Abstract
Our previous studies have shown that epithelial–mesenchymal transition (EMT) may be involved in the vasculogenic mimicry (VM) formation in hepatocellular carcinoma. Here, we hypothesize that zinc finger E‐box binding homeobox 1 (ZEB1) promotes VM formation in colorectal carcinoma (CRC) by inducing EMT. We identified VM in 39 (19.2%) out of 203 CRC patients. The presence of VM was associated with aggressive biological behavior and was an unfavorable prognostic indicator. By immunohistochemical analysis, we found that the VM‐positive CRC samples showed increased ZEB1 expression compared with the VM‐negative samples and the ZEB1 expression occurred concomitantly with features of EMT. In vitro, knockdown of ZEB1 in poorly differentiated HCT116 CRC cells destroyed the vessel‐like structures in the 3‐D culture, a property associated with VM formation. Knockdown of ZEB1 resulted in restoration of epithelial phenotypes and significantly inhibited the ability to migrate and invade. In addition, ZEB1 underexpression decreased the expression of vascular endothelial (VE)‐cadherin and Flk‐1, which are characteristics of endothelial cells. Taken together, our results suggest that ZEB1 can promote VM formation by inducing EMT in CRC and might represent an important target in CRC. (Cancer Sci 2012; 103: 813–820)
Colorectal carcinoma (CRC) is one of the most common causes of cancer‐related deaths worldwide.1 Although CRC diagnosis and treatment have significantly advanced over the past two decades, the death rate of CRC patients has not improved. Thus, advances in the treatment of this disease should come from a deeper understanding of its pathogenesis and biological features.2
Vasculogenic mimicry (VM) describes the unique ability of some tumor cells, particularly aggressive ones, to express multiple endothelial markers and resemble endothelial cell functions.3 VM is associated with the invasive and metastatic potential of tumor cells, as well as poor clinical outcomes.4, 5 Current anti‐angiogenic treatments cannot inhibit the VM process and can even potentially facilitate VM formation;6 therefore, such treatment strategies need to be reconsidered. Despite its clinical importance, the cellular and molecular events underlying the VM mechanism are not well understood. Current studies focus on the endothelial transdifferentiation of VM‐channel‐forming tumor cells,7 the lack of coagulation activity of VM channels, and certain tumor microenvironments.8 Although many molecules, including VE‐cadherin,9 Ephrin A2,10 matrix metalloproteinase,11 laminin,12 PI3K,13 TFPI2,8 FAK14 and CD147,15 are shown to contribute to VM formation, we still need to investigate the exact mechanisms underlying VM to further define potential targets.
Epithelial–mesenchymal transition (EMT) is a dynamic biological process where polarized epithelial cells lose their epithelial characteristics and acquire properties typical of mesenchymal cells.16 Epithelial tumor cells capable of VM imitate endothelial functions and display some endothelial phenotypes of mesenchymal cells. Therefore, VM formation in epithelial cancer is assumed to be associated with the EMT process, and the regulators that contribute to EMT may also modulate VM formation. Studies carried out in our laboratory have shown that Twist1, an EMT inducer, is closely associated with tumor cell plasticity to VM patterns in hepatocellular carcinoma (HCC).17 As a crucial EMT inducer, zinc finger E‐box binding homeobox 1 (ZEB1) not only promotes malignant progression of various epithelial tumors,18, 19, 20, 21 but is also necessary for the tumor‐initiating capacity of pancreatic cancer and CRC cells.22, 23
In the present study, we found that ZEB1 was increased in VM‐positive CRC samples and that its expression concomitantly occurred with EMT features. The ZEB1 knockdown in highly aggressive CRC cells inhibited VM formation and decreased VE‐cadherin and Flk‐1 expressions in vitro. These are all characteristics of endothelial cells.
Materials and Methods
Patient samples
The current study consisted of 203 CRC patients who had not undergone chemotherapy or radiotherapy prior to surgery between 2000 and 2004. Formalin‐fixed and paraffin‐embedded samples from the patients were obtained from the Department of Pathology, Tianjin Cancer Institute and Hospital, Tianjin Medical University, China. The sections from all cases were reviewed by two senior pathologists. Clinical and pathological variables were determined using well‐established criteria (see Table 1). The use of the tissue samples in this study was approved by the Institutional Research Committee.
Table 1.
Correlation between vasculogenic mimicry (VM) and clinicopathologic characteristics of patients with colorectal carcinoma
| Variable | Total | Tissue samples | χ2 | P | |
|---|---|---|---|---|---|
| VM | Non‐VM | ||||
| Age | |||||
| <45 | 25 | 7 | 18 | 1.419 | 0.277 |
| ≥45 | 178 | 32 | 146 | ||
| Sex | |||||
| Male | 95 | 19 | 76 | 0.71 | 0.859 |
| Female | 108 | 20 | 88 | ||
| Tumor size (cm) | |||||
| ≥10 | 21 | 6 | 15 | 1.322 | 0.250 |
| <10 | 182 | 33 | 149 | ||
| Histological differentiation | |||||
| Well differentiated | 14 | 1 | 13 | 65.377 | 0.000a |
| Moderately differentiated | 109 | 8 | 101 | ||
| Poorly differentiated | 53 | 30 | 23 | ||
| Mucinous carcinoma | 27 | 0 | 27 | ||
| TNM stage | |||||
| I | 10 | 0 | 9 | 22.453 | 0.000a |
| II | 138 | 16 | 110 | ||
| III | 57 | 16 | 41 | ||
| IV | 12 | 7 | 4 | ||
| Metastasis and recurrence | |||||
| Present | 77 | 24 | 53 | 14.099 | 0.000a |
| Absent | 126 | 15 | 111 | ||
| ZEB1 expression | |||||
| Positive | 58 | 17 | 41 | 5.335 | 0.029a |
| Negative | 145 | 22 | 123 | ||
Significantly different.
Antibodies and reagents
The primary antibodies were rabbit anti‐ZEB1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti‐CD34 (Santa Cruz Biotechnology), mouse anti‐E‐cadherin (BD Biosciences, San Deigo, CA, USA), rabbit anti‐claudin‐4 (Santa Cruz Biotechnology), rabbit anti‐vimentin (Epitomics, Burlingame, CA, USA), rabbit anti‐Fibronectin (Abcam, Cambridge, UK), rabbit anti‐VE‐cadherin (Abcam), rabbit anti‐Flk‐1 (Santa Cruz Biotechnology), rabbit anti‐Flt‐1 (Santa Cruz Biotechnology) and mouse anti‐β‐actin (Santa Cruz Biotechnology). Transwell cell inserts were purchased from Costar. Matrigel was purchased from BD Biosciences.
Immunohistochemistry and CD34/periodic acid Schiff double‐staining
Formalin‐fixed and paraffin‐embedded sections were deparaffinized by sequential washing with xylene, graded ethanol and PBS. The staining system was PicTure PV6000 (Zhongshan Chemical, Beijing, China). PBS was used as the primary antibody for the negative controls, whereas samples known to express ZEB1, E‐cadherin and vimentin served as the positive controls. After immunohistochemical (IHC) staining for CD34, the sections were washed with running water for 5 min and incubated with periodic acid Schiff (PAS) for 15 min. Finally, all of the sections were counterstained with hematoxylin, dehydrated and mounted. Normal human stomach mucous membrane was the positive control for PAS staining.
Cell culture and 3‐D culture
Human CRC cell lines, HT‐29, HCT116 and SW480, and human umbilical vein endothelial cells (HUVEC) were purchased from the Cell Resource Center, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences/Peking Union Medical College (Beijing, China). The HT29 cells were cultured in a DMEM/F12 medium supplemented with 5% FCS at 37°C and 5% CO2. The HCT116 and the SW480 cells were cultured in IMDM, supplemented with 10% FCS at 37°C and 5% CO2. The HUVEC were cultured in DMEM/H, supplemented with 10% FCS at 37°C and 5% CO2. The FCS and all cell cultures were purchased from Hyclone.
Vasculogenic mimicry formation was tested using 3‐D culture containing Matrigel in vitro. Twenty‐four wells of culture plates were coated with Matrigel (0.1 mL/well). The cells were trypsinized and suspended in the complete medium at 2.5 × 105 cells/mL, plated onto the surface of Matrigel at 1 mL/well, and incubated at 37°C for 48 h. The numbers of tube‐like structures were measured a in high‐power field.
Small RNA interfering and transfection
HCT116 cells were transfected with validated small interfering RNA (siRNA) ZEB1 or scrambled control ZEB1 siRNA, which were purchased from Genechem Technologies (Shanghai, China). The target sequence (5′‐CCUCUCUGAAAGAACACAU‐3′) was used to downregulate ZEB1 in vitro. A nonsilencing siRNA sequence (target sequence 5′‐UUCUCCGAACGUGUCACGU‐3′) was used as a negative control. Following the manufacturer's protocol, 200 nmol/L siRNA for either ZEB1 or control was transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Western blot analysis
Colorectal carcinoma cells were washed with PBS and the whole‐cell lysates were prepared using modified radioimmunoprecipitation assay buffer at 4°C for 15 min. Proteins were resolved by SDS‐PAGE and analyzed by Western blot using PVDF membranes (Millipore, Temecula, CA, USA). Subsequently, the membranes were incubated overnight with primary antibodies (ZEB1 1:100, E‐cadherin 1:1000, claudin‐4 1:200, vimentin 1:2000, fibronectin 1:500, VE‐cadherin 1:1000, Flt‐1 1:200 and Flk‐1 1:200) at 4°C. Blots were washed in TBS containing 0.1% Tween 20 and labeled with goat anti‐mouse IgG‐HRP (1:5000; Santa Cruz Biotechnology) or goat anti‐rabbit IgG‐HRP (1:5000; Santa Cruz Biotechnology). Equal loading of samples was confirmed by probing the membranes with β‐actin antibody.
Wound healing assay
HCT116 cells were plated in 35‐mm dishes to form a monolayer a day before the assay. A uniform scratch was made down the center of the well using a micropipette tip the following day, before washing with PBS. The cells were incubated at 37°C with 5% CO2. Cell motility was assessed by measuring the speed of wound closure at intervals.
To determine the role of ZEB1 on migration, HCT116 cells were transfected with ZEB1 siRNA or control oligos for 48 h before the induction of the scratch. Each experiment was performed in triplicate.
Matrigel invasion chamber assay
HCT116 cell invasion assay was performed using transwell cell culture inserts with 8‐μm membrane pores. Matrigel, with a final concentration of 1.5 mg/mL, was added to the upper surface of each transwell chamber filter and incubated at 37°C with 5% CO2 for 1 h. The cells were trypsinized, and 200 μL of cell suspension (5 × 105 cells/mL) contained in a serum‐free culture medium was added to the upper chamber. The culture medium at 300 μL, supplemented with 20% FBS, was added to the lower chamber. After incubation at 37°C with 5% CO2 for 48 h, the passed cells were fixed with formaldehyde and stained with 0.5% crystal violet. The number of invading cells was counted using an inverted light microscope (Olympus, Tokyo, Japan).
Immunofluorescence and confocal microsocopy
HCT116 cells were cultured on sterile glass cover slips 1 day prior to staining. Cells were fixed with 10% formalin in PBS for 10 min, quenched with 50 mM NH4Cl for 10 min and 0.2% triton for 10 min, and then blocked with 3% BSA for 1 h. The slips were incubated overnight with the primary antibodies (E‐cadherin 1:50, claudin‐4 1:50, vimentin 1:250 and fibronectin 1:50) at 4°C. Slides were washed in PBS and labeled with specific secondary antibodies for 1 h in the dark. The cells were counterstained with DAPI and then viewed with fluorescent microscopy (Olympus).
Statistical analysis
SPSS was used for all statistical analyses. P < 0.05 was considered statistically significant. The statistical significance of the relationships between VM, ZEB1 and clinicopathological parameters were assessed using Fisher's exact and χ2 tests. Survival analysis was carried out according to Kaplan–Meier. Differences in survival curves were assessed using the log rank test. The data for scratch, invasion and VM formation assays were analyzed using one‐way anova.
Results
Vasculogenic mimicry (VM) was detected in high‐grade colorectal carcinoma tissues and related to poor prognosis
Using HE staining (Fig. 1A,B) and CD34/PAS double‐staining (Fig. 1C,D), VM was distinguished by channels lined with tumor cells instead of shuttle‐like endothelial cells. These tumor cells were negative for CD34 and the base membrane‐like structure between red blood cells and tumor cells was positive for PAS. Cells lining VM channels were negative for CD34, confirming that cells around the channels were not composed of endothelium. Necrosis and infiltrating inflammatory cells around the channels were not observed, and red blood cells were found inside the channels.
Figure 1.
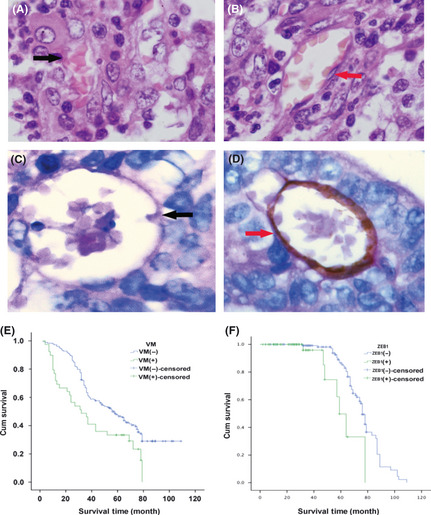
(A) Vasculogenic mimicry (VM) channel (black arrow) lined with tumor cells and containing red blood cells (HE staining, ×400). (B) Endothelial‐dependent vessel lined with shuttle‐like endothelium cells (red arrow) (HE staining, ×400). (C) The VM channel formed by tumor cells was negative for CD34 and the base membrane‐like structure between red blood cells and tumor cells was positive for periodic acid Schiff (PAS) (black arrow) (CD34/PAS double‐staining, ×400). (D) The endothelial‐dependent vessel was both positive for CD34 and PAS (red arrows) (CD34/PAS double‐staining, ×400). (E) Kaplan–Meier survival analysis showing that the VM‐positive patients have shorter survival time than VM‐negative patients. (F) Kaplan–Meier survival analysis showing the zinc finger E‐box binding homeobox 1 (ZEB1)‐positive patients have shorter survival time than ZEB1‐negative patients.
Vasculogenic mimicry (VM) was detected in 39 (19.2%) out of 203 CRC cases. Vasculogenic mimicry (VM) was commonly characterized by histological differentiation, metastasis or recurrence, and TNM stages. The frequency of VM was significantly higher in poorly differentiated CRC (30/53, 57%) than in well (1/14, 7%) and moderately (8/109, 7%) differentiated CRC. It was also significantly higher in advanced‐stage carcinomas (TNM stages III and IV, 23/69, 33.3%) than in early‐stage carcinomas (TNM stages I and II, 16/148, 10.8%). However, no VM was observed in mucinous adenocarcinoma. A total of 77 (38%) CRC patients experienced metastasis or recurrence. The VM‐positive group had a higher rate of metastasis or recurrence (24/39, 61.5%) than the VM‐negative group (53/164, 32.3%). However, no correlation was found between the presence of VM and patient characteristics, such as age, gender and tumor size. The clinical and pathological features of VM in all 203 CRC cases are summarized in Table 1.
To determine the clinical significance of VM, we examined the clinical outcomes associated with VM formation. The total survival period of patients with VM was found to be significantly shorter than that of patients without VM (P < 0.05). The average survival period for VM‐positive patients was 32.5 months, whereas that for VM‐negative patients was 56.49 months (Fig. 1E).
Vasculogenic mimicry (VM) was associated with ZEB1 expression and epithelial–mesenchymal transition marker
The expressions of ZEB1 and EMT markers were examined using IHC to investigate whether a differential expression exists between the VM‐positive and VM‐negative groups of CRC tissue samples. The ZEB1‐positive samples were identified as those with brown stains in the nuclei of neoplastic epithelial cells and non‐stromal cells (Fig. 2A). Statistical analysis revealed a significant difference in the ZEB1 expressions of the VM‐positive (17/39, 43.6%) and VM‐negative groups (41/164, 25%) (P < 0.05) (Table 1).
Figure 2.
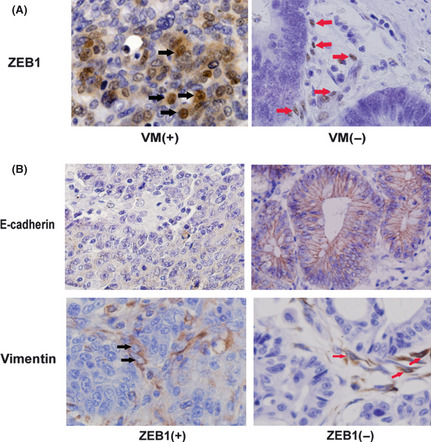
Expression of zinc finger E‐box binding homeobox 1 (ZEB1) and epithelial–mesenchymal transition (EMT) markers in colorectal carcinoma (CRC) samples. (A) The ZEB1 expression (black arrows) in the vasculogenic mimicry (VM)‐positive group was higher than in the VM‐negative group. In contrast, except for some stromal cells (red arrows), tumor cells in the VM‐negative group showed almost no ZEB1 expression (immunohistochemical staining, ×400). (B) E‐cadherin expression was lower in the ZEB1‐positive CRC tissue samples than in the ZEB1‐negative ones. Some tumor cells showed vimentin expression in ZEB1‐positive CRC samples (black arrow), whereas the tumor cells showed no vimentin expression and only some stromal cells showed vimentin expression (red arrow) in ZEB1‐negative CRC samples (IHC staining, ×400).
Furthermore, Kaplan–Meier survival analysis showed that ZEB1 had an effect similar to VM on the survival of CRC patients. The total survival time for patients in the ZEB1‐positive group was significantly shorter than for those in the ZEB1‐negative group (P < 0.05). The average survival time for ZEB1‐positive patients was 30.8 months, whereas the average survival time for ZEB1‐negative patients was 67.32 months (Fig. 1F).
To assess the relationship between ZEB1 and EMT in CRC, we determined the expression of EMT‐associated markers, including E‐cadherin and vimentin, in the ZEB1‐negative and the ZEB1‐positive groups. E‐cadherin is usually found in the exclusive membranes of epithelial cells, whereas vimentin is usually expressed in the cytoplasms of stromal cells. However, the down‐expression of E‐cadherin was more frequently detected in the ZEB1‐positive group (18.97%) than in the ZEB1‐negative group (4.14%). Meanwhile, the expression of vimentin was found to be higher in some cancer cells in the ZEB1‐positive group (18.97%) than in the ZEB1‐negative group (4.83%) (Fig. 2B, Table 2).
Table 2.
Relationship between expression of zinc finger E‐box binding homeobox 1 (ZEB1), E‐cadherin and vimentin
| Variable | Total | ZEB1 expression | χ2 | P | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| E‐cadherin | |||||
| Positive | 186 | 47 | 139 | 11.871 | 0.001a |
| Negative | 17 | 11 | 6 | ||
| Vimentin | |||||
| Positive | 18 | 11 | 7 | 10.248 | 0.001a |
| Negative | 185 | 47 | 138 | ||
Significantly different.
In addition, the relationship between VM formation and the expressions of E‐cadherin and vimentin was analyzed. We found that the VM‐positive tissue sections showed less E‐cadherin expression than the VM‐negative sections (33.33 vs 2.44%, P < 0.05). In contrast, vimentin showed higher expression in the VM‐positive sections than in the VM‐negative sections (35.90 vs 2.44%, P < 0.05). The results indicate that VM is associated with EMT in CRC (Table 3).
Table 3.
Relationship between expression of vasculogenic mimicry (VM), E‐cadherin and vimentin
| Variable | Total | VM | χ2 | P | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| E‐cadherin | |||||
| Positive | 186 | 26 | 160 | 35.269 | 0.000a |
| Negative | 17 | 13 | 4 | ||
| Vimentin | |||||
| Positive | 18 | 14 | 4 | 39.606 | 0.000a |
| Negative | 185 | 25 | 160 | ||
Significantly different.
Knockdown of ZEB1 inhibited the VM formation of HCT116 cells in vitro and induced decreased expression of VE‐cadherin and Flk‐1
We initially chose three different differentiated CRC cell lines, namely, HT29 (well differentiated), SW480 (moderately differentiated) and HCT116 (poorly differentiated), and used a well‐established in vitro 3‐D culture model to investigate VM formation. Among these cell lines, only HCT116 cells could form typical vessel‐like tubes. In contrast, the SW480 and HT29 cells, which were less invasive, did not form vascular networks in vitro under the same 3‐D culture conditions (Fig. 3A). Thus, we used HCT116 cells to investigate the effect of ZEB1 on EMT and VM formation.
Figure 3.
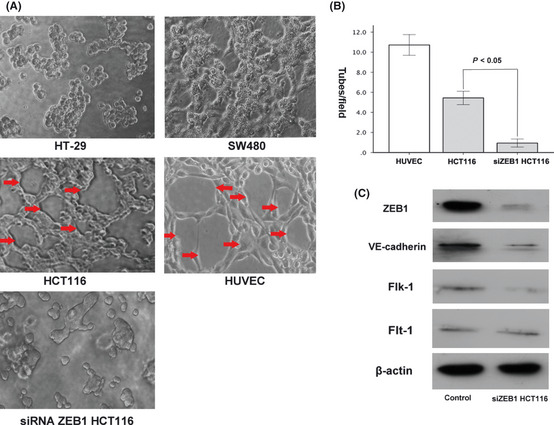
Effect of zinc finger E‐box binding homeobox 1 (ZEB1) on the vasculogenic mimicry (VM) formation of three colorectal carcinoma (CRC) cell lines in Matrigel. (A) HCT116 cells can form typical tube‐like structures in the 3‐D culture (red arrow), whereas HT‐29 and SW480 cannot. The typical tube‐like structures formed by human umbilical vein endothelial cells (HUVEC) were regarded as positive control. After ZEB1 siRNA treatment, VM formation was significantly reduced in HCT116 cells. (B) Quantitative analysis of the mean number of tube‐like structures formed by HUVEC, control HCT116 and siZEB1 HCT116 cells in 3‐D culture. (C) Western blot analysis showed that the ZEB1 knockdown in HCT116 cells results in reduced VE‐cadherin and Flk‐1 expression. No significant change in Flt‐1 expression was observed.
The functional role of ZEB1 in the formation of vessel‐like tubes in vitro was determined by transiently transfecting HCT116 cells with ZEB1 siRNA. The decreased ZEB1 expression was confirmed using Western blot analysis (Fig. 3C). HCT116 cells formed a few tubular structures (5.44 ± 1.44/high power field), whereas siZEB1 HCT116 cells formed hardly any similar structures (0.94 ± 0.8/high power field), indicating that ZEB1 is a requirement for VM formation in HCT116 cells (Fig. 3A,B).
Compared with the controls, the ZEB1‐underexpressed HCT116 cells showed reduced VE‐cadherin and Flk‐1 expression. However, we did not observe a significant change in the Flt‐1 expression in the same cells (Fig. 3C).
Knockdown of ZEB1 resulted in restoration of epithelial phenotypes and inhibited epithelial–mesenchymal transition in HCT116 cells
We also found that the ZEB1 knockdown in HCT116 cells led to significant morphologic changes resembling the restoration of epithelial phenotypes (the cells became cobblestone‐like with clear cell–cell contacts) (Fig. 4A). Immunofluorescence showed that the HCT116 cells with ZEB1 siRNA had significantly increased epithelial marker (E‐cadherin, claudin‐4) and decreased mesenchymal marker (vimentin and fibronectin) expressions, respectively, compared with those in the controls (Fig. 4B).
Figure 4.
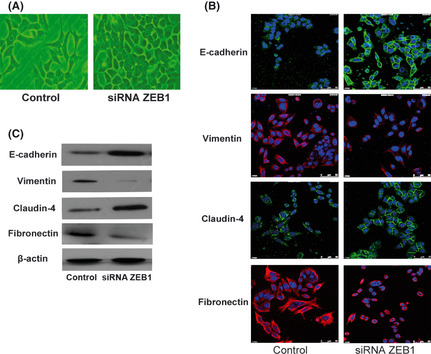
The zinc finger E‐box binding homeobox 1 (ZEB1) knockdown resulted in the restoration of epithelial phenotype in HCT116 cells. (A) The ZEB1 knockdown in HCT116 cells resulted in a morphological change from a fibroblastic to an epithelial appearance. (B) Immunofluorescence and (C) western blot analysis revealed upregulated epithelial marker (E‐cadherin, claudin‐4) and downregulated mesenchymal marker (vimentin, fibronectin) expression, respectively, after ZEB1 siRNA treatment.
The expressions of the EMT‐associated proteins E‐cadherin, claudin‐4, vimentin and fibronectin were also detected using Western blot analysis, and the results were consistent with those of the immunofluorescence (Fig. 4C).
Knockdown of ZEB1 induced decreased ability of migration and invasion in HCT116 cells in vitro
Based on the effects of ZEB1 siRNA on EMT reversal, the effect of ZEB1 knockdown on the migratory and invasive capabilities of HCT116 cells was examined. As shown in Figure 5(A,B), the migratory ability of the ZEB1‐downregulated HCT116 cells was reduced compared with that of the control (P < 0.05). Thus, ZEB1 is an important positive mediator of cell migration. To determine the role of ZEB1 in cancer cells invasion, we also investigated the ability of HCT116 cells to invade an extracellular matrix. The ZEB1 knockdown caused by siRNA resulted in the reduced ability of the cells to invade through Matrigel, compared with that of the controls (Fig. 5C,D).
Figure 5.
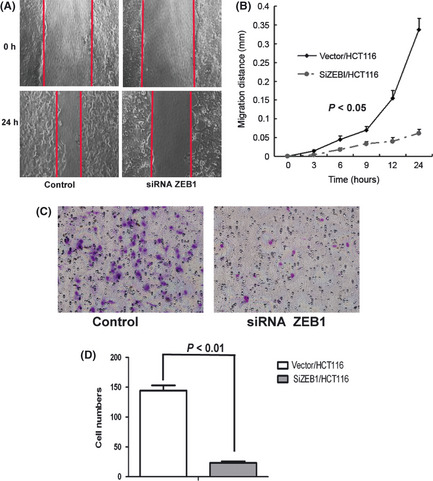
The zinc finger E‐box binding homeobox 1 (ZEB1) knockdown in HCT116 cells resulted in reduced migratory and invasive capabilities. (A) Scratch wounds were made at 0 and 24 h after wounds (×200). (B) Quantitative measurement of wound gaps showed a larger reduction in the cellular motility of ZEB1 siRNA‐transfected HCT116 cells than control cells. (C,D) There were much fewer ZEB1 knockdown HCT116 cells that invaded through Matrigel compared to the control cells (×200).
Discussion
In 1999, Maniotis et al first reported the existence of VM in highly aggressive uveal melanoma.5 In recent years, VM has been identified in other tumors, including breast,24 prostate,25 ovarian26 and liver cancers,27 glioblastoma,28 mesothelial sarcomas and alveolar rhabdomyosarcomas.4 In the current study, the incidence of VM in human CRC (19.2%) is similar to that reported by Coen,29 who detected VM in 23 (19.7%) out of 117 CRC samples. The unique structure of VM directly exposes tumor cells to the blood flow, allowing them to enter the microcirculation and to metastasize to other organs. This finding may explain the increased risk of metastasis or recurrence and the shorter survival period of VM‐positive CRC patients. In addition, the relationship between VM and patient characteristics, such as gender, age and tumor size, were insignificant. This result is similar to those of previous studies on VM.27, 29, 30
Vasculogenic mimicry (VM) is usually regarded as an example of aggressive tumor cells with remarkable differentiation plasticity, because VM‐forming tumor cells alter their cell markers and form vessel‐like structures with a similar pattern to that of embryonic vasculogenesis. The microarray analysis indicated that the VM‐positive tumor cells of aggressive melanoma displayed increased expressions of genes associated with undifferentiated embryonic phenotypes.31 Considering these tumor cells can transdifferentiate into endothelium‐like cells which are mesenchymal cells, we can assume that the activation of EMT may be implicated in VM formation.
Evidence accumulated over the past decade indicates that the aberrant activation of EMT contributes to tumor progression and metastasis.16 As an EMT transcription factor, ZEB1 plays an important regulatory role in the induction of EMT in many human cancers, such as prostate, colon, breast and pancreatic cancers, and suppresses the expression of basement membrane components and cell polarity factors.22, 23, 32, 33, 34 In the current study, we observed that the VM was associated with ZEB1 expression concomitantly occurred with EMT features in CRC. Our results confirm that the EMT‐inducer ZEB1 plays an important role in VM formation and that EMT is the important pathway for VM formation in CRC.
Some studies have established a crucial link between EMT and the acquisition of stemness.35 ZEB1 not only regulates EMT but also controls other crucial cellular functions and states, such as stemness and differentiation. Wellner et al show that ZEB1 and miR‐200 family members mutually repress each other in a reciprocal feedback loop and that ZEB1 indirectly induces stemness maintenance.23 Recently, cancer stem cells or dedifferentiated stem‐like cells have been shown to contribute to VM formation. Some studies on glioblastoma indicate that the cells engaged in VM may come from cancer cells endowed with stem‐cell plasticity and may represent an incomplete differentiation of cancer stem‐like cells toward the endothelial lineage.7, 28 We also found that ZEB1, which indirectly induces stemness maintenance, was more upregulated in VM‐positive CRC than in VM‐negative CRC. The relationship between the EMT‐inducer ZEB1 and cellular stemness may facilitate the understanding of the mechanism of VM formation.
Expression profiling studies reveal that VM‐engaging tumor cells involve the transdifferentiation of aggressive epithelial cancer cells into endothelium‐like cells.36, 37 We found that the poorly differentiated HCT116 cells are not only capable of in vitro VM formation but also induced the expressions of VE‐cadherin, Flt‐1 (VEGFR‐1) and Flk‐1 (VEGFR‐2), which are important endothelium‐associated markers. In the current study, we observed that si‐ZEB1‐transfected HCT116 cells showed decreased expression of Flk‐1, which is responsible for the VEGF‐induced differentiation of vascular endothelial cells.38, 39 In addition, VE‐cadherin, one of the first molecules identified as an important player in melanoma VM,10, 40 was also found downregulated in si‐ZEB1‐transfected HCT116 cells. Coincident with decreased endothelial‐related markers, the si‐ZEB1‐transfected HCT116 cells formed less tube‐like structures in 3‐D culture, indicating that ZEB1 is an important regulator of VM.
The current paper is the first to report the previously unrecognized role of ZEB1 as an important mediator for VM formation through EMT induction. Moreover, the findings of the paper might hold a number of implications for future drug‐mediated therapeutic modifications aimed at VM.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
This study was supported by the Key Project of Nature Science Foundation of China (No. 30830049), the cooperation of China–Sweden (No. 09ZCZDSF04400) and the Tianjin Natural Science Foundation (No. 08JCZDJC23500 and No. 09JCYBJC12100).
References
- 1. Ong CW, Kim LG, Kong HH et al CD133 expression predicts for non‐response to chemotherapy in colorectal cancer. Modern Pathology 2010; 23: 450–7. [DOI] [PubMed] [Google Scholar]
- 2. Franci C, Gallen M, Alameda F et al Snail1 protein in the stroma as a new putative prognosis marker for colon tumours. PLoS ONE 2009; 4: e5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seftor EA, Meltzer PS, Schatteman GC et al Expression of multiple molecular phenotypes by aggressive melanoma tumor cells: role in vasculogenic mimicry. Critical Reviews in Oncology/hematology 2002; 44: 17–27. [DOI] [PubMed] [Google Scholar]
- 4. Sun B, Zhang S, Zhao X, Zhang W, Hao X. Vasculogenic mimicry is associated with poor survival in patients with mesothelial sarcomas and alveolar rhabdomyosarcomas. International Journal of Oncology 2004; 25: 1609–14. [PubMed] [Google Scholar]
- 5. Maniotis AJ, Folberg R, Hess A et al Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. American Journal of Pathology 1999; 155: 739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Schaft DW, Seftor RE, Seftor EA et al Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. Journal of the National Cancer Institute 2004; 96: 1473–7. [DOI] [PubMed] [Google Scholar]
- 7. Wang R, Chadalavada K, Wilshire J et al Glioblastoma stem‐like cells give rise to tumour endothelium. Nature 2010; 468: 829–33. [DOI] [PubMed] [Google Scholar]
- 8. Ruf W, Seftor EA, Petrovan RJ et al Differential role of tissue factor pathway inhibitors 1 and 2 in melanoma vasculogenic mimicry. Cancer Research 2003; 63: 5381–9. [PubMed] [Google Scholar]
- 9. Hendrix MJ, Seftor EA, Meltzer PS et al Expression and functional significance of VE‐cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proceedings of the National Academy of Sciences of the United States of America 2001; 98: 8018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hess AR, Seftor EA, Gruman LM, Kinch MS, Seftor RE, Hendrix MJ. VE‐cadherin regulates EphA2 in aggressive melanoma cells through a novel signaling pathway: implications for vasculogenic mimicry. Cancer Biology & Therapy 2006; 5: 228–33. [DOI] [PubMed] [Google Scholar]
- 11. Seftor RE, Seftor EA, Kirschmann DA, Hendrix MJ. Targeting the tumor microenvironment with chemically modified tetracyclines: Inhibition of laminin 5 gamma2 chain promigratory fragments and vasculogenic mimicry. Molecular Cancer Therapeutics 2002; 1: 1173–9. [PubMed] [Google Scholar]
- 12. Seftor RE, Seftor EA, Koshikawa N et al Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase‐2, and membrane type‐1‐matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Research 2001; 61: 6322–7. [PubMed] [Google Scholar]
- 13. Hess AR, Seftor EA, Seftor RE, Hendrix MJ. Phosphoinositide 3‐kinase regulates membrane type 1‐matrix metalloproteinase (MMP) and MMP‐2 activity during melanoma cell vasculogenic mimicry. Cancer Research 2003; 63: 4757–62. [PubMed] [Google Scholar]
- 14. Hess AR, Postovit LM, Margaryan NV et al Focal adhesion kinase promotes the aggressive melanoma phenotype. Cancer Research 2005; 65: 9851–60. [DOI] [PubMed] [Google Scholar]
- 15. Millimaggi D, Mari M, S DA, Giusti I, Pavan A, Dolo V. Vasculogenic mimicry of human ovarian cancer cells: role of CD147. International Journal of Oncology 2009; 35: 1423–8. [DOI] [PubMed] [Google Scholar]
- 16. Iwatsuki M, Mimori K, Yokobori T et al Epithelial–mesenchymal transition in cancer development and its clinical significance. Cancer Science 2010; 101: 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun T, Zhao N, Zhao XL et al Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology 2010; 51: 545–56. [DOI] [PubMed] [Google Scholar]
- 18. Brabletz S, Brabletz T. The ZEB/miR‐200 feedback loop–A motor of cellular plasticity in development and cancer? EMBO Rep 2010; 11: 670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dohadwala M, Wang G, Heinrich E et al The role of ZEB1 in the inflammation‐induced promotion of EMT in HNSCC. Otolaryngology ‐ Head and Neck Surgery 2010; 142: 753–9. [DOI] [PubMed] [Google Scholar]
- 20. Drake JM, Strohbehn G, Bair TB, Moreland JG, Henry MD. ZEB1 enhances transendothelial migration and represses the epithelial phenotype of prostate cancer cells. Molecular Biology of the Cell 2009; 20: 2207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spoelstra NS, Manning NG, Higashi Y et al The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Research 2006; 66: 3893–902. [DOI] [PubMed] [Google Scholar]
- 22. Aigner K, Dampier B, Descovich L et al The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 2007; 26: 6979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wellner U, Schubert J, Burk UC et al The EMT‐activator ZEB1 promotes tumorigenicity by repressing stemness‐inhibiting microRNAs. Nature Cell Biology 2009; 11: 1487–95. [DOI] [PubMed] [Google Scholar]
- 24. Shirakawa K, Wakasugi H, Heike Y et al Vasculogenic mimicry and pseudo‐comedo formation in breast cancer. International Journal of Cancer 2002; 99: 821–8. [DOI] [PubMed] [Google Scholar]
- 25. Sharma N, Seftor RE, Seftor EA et al Prostatic tumor cell plasticity involves cooperative interactions of distinct phenotypic subpopulations: role in vasculogenic mimicry. Prostate 2002; 50: 189–201. [DOI] [PubMed] [Google Scholar]
- 26. Sood AK, Fletcher MS, Coffin JE et al Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. American Journal of Obstetrics and Gynecology 2004; 190: 899–909. [DOI] [PubMed] [Google Scholar]
- 27. Sun B, Zhang S, Zhang D et al Vasculogenic mimicry is associated with high tumor grade, invasion and metastasis, and short survival in patients with hepatocellular carcinoma. Oncology Reports 2006; 16: 693–8. [PubMed] [Google Scholar]
- 28. Ricci‐Vitiani L, Pallini R, Biffoni M et al Tumour vascularization via endothelial differentiation of glioblastoma stem‐like cells. Nature 2010; 468: 824–8. [DOI] [PubMed] [Google Scholar]
- 29. Baeten CI, Hillen F, Pauwels P, de Bruine AP, Baeten CG. Prognostic role of vasculogenic mimicry in colorectal cancer. Diseases of the Colon and Rectum 2009; 52: 2028–35. [DOI] [PubMed] [Google Scholar]
- 30. El Hallani S, Boisselier B, Peglion F et al A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain 2010; 133: 973–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene 2003; 22: 3070–5. [DOI] [PubMed] [Google Scholar]
- 32. Wu Y, Zhou BP. New insights of epithelial‐mesenchymal transition in cancer metastasis. Acta Biochimica et Biophysica Sinica (Shanghai) 2008; 40: 643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grooteclaes ML, Frisch SM. Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene 2000; 19: 3823–8. [DOI] [PubMed] [Google Scholar]
- 34. Shirakihara T, Saitoh M, Miyazono K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF‐beta. Molecular Biology of the Cell 2007; 18: 3533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mani SA, Guo W, Liao MJ et al The epithelial‐mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paulis YW, Soetekouw PM, Verheul HM, Tjan‐Heijnen VC, Griffioen AW. Signalling pathways in vasculogenic mimicry. Biochimica et Biophysica Acta 2010; 1806: 18–28. [DOI] [PubMed] [Google Scholar]
- 37. Monzani E, La Porta CA. Targeting cancer stem cells to modulate alternative vascularization mechanisms. Stem Cell Rev 2008; 4: 51–6. [DOI] [PubMed] [Google Scholar]
- 38. Winder T, Lenz HJ. Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology 2010; 138: 2163–76. [DOI] [PubMed] [Google Scholar]
- 39. Shibuya M. Differential roles of vascular endothelial growth factor receptor‐1 and receptor‐2 in angiogenesis. J Biochem Mol Biol 2006; 39: 469–78. [DOI] [PubMed] [Google Scholar]
- 40. Zhang LZ, Mei J, Qian ZK, Cai XS, Jiang Y, Huang WD. The role of VE‐cadherin in osteosarcoma cells. Pathol Oncol Res 2010; 16: 111–7. [DOI] [PubMed] [Google Scholar]


