |
|
Median LDH (range) (IU/L) |
730 (128–5106) | 518 (91–6041) | 0.500 |
| FAB classification | |||
| M0 | 2 (5.1) | 16 (4.3) | 0.799 |
| M1 | 7 (17.9) | 55 (14.6) | 0.580 |
| M2 | 10 (25.6) | 157 (41.8) | 0.051 |
| M3 | 1 (2.6) | 53 (14.1) | 0.042 |
| M4 | 12 (30.8) | 45 (12.0) | 0.001 |
| M5 | 7 (17.9) | 31 (8.2) | 0.045 |
| M6 | 1 (2.6) | 12 (3.2) | 0.830 |
| M7 | 0 (0.0) | 7 (1.9) | 0.390 |
GS, granulocytic sarcoma; LDH, lactate dehydrogenase; WBC, white blood cells.
Sites of GS
As shown in Table 2, the most frequent site of GS was the lymph nodes (25 cases, 55.6%) followed by the central nervous system (four cases, 8.9%) and bones (four cases, 8.9%). Other specific organs involved with GS were the mediastinum (three cases, 6.7%), skin (two cases, 4.4%), testis (two cases, 4.4%), mammary glands (two cases, 4.4%), parotid gland (two cases, 4.4%), uterus (one case, 2.2%), pancreas (one case, 2.2%) and the small intestine (one case, 2.2%). Two patients had GS at two distinct sites concomitantly: bones and mammary glands.
Table 2.
Site of GS in 45 acute myeloid leukemia patients
| Site of GS | n (%) |
|---|---|
| Lymph node | 25 (55.6) |
| Central nervous system | 4 (8.9) |
| Bone | 4 (8.9) |
| Mediastinum | 3 (6.7) |
| Skin | 2 (4.4) |
| Testis | 2 (4.4) |
| Mammary gland | 2 (4.4) |
| Parotid gland | 2 (4.4) |
| Uterus | 1 (2.2) |
| Pancreas | 1 (2.2) |
| Small intestine | 1 (2.2) |
GS, granulocytic sarcoma.
French–American–British classification, surface markers and cytogenetic abnormalities
French–American–British classification of the GS and non‐GS groups is shown in Table 1. Patients in the GS group were categorized more frequently as FAB M4 (P = 0.001) and M5 (P = 0.045) and less frequently as M3 (P = 0.042) than those in the non‐GS group. As indicated by immunophenotypic analysis shown in Table 3, no significant differences between the GS and non‐GS groups were observed regarding the expression of each surface marker, including CD7 and CD56. Additionally, no specific surface marker to each GS site was identified. Likewise, cytogenetic analysis, as shown in Table 3, revealed that no significant differences were found between the GS and non‐GS groups regarding the distribution of cytogenetic subgroups or the frequency of individual cytogenetic abnormalities, including t(8;21), 11q23, inv(16) and complex karyotypes.
Table 3.
Surface markers and cytogenetic abnormalities of 434 acute myeloid leukemia patients
| GS group, n (%) | Non‐GS group, n (%) | P‐value | |
|---|---|---|---|
| Surface marker | |||
| CD13 | 40 (90.9) | 327 (88.6) | 0.648 |
| CD33 | 41 (93.2) | 342 (92.7) | 0.904 |
| CD34 | 27 (67.5) | 200 (58.1) | 0.254 |
| HLA‐DR | 34 (79.1) | 278 (75.5) | 0.609 |
| CD11b | 12 (34.3) | 68 (24.5) | 0.213 |
| CD14 | 5 (12.5) | 22 (6.3) | 0.146 |
| CD15 | 20 (57.1) | 140 (52.4) | 0.600 |
| CD7 | 13 (29.5) | 88 (23.9) | 0.412 |
| CD19 | 4 (9.1) | 40 (17.0) | 0.185 |
| CD56 | 13 (33.3) | 96 (29.2) | 0.591 |
| Cytogenetic abnormalities | |||
| Favorable | 6 (13.3) | 103 (27.2) | 0.051 |
| t(8;21) | 3 (6.7) | 42 (10.7) | 0.379 |
| inv(16) | 2 (4.4) | 10 (2.6) | 0.477 |
| t(15;17) | 1 (2.2) | 47 (12.1) | 0.044 |
| Intermediate | 31 (68.9) | 239 (61.4) | 0.371 |
| Normal | 17 (37.8) | 162 (41.6) | 0.580 |
| 11q23 | 3 (6.7) | 14 (3.6) | 0.324 |
| Other | 11 (24.4) | 63 (16.2) | |
| Adverse | 8 (17.8) | 36 (9.3) | 0.078 |
| Complex | 6 (13.3) | 25 (6.4) | 0.093 |
| Other | 2 (4.4%) | 11 (2.8) | |
GS, granulocytic sarcoma.
Treatment outcomes
Of the 260 patients enrolled in the analysis of treatment outcomes, 162 were men and 98 were women and the median age was 50 years (range, 15–65 years). Of the 260 patients, 40 had GS and 220 did not. There were no significant differences in age, gender, allo‐SCT treatment or cytogenetic abnormalities between the GS and non‐GS groups.
Among the 221 patients who had achieved CR, 165 (63.5%) had achieved CR after the first induction therapy, 39 (15.0%) after the second induction therapy and 17 (6.5%) after more than two courses of induction therapy. Of these 221 patients, 128 (57.9%) had experienced a relapse. Although the CR rates within two courses of induction therapy did not differ significantly between the GS and non‐GS groups (75.0% vs 79.1%, respectively; P = 0.563), the GS group experienced a higher rate of relapse than the non‐GS group (74.2% vs 55.3%, respectively; P = 0.048). After the first relapse, the probabilities of second CR were not significantly different between the GS and non‐GS groups (66.7% vs 62.4%, respectively; P = 0.678). Regarding the sites of relapse, patients in the GS group showed a significantly higher frequency of relapse with GS than those in the non‐GS group (26.1% vs 6.7%, respectively; P = 0.005).
Among 260 patients examined, the 5‐year OS and 5‐year DFS rates were 40.7% and 25.1%, respectively. As shown in Figure 1, the 5‐year OS rate did not differ significantly between the GS and non‐GS groups (39.9% vs 38.7%, respectively; P = 0.749), whereas the 5‐year DFS rate of the GS group was significantly lower than that of the non‐GS group (8.2% vs 25.7%, respectively; P = 0.005). Among five patients who received local irradiation for GS, three achieved continuous CR. Although the other two patients experienced relapses at both bone marrow and extramedullary sites, relapses at the same extramedullary sites were not observed.
Figure 1.
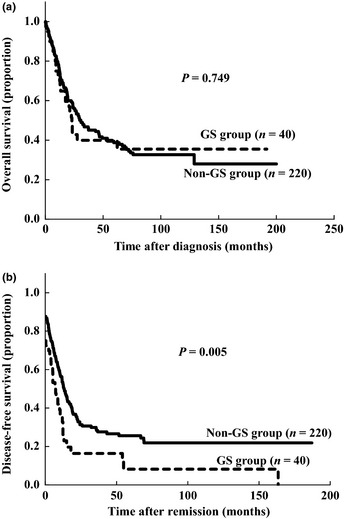
Overall survival rates (a) and disease‐free survival rates (b) of 260 acute myeloid leukemia patients with or without granulocytic sarcoma (GS) whose outcomes were examined.
Impact of allo‐SCT
Of the 40 patients in the GS group in the present study, 19 had undergone allo‐SCT using myeloablative conditioning regimens that included total body irradiation. Of these 19 patients, four received allo‐SCT from a related human leukocyte antigen (HLA)‐matched donor, 11 from an unrelated HLA‐matched donor and four from cord blood. Regarding disease status, 10 patients had been in CR at the time of allo‐SCT. Although the patients who received allo‐SCT were significantly younger than those who did not (median 29 years vs 53 years, respectively; P < 0.001), the 5‐year OS rate after diagnosis of the patients treated with allo‐SCT was significantly higher than that with chemotherapy alone (52.6% vs 20.8%, respectively; P = 0.029) (Fig. 2a). Furthermore, of the 10 patients who had undergone allo‐SCT while in CR, including second and third CR, nine remained in continuous CR at the time of analysis, whereas of the nine patients who had undergone allo‐SCT in non‐CR only one was a DFS.
Figure 2.
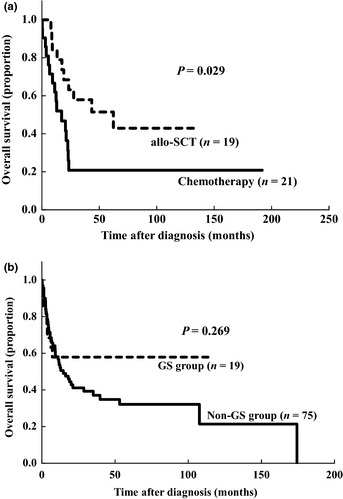
Impact of allogeneic stem cell transplantation. Overall survival rates of 40 acute myeloid leukemia patients with granulocytic sarcoma (GS) treated with or without allogeneic stem cell transplantation (allo‐SCT) (a). Overall survival rates of 94 patients with or without GS who had undergone allo‐SCT (b).
Of the 220 patients in the non‐GS group, 75 had undergone allo‐SCT. Of all 94 patients who had undergone allo‐SCT, those in the GS group were significantly younger than those in the non‐GS group (median age, 29 years vs 49 years, respectively; P = 0.013), but the conditioning regimen, the stem cell source and disease status were not significantly different between the groups (data not shown). Moreover, as shown in Figure 2b, the 5‐year OS rate after allo‐SCT was not significantly different between the GS and non‐GS groups (52.6% vs 32.1%, respectively; P = 0.269).
Multivariate analysis
Although multivariate analysis of OS revealed age and cytogenetic risk groups but not GS as independent prognostic factors, GS was an independent prognostic factor for DFS in addition to age and cytogenetic risk groups (Table 4). These results indicate that the presence of GS was predictive for DFS but not for OS.
Table 4.
Multivariate analysis of OS and DFS in 260 acute myeloid leukemia patients examined for treatment outcomes
| OS | DFS | |||
|---|---|---|---|---|
| P‐value | HR (95% CI) | P‐value | HR (95% CI) | |
| GS vs non‐GS | 0.651 | 1.11 (0.70–1.76) | 0.008 | 1.72 (1.16–2.57) |
| Age (≤50 vs > 50) (years) | 0.007 | 1.60 (1.13–2.25) | 0.433 | 1.14 (0.83–1.56) |
| Sex | 0.229 | 1.23 (0.88–1.71) | 0.363 | 1.16 (0.84–1.59) |
| WBC (≤20 000 vs > 20 000) | 0.079 | 0.74 (0.53–1.04) | 0.683 | 0.94 (0.68–1.29) |
| Cytogenetic subgroups | <0.001 | 2.19 (1.58–3.02) | <0.001 | 2.00 (1.46–2.73) |
| FAB (M4, or M5 vs others) | 0.877 | 0.97 (0.66–1.43) | 0.900 | 1.02 (0.72–1.45) |
CI, confidence interval; DFS, disease‐free survival; FAB, French–American–British; GS, granulocytic sarcoma; HR, hazard ratio; OS, overall survival; WBC, white blood cells.
Discussion
Most research on GS has primarily focused on examining distinct subpopulations of AML patients, such as young adults or patients with t(8;21).18, 19 To fill the research need for broad examination of a larger AML patient population, the present study conducted a large‐scale analysis of adult patients with AML to explore the clinical significance of GS among this general population.
The present study confirmed that GS develops predominantly in younger patients and is associated with higher WBC counts. It also showed that patients in the GS group presented with higher frequencies of FAB M4 and M5 morphology, which is consistent with previous reports.12, 13 In contrast, GS occurred less frequently in M3 AML. These findings indicate that AML with accompanying GS might be a distinctive subgroup among adult AML patients from a biological aspect.
A close association between the occurrence of GS and the expressions of CD7 or CD56 has been described and CD56 is an adhesion molecule that is considered to play a role in the formation of GS.13, 14 However, no specific surface makers associated with GS were identified.
Moreover, we did not find any cytogenetic abnormalities specific for AML with GS. In a study of 53 AML M2 patients, Tallman et al.17 reported a high incidence of GS among patients carrying t(8;21), finding that three of eight patients carrying t(8;21) developed GS (38%), whereas none of the others develop GS. In a similar study, Swirsky et al.18 reported that seven of 33 patients with t(8;21) developed GS (21%). Several investigators have reported an association between inv16 and the development of GS in the abdominal cavity, especially in the small intestine.19, 20 However, only one patient in the present study developed GS in the pancreas and no patient developed GS in the small intestine.
Prevalence of GS at a time of AML diagnosis was 10.4% in this group of patients and 10.4% is slightly higher than that reported previously.1, 2 Lymph nodes were the most frequently affected organ, by 53.2%, which is higher than the rates in previous reports.4 These higher rates might be explained by the criteria used to define GS, which was based on the physician's examination, imaging studies and the response to chemotherapy, but did not require pathological confirmation. Therefore, we might have overestimated the incidence of GS, especially in the lymph nodes. However, patients with enlarged lymph nodes as the only site of GS showed similar clinical presentations and treatment outcomes to other patients in the GS group (data not shown). As it is often difficult to obtain pathological samples at the time of AML diagnosis because of bleeding and the risk of infection, we are confident that our criteria for GS is acceptable in clinical practice.
The most important implication in the present study is that if treated only with chemotherapy the adult AML patients with GS are at a higher risk of relapse than those without GS. Although the CR rate did not differ significantly between the GS and non‐GS groups, the relapse rate of the GS group was significantly higher (74.2% vs 55.3%, respectively; P = 0.048) and the 5‐year DFS was significantly lower (8.2% vs 25.7%, respectively; P = 0.005) than those of the non‐GS group. The implication of these findings, that GS is an independent predictive factor for the DFS period, was confirmed using multivariate analysis.
In addition, the difference in the pattern of relapse could be identified between both groups. Patients in the GS group was more likely to experience relapses with GS, so vigilance is required regarding development of extramedullary relapse, especially during follow up of patients with GS at the time of diagnosis.
To describe the explanation for comparable OS despite lower DFS in the GS group, we examined the impact of allo‐SCT. Although patients with GS who underwent allo‐SCT were in a significantly younger cohort and selection bias existed, a favorable prognosis for them compared with patients treated with chemotherapy alone was shown. Furthermore, comparable survival rates after allo‐SCT between the GS and non‐GS groups indicated that GS was not a negative prognostic factor after allo‐SCT. Of course further investigation is essential, but a potential reason for the discrepancy between the OS and DFS rates is that the relapsed patients in the GS group were salvaged with allo‐SCT.
In a study of 51 patients (37 adults and 14 children) with GS who had undergone allo‐SCT, Chevallier et al. found that the 5‐year OS and DFS rates were 47% and 36%, respectively.30 These results led them to propose that performing allo‐SCT early in the disease course of AML might decrease the risk of adverse outcomes. Regarding the 19 patients with GS who had undergone allo‐SCT, whose 5‐year OS rate was 57%, the findings of the present study provide support for their proposal. In addition, the patients who received an allo‐SCT during CR achieved an excellent prognosis.
In conclusion, the present study showed that adult patients diagnosed with AML and concomitant GS were younger and presented with higher WBC counts and were more likely to present with FAB M4 and M5 morphology and less likely to present with M3 morphology than patients diagnosed only with AML. Particularly significant, the current study found that the presence of GS adversely affected the relapse and DFS rates if treated with chemotherapy alone, indicating the potential importance of using detection of GS as a means of identifying a high‐risk subset of adult patients with AML.
Disclosure Statement
The authors declare no competing financial interests.
Acknowledgments
The authors thank the hematologists at the three participating hospitals for providing access to the clinical data.
References
- 1. Liu PI, Ishimaru T, McGregor DH, Yamamoto T, Steer A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima‐Nagasaki 1949–1969. Cancer 1973; 31: 948–55. [DOI] [PubMed] [Google Scholar]
- 2. Muss HB, Moloney WC. Chloroma and other myeloblastic tumors. Blood 1973; 42: 721–8. [PubMed] [Google Scholar]
- 3. Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol 1995; 13: 1800–16. [DOI] [PubMed] [Google Scholar]
- 4. Pileri SA, Ascani S, Cox MC et al Myeloid sarcoma: clinico‐pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007; 21: 340–50. [DOI] [PubMed] [Google Scholar]
- 5. Struhal W, Oberndorfer S, Lahrmann H et al Myeloid sarcoma in the central nervous system: case report and review of the literature. Acta Clin Croat 2008; 47: 19–24. [PubMed] [Google Scholar]
- 6. Widhalm G, Dietrich W, Müllauer L et al Myeloid sarcoma with multiple lesions of the central nervous system in a patient without leukemia. Case report. J Neurosurg 2006; 105: 916–9. [DOI] [PubMed] [Google Scholar]
- 7. Kumar J, Seith A, Bakhshi S, Kumar R, Kumar A, Sen S. Isolated granulocytic sarcoma of the orbit. Eur J Haematol 2007; 78: 456. [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharjee K, Bhattacharjee H, Das D et al Chloroma of the orbit in a non‐leukemic adult: a case report. Orbit 2003; 22: 293–7. [DOI] [PubMed] [Google Scholar]
- 9. Kamble R, Kochupillai V, Sharma A et al Granulocytic sarcoma of uterine cervix as presentation of acute myeloid leukemia: a case report and review of literature. J Obstet Gynaecol Res 1997; 23: 261–6. [DOI] [PubMed] [Google Scholar]
- 10. Eggener SE, Abrahams A, Keeler TC. Granulocytic sarcoma of the testis. Urology 2004; 63: 584–5. [DOI] [PubMed] [Google Scholar]
- 11. van der Weide M, Imandt LM, Langenhuijsen MM. Motility of leukaemic cells in acute leukaemia related to tumour mass, maturation and FAB classification. Acta Haematol 1988; 80: 34–9. [DOI] [PubMed] [Google Scholar]
- 12. Yamazaki M, Komiyama A, Yamazaki T et al Leukaemia cell mobility in childhood acute myeloid leukaemia based on the FAB classification. Scand J Haematol 1985; 35: 481–6. [DOI] [PubMed] [Google Scholar]
- 13. Iizuka Y, Aiso M, Oshimi K et al Myeloblastoma formation in acute myeloid leukemia. Leuk Res 1992; 16: 665–71. [DOI] [PubMed] [Google Scholar]
- 14. Seymour JF, Pierce SA, Kantarjian HM, Keating MJ, Estey EH. Investigation of karyotypic, morphologic, and clinical features in patients with acute myeloid leukemia blast cells expressing the neural cell adhesion molecule (CD56). Leukemia 1994; 8: 823–6. [PubMed] [Google Scholar]
- 15. Cross AH, Goorha RM, Nuss R et al Acute myeloid leukemia with t‐lymphoid features: a distinct biologic and clinical entity. Blood 1988; 72: 579–87. [PubMed] [Google Scholar]
- 16. Schwonzen M, Kuehn N, Vetten B, Diehl V, Pfreundschuh M. Phenotyping of acute myelomonocytic (AMMOL) and monocytic leukemia (AMOL): association of T‐cell‐related antigens and skin‐infiltration in AMOL. Leuk Res 1989; 13: 893–8. [DOI] [PubMed] [Google Scholar]
- 17. Tallman MS, Hakimian D, Shaw JM, Lissner GS, Russell EJ, Variakojis D. Granulocytic sarcoma is associated with the 8;21 translocation in acute myeloid leukemia. J Clin Oncol 1993; 11: 690–7. [DOI] [PubMed] [Google Scholar]
- 18. Swirsky DM, Li YS, Mathews JG, Flemans RJ, Rees JK, Hayhoe FG. 8;21 chromosomal translocation in acute granulocytic leukemia: cytogenetical, cytochemical, and clinical features. Br J Haematol 1984; 56: 199–213. [DOI] [PubMed] [Google Scholar]
- 19. Xavier SG, Fagundes EM, Hassan R et al Granulocytic sarcoma of the small intestine with CBFbeta/MYH11 fusion gene: report of an aleukaemic case and review of the literature. Leuk Res 2003; 27: 1063–6. [DOI] [PubMed] [Google Scholar]
- 20. Fujieda A, Nishii K, Tamaru T et al Granulocytic sarcoma of mesentery in acute myeloid leukemia with CBFB/MYH11 fusion gene but not inv(16) chromosome: case report and review of literature. Leuk Res 2006; 30: 1053–7. [DOI] [PubMed] [Google Scholar]
- 21. Byrd JC, Weiss RB, Arthur DC et al Extramedullary leukemia adversely affects hematologic complete remission rate and overall survival in patients with t(8;21)(q22;q22): results from Cancer and Leukemia Group B 8461. J Clin Oncol 1997; 15: 466–75. [DOI] [PubMed] [Google Scholar]
- 22. Felice MS, Zubizarreta PA, Alfaro EM et al Good outcome of children with acute myeloid leukemia and t(8;21)(q22;q22), even when associated with granulocytic sarcoma: a report from a single institution in Argentina. Cancer 2000; 88: 1939–44. [PubMed] [Google Scholar]
- 23. Bennett JM, Catovsky D, Daniel MT et al Proposals for the classification of the acute leukaemias. French‐American‐British (FAB) Co‐Operative Group. Br J Haematol 1976; 33: 451–8. [DOI] [PubMed] [Google Scholar]
- 24. Swerdlow SH, Campo E, Harris NL et al WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC, 2008. [Google Scholar]
- 25. Grimwade D, Walker H, Oliver F et al The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood 1998; 92: 2322–33. [PubMed] [Google Scholar]
- 26. Miyawaki S, Tanimoto M, Kobayashi T et al No beneficial effect from addition of etoposide to daunorubicin, cytarabine, and 6‐mercaptopurine in individualized induction therapy of adult acute myeloid leukemia: the JALSG‐AML92 Study. Japan Adult Leukemia Study Group. Int J Hematol 1999; 70: 97–104. [PubMed] [Google Scholar]
- 27. Ohtake S, Miyawaki S, Kiyoi H et al Randomized trial of response‐oriented individualized versus fixed‐schedule induction chemotherapy with idarubicin and cytarabine in adult acute myeloid leukemia: the JALSG AML95 Study. Int J Hematol 2010; 91: 276–83. [DOI] [PubMed] [Google Scholar]
- 28. Sakamaki H, Miyawaki S, Ohtake S et al Allogeneic stem cell transplantation versus chemotherapy as post‐remission therapy for intermediate or poor risk adult acute myeloid leukemia: results of the JALSG AML97 Study. Int J Hematol 2010; 91: 284–92. [DOI] [PubMed] [Google Scholar]
- 29. Ohtake S, Miyawaki S, Fujita H et al Randomized study of induction therapy comparing standard‐dose idarubicin with high‐dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: JALSG AML201 Study. Blood 2011; 117: 2358–65. [DOI] [PubMed] [Google Scholar]
- 30. Chevallier P, Mohty M, Lioure B et al Allogeneic hematopoietic stem‐cell transplantation for myeloid sarcoma: a retrospective study from the SFGM‐TC. J Clin Oncol 2008; 26: 4940–3. [DOI] [PubMed] [Google Scholar]


