Summary
Oral cavity squamous cell carcinoma (OCSCC) is a heterogeneous and complex disease that arises due to dysfunction of multiple molecular signaling pathways. Recent advances in high-throughput genetic sequencing technologies coupled with innovative analytical techniques have begun to characterize the molecular determinants driving OCSCC. An understanding of the key molecular signaling networks underlying the initiation and progression of is essential for informing treatment of the disease. In this chapter, we discuss recent findings of key genes altered in OCSCC and potential treatments targeting these genes.
Introduction
All malignancies are endowed with specific capabilities that allow for continued proliferation, invasion and metastasis. In a hallmark review by Hanahan and Weinberg, it is suggested that there are six essential fundamental processes involved in the maintenance of any malignancy, including the resistance of cell death, continued proliferation, evasion of growth suppressors, enabling replicative immortality, inducing angiogenesis and activating invasion and metastasis [1]. These processes are driven by a complex network of molecular signaling pathways that are daunting to scientists and physicians alike.
The discovery of human papillomavirus (HPV) as a key driver of a subset of oropharyngeal squamous cell carcinomas has recently sparked a significant amount of research and interest. HPV status informs treatment, as overall survival and prognosis are greater for HPV-positive oropharyngeal squamous cell carcinomas compared to those that are HPV-negative. However, whereas 70.1% of oropharyngeal squamous cell carcinomas are caused by HPV [2], oral cavity squamous cell carcinoma (OCSCC) has proven itself to be a separate disease in that it is almost always negative for HPV. As such, designing treatments are difficult as there is no single predominant pattern or molecular driver of the OCSCC progression. However, the same key processes described by Hanahan and Weinberg are in play in oral cavity squamous cell carcinomas. Recent research has begun to reveal the ways in which the molecular players controlling these processes are dysregulated in OCSCC. For instance, loss of function of p16 and p53 is associated with proliferation and evasion of growth suppressors, dysregulation of the Notch and WNT pathways may assist in enabling replicative immortality and avoiding cell differentiation, and the AKT pathway plays key roles in invasion and metastasis. In this review, we provide an overview of our current understanding of these and other molecular players involved in OCSCC tumorigenesis, both at the genetic and epigenetic levels. We also discuss factors that contribute to invasion and metastasis of established oral malignancies, as well as recent advances in targeted therapy and biomarkers predicting response to treatment. It is essential for any practicing clinician who takes care of oral cancer patients to have a basic understanding of these pathways, as these will likely form the basis for therapies down the road.
Molecular Genetics
p16/Cyclin D1/pRb/p53
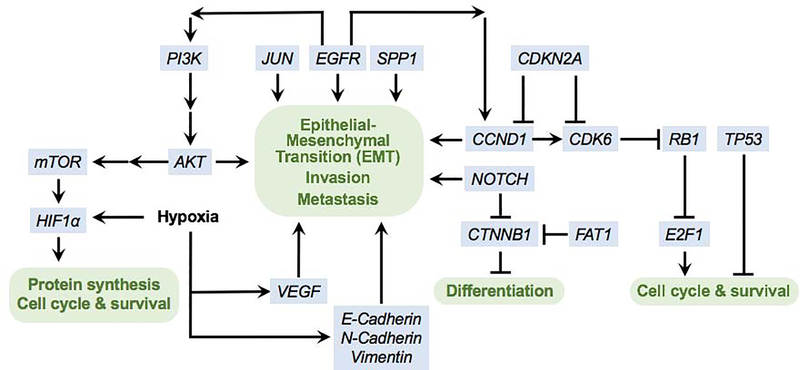
Two major tumor suppressor proteins, p16 and p53, are frequently inactivated in head and neck cancer (Figure 1) [3–6]. p16, which is encoded by the CDKN2A gene located on chromosome 9p21, is a tumor suppressor protein that is involved in cell growth and cell cycle control. Specifically, it blocks progression from the G1 to S phase of the cell cycle by inhibiting another protein called Cyclin D1. Thus, disruption of p16 activity results in a loss of cell senescence, subsequently leading to dysplasia. Similar to other types of H&N malignancies, OCSCC is often negative for p16 [7,8]. Moreover, OCSCC patients with p16 inactivation have significantly lower survival than patients with normal or amplified levels of p16 [9].
Figure 1. Signaling pathways in oral cavity squamous cell carcinoma.
Partial list of key affected pathways.
CCND1 is a gene which encodes Cyclin D1 protein, whose activity is inhibited by p16. CCND1 is amplified in 25–43% of OCSCCs [10–12], whereas its product, Cyclin D1, was found to be overexpressed at early stages of oral carcinogenesis and is associated with tumorigenic proliferation [13]. Specifically, the cytoplasmic Cyclin D1 expression was increased in OCSCC tumors with advanced stages, poor differentiation, increased mitosis, and invasive cell morphology, suggesting that elevated cytoplasmic cyclin D1 levels may promote cell migration and invasion [14]. Moreover, several studies showed that CCND1 amplification and cyclin D1 expression were associated with decreased survival and poor prognosis in OCSCC patients [10,14–16].
Inhibition of Cyclin D1 suppresses activation of the cyclin dependent kinases CDK4 and CDK6, which drive cell cycle progression by preventing the phosphorylation and inactivation of pRb. pRb is a tumor suppressor protein encoded by RB1; in its hypophosphorylated state, pRb prevents cell cycle progression from the G1 to S phase [17,18]. Dysregulation of pRb occurs early in oral epithelial dysplasia and is associated with higher likelihood of transformation to malignant carcinoma [19].
Inactivation of the p53 protein, which is encoded by the gene TP53 (located on chromosome 17p12), plays a prominent role in the pathogenesis of various solid malignancies including head and neck cancers. p53 regulates the cell cycle; cellular stress such as DNA damage causes translocation of p53 to the nucleus, where it plays role in multiple cellular processes including cell growth arrest or apoptosis [5,20]. Mutations in exon 4 or intron 6 of the TP53 [21,22] are commonly detected in the majority (84%) of H&N cancers, including OCSCC [5],
p53 is a useful prognosticator of OCSCC. Its expression is correlated with tumor stage and grade, and also predicts the presence of dysplastic surgical margins in early OCSCC [23,24]. However, TP53 expression is not correlated with lymph node metastasis [23]. p53 interacts with a complex network of proteins to regulate the cell cycle progression, apoptosis and differentiation. It was recently found to be co-expressed with platelet-derived growth factor receptor A (PDGFRα), a stimulator of cell growth, in many poorly differentiated OCSCCs, suggesting that the two proteins may cooperate to increase tumor aggressiveness [25]. Thus, potential treatments restoring the activity of p53 are currently being explored. Operculina turpathum extract has been found to upregulate p53, thus inducing apoptosis, and also downregulates cyclin D1, causing cell cycle arrest [26]. Cis-3-O-p-hydroxycinnamoyl ursolic acid (HCUA) also causes p53-mediated apoptosis in oral cancer cells, and thus may have an antitumor effect [27].
Notch
Notch signaling is a highly conserved pathway of communication between neighboring cells, regulating cell proliferation and fate [28]. Activating mutations in NOTCH1 are present in some cancers, particularly in human T cell acute lymphoblastic leukemia [29,30]. On the other hand, inactivating mutations in NOTCH1 are present in 11–19% of HNSCC tumors, suggesting that NOTCH1 may act as a tumor suppressor in HNSCC, in contrast with its proto-oncogenic role in other cancers [5,20,31].
NOTCH1 is physiologically expressed in basal cells of the oral squamous epithelium, and its expression is inhibited in oral cancer and oral epithelial dysplasia [32]. Pickering and colleagues found that 9% of patients with OCSCC harbor inactivating mutations in NOTCH1 and that OCSCC proliferation in vitro was inhibited by functional NOTCH1 signaling [33]. In their study, 66% of OCSCC patients had defective signaling in at least one member of the Notch signaling pathway. Higher rates of NOTCH1 mutation (43%) were found in Chinese patients with OSC [34], whereas NOTCH1 mutations were uncommon in Singaporean patients with oral tongue squamous cell carcinoma [35], suggesting that NOTCH1 inactivation may have complex genetic interactions in the pathogenesis of OCSCC.
Interestingly, activation of the Notch signaling pathway may also promote OCSCC progression in some cases. Two groups found that NOTCH1 expression was increased in OCSCCs [36,37]. In these studies, NOTCH1 expression was correlated with T-stage and clinical stage, and depletion of Notch1 caused decreased cell proliferation. Additionally, overexpression of Nrf2 (which activates the Notch signaling pathway among its other activities) in OCSCC cells was found to promote a cancer phenotype [38]. Thus, tumorigenesis may arise from multiple forms of dysregulation of the Notch signaling pathway, suggesting that its careful regulation is essential for proper cell function.
Wnt/β-catenin
The Wnt/β-catenin signaling pathway is a conserved pathway that regulates cell fate determination, proliferation, and differentiation. Upregulation of the pathway signaling leads to oncogenesis in OCSCC, often through multiple mechanisms stemming from aberrant activation of β-catenin. Mutations in CTNNB1, which encodes β-catenin, are rare in HNSCC; however, inactivating mutations in NOTCH1 and FAT1 signaling prevent their inhibition of CTNNB1 expression [5].
Epidermal Growth Factor Receptor
The epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase that, when activated, upregulates several downstream signaling pathways, including MAPK, AKT, ERK, and Jak/STAT, which are essential for tumor growth, proliferation, apoptosis, survival, angiogenesis, invasion, and metastasis [39–43]. EGFR levels are elevated in more than 90% of patients with HNSCC [44]. High expression of EGFR is associated with tumor aggressiveness and poor survival of patients with OCSCC [45–47], including specifically oral tongue cancers [48,49]. Additionally, EGFR expression in the nucleus of more than 5% of tumor cells was found in 24.3% of OCSCC patients, and was associated with poor survival [50].
The high expression of EGFR in oral cancers makes it an attractive molecular target for treatment. Strategies to target EGFR have been developed, including specific tyrosine kinase inhibitors such as gefitinib and erlotinib; monoclonal antibodies such as cetuximab and panitumumab; and siRNAs, which inhibit mRNA expression [51]. Cetuximab, a structural inhibitor of EGFR signaling [52], has been highly studied, and is the only approved targeted therapy for HNSCCs, including advanced OCSCCs. The combination of cetuximab with platinum-based chemotherapy is approved for first-line use to treat recurrent and metastatic HNSCCs, including OCSCC, and cetuximab alone is a second-line treatment for platinum-resistant HNSCCs [53–55].
Several inhibitors of the tyrosine kinase domain of EGFR have been developed; however, none have shown survival benefit for HNSCCs. Gefitinib has not shown survival benefit for HNSCCs, whether given alone or with chemoradiotherapy [56–58]. Similarly, lapatinib does not show a survival benefit [59,60], and cisplatin and radiotherapy with or without erlotinib showed no difference in progression-free survival or complete response rate [61].
PI3K/AKT/mTOR
The PI3K/AKT/mTOR pathway is a well characterized signaling axis that is known to be involved in proliferation, growth, survival, and drug resistance of multiple cancer types [62]. In the pathway, activation of the PI3 family of protein kinases by tyrosine kinase receptors leads to the formation of second messenger PIP3, which in turn activates AKT and results in activation of multiple downstream targets including mammalian target of rapamycin (mTOR).
Studies have clearly implicated a role of AKT signaling activation in OCSCC. For example, immunohistochemistry analyses have identified staining of AKT, pAKT and pmTOR in a substantial number of OCSCC tumors [63,64], and gene expression profiling has identified PI3K-AKT as one of the pathways commonly enhanced in OCSCC malignancies [65]. Furthermore, somatic copy number alterations in genes encoding the members of the PI3K/AKT/mTOR signaling network are also more commonly seen in patients with OCSCC [66].
Multiple other tumor associated pathways that have been shown to promote growth, survival, invasion or drug resistance via activation of AKT signaling have also been studied in OCSCC. Recently identified contributors activating AKT include ZNF703 [67], FoxM1 [68], PDGF-D [69], Nox1 [70], RACK1 [71], CCL18 [72], and Muc1 [73]. These observations demonstrate that many players impinge on the AKT pathway, which must be tightly controlled to prevent tumorigenesis.
Finally, investigators have demonstrated efficacy of inhibitors of the PI3K/AKT/mTOR pathway in preclinical studies [74–76]. Rapamycin, the canonical inhibitor of mTOR from which the molecule owes its name, has been shown to inhibit oral cavity cancer growth [77]. Conversely, activation of pAKT predicts poorer responses to cetuximab chemotherapy [78].
Epigenetics
Epigenetic modifications to the genome provide one of the major mechanisms underlying gene expression regulation. Processes such as methylation or demethylation of the gene promoter regions play a crucial role in gene silencing or gene expression, respectively [79].The silencing of tumor suppressor genes due to methylation is one of the hallmarks of carcinogenesis [80,81].
Importantly, p16 is frequently inactivated due to promoter hypermethylation in OCSCC, with values between 22–76% of patients with OCSCC having been reported [82–89]. In contrast, the p16 promotor region was found to be methylated in 5.4% of normal mucosa samples, emphasizing the prominence of methylation in the pathogenesis of OCSCC [82]. Methylation of the p16 promoter, as well as other tumor suppressor genes, is likely due to the upregulated expression of the DNA methyltransferases as a result of continuous tobacco use.[90,91]. In tobacco users, 28–58% of precancerous oral tissues demonstrated genome-wide DNA methylation, with levels of methylation increasing throughout the progression to oral cancers [82,92–95].
Many other genes are also hypermethylated at promoter regions in OCSCC, including p14, p15, RASSF1A, RASSF2A, DAPK, SFRP1, CDH1, and MLH1. These genes play important roles in various cancer-promoting signaling networks, such as those including cell cycle arrest and apoptosis, Wnt signaling, cell adhesion, and DNA repair. [85,95–104]. Additionally, in OCSCC, certain microRNAs undergo hypermethylation (i.e. miR-200ab-429, miR-200c-141, miR-205, miR-137) or hypomethylation (i.e. miR-127), which may change their expression and play a role in tumorigenesis [105]. The field of microRNA research is still relatively young; the advent of new technologies for profiling RNA will likely bring about greater understanding of the contributions of microRNA hypermethylation to tumor growth.
The prominence of epigenetic alterations in OCSCC suggests that treatments targeting methyltransferases may prove to be effective. Indeed, the DNA methyltransferase inhibitor azacitidine is approved for myelodysplastic syndrome and acute myeloid leukemia [106]. In vitro studies using decitabine (5-aza-2’-deoxycytidine), the deoxy derivative of azacitidine, have shown increased expression of tumor suppressor genes, subsequently decreasing OCSCC growth; thus, potential translational applications may be possible [107,108]. Recent research has also explored the use of the epigenetic modification 5-hydroxymethylcytosine, the oxidative product of methylated cytosine (methylcytosine), as a diagnostic biomarker of HNSCC. For example, circulating tumor DNA of esophageal squamous cell carcinoma, which can be isolated from plasma, has been shown to have a signature genomic pattern of 5-hydroxymethylcytosine on certain genes, including NXN, KIAA0040, and LRRC3B [109], supporting its potential as an avenue to diagnose the disease [109]. Further research on epigenetic alterations in circulating tumor DNA in patients with OCSCC and other HNSCCs may allow early and non-invasive diagnosis of the disease using plasma samples.
Invasion/Metastasis
Metastasis is a complex process where selected malignant cells gain the ability to survive in a distant environment. Its main steps include angiogenesis, or the recruitment of disorganized vasculature, and the epithelial-mesenchymal transition (EMT), the process by which epithelial cells lose cell adhesive properties and gain migratory and invasive properties, thus allowing for seeding of metastatic foci. An understanding of how genomic markers predict risk of both nodal and distant metastasis can be used to inform the approach to treatment.
Angiogenesis is a process that centers around the activation of a protein called vascular endothelial growth factor (VEGF). VEGFA, a member of the VEGF protein family, has shown to be a useful prognosticator for oral tongue SCC [110]. Its overexpression has been shown to be associated with poor survival [111]. In addition, Bevacizumab, a monoclonal antibody against VEGF, shows efficacy in preclinical studies in oral cancer [112,113]. Its concomitant use with irradiation dramatically decreases tumor growth and reduces the number of metastatic nodes. Mechanistically, Bevacizumab has been shown to lower cell migration in vitro, in accordance with its role inhibiting VEGF.
EMT specifically involves the reduction in molecules that govern cell adhesion, such as E-cadherin, and the upregulation of mesenchymal proteins such as N-cadherin and cytoskeletal proteins such as Vimentin. In immunohistochemical studies, oral cavity cancers have been shown to have decreased E-cadherin and increased Vimentin expression [114,115], thus suggesting that cancers utilize EMT to invade and metastasize. EMT is also prominently driven by the expression of transforming growth factor beta (TGF-β), which activates cell spreading and separation of cell borders. Overexpression of TGF-β1 increases the migration and invasiveness of OCSCC cells and promotes malignancy [116,117].
Processes such as EMT, angiogenesis, invasion and metastasis are frequently driven by a combination of tumor cell centric factors and the tumor microenvironment. Most notably, hypoxia is well documented in HNSCC and likely to be central to the metastatic process by induction of EMT [118]. Hif1alpha is a key protein whose expression is promoted by hypoxia, and its expression is associated with lower patient survival [119]. In addition, its activation is correlated with EMT as assessed by in-vitro assays for invasion [120].
Invasion and metastasis are driven by complex molecular signaling networks, with multiple interacting pathways that interplay in the process. The PI3K/AKT/mTOR pathway is increasingly being studied as one of the major pathways involved. Overexpression of many of the molecules activating AKT described earlier cause an increase in invasion and migration as demonstrated by EMT marker expression and in vitro invasion assays. These include ZNF703, FoxM1, PDGF-D, and CXCL9 [67–69,121].
Although inactivation of the Notch pathway is often seen in OCSCC, activation of the Notch pathway may promote the migration and invasion of OCSCC cell lines and squamous cell carcinoma of the tongue. Weaver and colleagues found that activation of Notch signaling in OCSCC correlates with increased expression of FGF-1, which causes increased cell migration and invasion, as well as increased patient mortality [122]. Notch activity also leads to increased angiogenesis of OCSCCs, contributing to invasion [123]. Moreover, hypoxia upregulates the expression of Notch receptors, whereas inhibition of Notch by γ-secretase inhibitor prevents the invasive effects of hypoxia [124]. Similarly, several other groups found that inhibition of Notch activity due to depletion of genes such as HNF1A-AS1 and Glutaredoxin 3 consequently decreases cell migration and invasion [125,126].
Phosphorylation of β-catenin leads to its dissociation from a complex with E-cadherin, resulting in loss of cell adhesion and subsequent tumor cell invasion, while translocation of β-catenin to the nucleus allows it to act as an oncogenic transcription factor [127,128].
EGFR also plays important roles in tumor invasion, demonstrating overexpression in over 80% of invasive HNSCCs [129,130]. Both EGFR gene copy number and elevated protein levels are associated with tumor stage, depth of invasion, lymph node metastasis, bone invasion, and perineural invasion [47]. Mechanistically, EGFR orchestrates various processes involved in angiogenesis and invasion via several pathways, including Ras/Raf/MAPK, PI3K/AKT/mTOR, and JAK/STAT. EGFR may also cause invasion by transforming neighboring epithelial cells; EGFR-containing extracellular vesicles derived from OCSCCs transform neighboring epithelial cells into mesenchymal cells, an effect that can be targeted and inhibited by the anti-EGFR antibody cetuximab [131].
Immunotherapy
The immune system protects against cancer by identifying and eliminating premalignant tumor cells. Thus, to avoid detection by the immune system, tumor cells may upregulate expression of inhibitory checkpoint receptors such as cytotoxic T lymphocyte antigen 4 (CTLA4), programmed death 1 (PD1), and its ligand PD-L1 [132]. Like other cancers, HNSCCs demonstrate suppression of the immune system, marked by low numbers of white blood cells in the periphery and a suppressive population of tumor infiltrating lymphocytes [133–136]. Thus, recent research has focused on the effectiveness of immunotherapies targeting inhibitory checkpoint receptors in treating HNSCCs [137].
Among the most promising immunotherapies are inhibitors of PD-1 and PD-L1, which cause a durable anti-tumor response and disease stabilization [138]. Pembrolizumab and nivolumab are anti-PD-1 receptor antibodies approved in 2016 by the Food and Drug Administration for treatment of recurrent or metastatic HNSCC that did not respond to treatment with a platinum-based systemic agent. Pembrolizumab demonstrates a modest increase in overall survival and response duration, as well as a less severe side effect profile, compared to standard therapy [139,140]. Similarly, patients with recurrent HNSCC refractory to platinum who were treated with nivolumab had longer overall survival compared to patients treated with standard, single-agent therapy [141]. In addition, the combination of nivolumab and ipilimumab, an antibody against CTLA4, which has been approved by the FDA for treatment of patients with unresectable or metastatic melanoma, has been shown to successfully treat a case of refractory oral tongue SCC [142].
As the majority of cancers continue to progress on PD-1 and PD-L1 inhibitors, the identification of predictive biomarkers is essential for improved treatment. Several prognostic biomarkers that have been extensively studied in HNSCC and other malignancies include PD-L1 expression, tumor mutational burden, and immune gene signatures.
PD-L1 expression has been shown to moderately predict response to treatment across several solid tumors [143], and multiple trials have evaluated its predictive value specifically in HNSCC. Checkmate 141 showed that treatment with nivolumab caused a greater reduction in risk of death in HNSCC patients with ≥1% of tumor cells expressing PD-L1 compared to patients with PD-L1 negative tumors [141]. Importantly, expression of PD-L1 on tumor-associated immune cells was a better predictor of benefit than tumor cell PD-L1 expression alone [144]. Similarly, KEYNOTE-40 showed that HNSCC patients with ≥50% of tumor cells expressing PD-L1 who were treated with pembrolizumab, but not those with <50% of tumor cells expressing PD-L1, had increased overall survival and progression free survival compared to standard of care [139]. KEYNOTE-012 also demonstrated that pembrolizumab treatment showed greater overall response rate in PD-L1-positive versus -negative patients [145].
Tumor mutational burden, a measure of the total number of mutations per coding area of a tumor genome, is also emerging as a prominent biomarker of the response to immunotherapy with PD-1 inhibitors [146,147]. Cancers with greater mutational burden demonstrate increased response to immunotherapy with PD-1 inhibitors, perhaps due to augmented formation of tumor-specific antigens (neoantigens), which may provide targets recognized by the immune system[148–152]. Overall, HNSCCs contain high mutational burden; although the effect of tumor mutational burden on treatment of HNSCCs is still unclear, immunotherapies such as anti-PD-1 or anti-PD-L1 inhibitors may serve as promising approaches [146,147,153].
Finally, the extent to which immune cells in the tumor microenvironment are activated has been characterized as a biomarker for response to treatment with PD-1 and PD-L1 inhibitors. KEYNOTE-012 demonstrated that in HNSCC patients treated with pembrolizumab, expression of six immune genes (interferon gamma and the related genes CXCL9, CXCL10, IDO1, HLA-DRA, and STAT1) showed correlation with response rate and progression free survival [154]. Supporting these observations, an analysis of patients treated with pembrolizumab in KEYNOTE-012 and −055 demonstrated that expression of 18 immune genes correlated with increased response, progression free survival, and overall survival [155].
Conclusion
Here, we have reviewed some of the current understanding and recent findings of molecular pathways prominent in OCSCC. As OCSCC is a complex and heterogeneous disease process (Figure 1), treatment must be guided by identification of the patient-specific aberrations that underlie the disease. Recent advances in high-throughput genomic sequencing technologies and molecular research techniques have shown great potential, both for directing current treatments and identifying new etiologies of the disease. Moreover, new techniques to detect changes at the genomic and epigenetic level early on in the disease may allow more robust diagnosis, further contributing to improved treatment options.
Highlights.
We review the current understanding and recent findings of molecular pathways prominent in OCSCC. As OCSCC is a heterogeneous and complex disease, treatment must be guided by identification of the patient-specific aberrations that underlie the disease. Recent advances in high-throughput genomic sequencing technologies and molecular research techniques have shown great potential, both for directing current treatments and identifying new etiologies of the disease. Moreover, new techniques to detect changes at the genomic and epigenetic level early on in the disease may allow more robust diagnosis, further contributing to improved treatment options.
Acknowledgements
PJH is supported by NIH Medical Scientist National Research Service Award T32 GM007281. NA is supported by a Team Science Grant from the University of Chicago Comprehensive Cancer Center.
Footnotes
Conflict of interest
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74. [DOI] [PubMed] [Google Scholar]
- 2.Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV Types in cancers: Implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015; 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Riet P, Nowroz H, Hruban RH, et al. Frequent Loss of Chromosome 9p21–22 Early in Head and Neck Cancer Progression. Cancer Res 1994; 54:1156–1158. [PubMed] [Google Scholar]
- 4.Cairns P, Polascik TJ, Eby Y, et al. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet 1995; 11:210–212. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Network T. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015; 517:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padhi SS, Roy S, Kar M, et al. Role of CDKN2A/p16 expression in the prognostication of oral squamous cell carcinoma. Oral Oncol 2017; 73:27–35. [DOI] [PubMed] [Google Scholar]
- 7.Salehinejad J, Sharifi N, Amirchaghmaghi M, Ghazi N, Shakeri MT, Ghazi A. Immunohistochemical expression of p16 protein in oral squamous cell carcinoma and lichen planus. Ann Diagn Pathol 2014; 18:210–213. [DOI] [PubMed] [Google Scholar]
- 8.Nemes JA, Deli L, Nemes Z, Márton IJ. Expression of p16INK4A, p53, and Rb proteins are independent from the presence of human papillomavirus genes in oral squamous cell carcinoma. Oral Surgery, Oral Med Oral Pathol Oral Radiol Endodontology 2006; 102:344–352. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki H, Sugimura H, Hashimoto K. p16INK4A in Oral Squamous Cell Carcinomas-A Correlation With Biological Behaviors: Immunohistochemical and FISH Analysis. J Oral Maxillofac Surg 2006; 64:1617–1623. [DOI] [PubMed] [Google Scholar]
- 10.Hanken H, Gröbe A, Cachovan G, et al. CCND1 amplification and cyclin D1 immunohistochemical expression in head and neck squamous cell carcinomas. Clin Oral Investig 2014; 18:269–276. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro LS, Diniz-Freitas M, Warnakulasuriya S, Garcia-Caballero T, Forteza-Vila J, Fraga M. Prognostic Significance of Cyclins A2, B1, D1, and E1 and CCND1 Numerical Aberrations in Oral Squamous Cell Carcinomas. Anal Cell Pathol (Amst) 2018; 2018:7253510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-García P, González-Moles MÁ, González-Ruiz L, et al. Clinicopathological significance of tumor cyclin D1 expression in oral cancer. Arch Oral Biol 2019; 99:177–182. [DOI] [PubMed] [Google Scholar]
- 13.Ramos-García P, González-Moles MÁ, Ayén Á, et al. Asymmetrical proliferative pattern loss linked to cyclin D1 overexpression in adjacent non-tumour epithelium in oral squamous cell carcinoma. Arch Oral Biol 2019; 97:12–17. [DOI] [PubMed] [Google Scholar]
- 14.Ramos-García P, Bravo M, González-Ruiz L, González-Moles M. Significance of cytoplasmic cyclin D1 expression in oral oncogenesis. Oral Dis 2018; 24:98–102. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-García P, González-Moles MÁ, González-Ruiz L, Ruiz-Ávila I, Ayén Á, Gil-Montoya JA. Prognostic and clinicopathological significance of cyclin D1 expression in oral squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol 2018; 83:96–106. [DOI] [PubMed] [Google Scholar]
- 16.Jayasurya R, Sathyan KM, Lakshminarayanan K, et al. Phenotypic alterations in Rb pathway have more prognostic influence than p53 pathway proteins in oral carcinoma. Mod Pathol 2005; 18:1056–1066. [DOI] [PubMed] [Google Scholar]
- 17.Buchkovich K, Duffy LA, Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell 1989; 58:1097–1105. [DOI] [PubMed] [Google Scholar]
- 18.Cordon-Cardo C Review Mutation of Cell Cycle Regulators Biological and Clinical Implications for Human Neoplasia; 1995. [PMC free article] [PubMed] [Google Scholar]
- 19.Soni S, Kaur J, Kumar A, et al. Alterations of Rb pathway components are frequent events in patients with oral epithelial dysplasia and predict clinical outcome in patients with squamous cell carcinoma. Oncology 2005; 68:314–325. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal N, Westra WH, Koch WM, et al. Exome Sequencing of Head and Neck Squamous Cell Carcinoma Reveals Inactivating Mutations in NOTCH1. Science (80- ) 2011; 1464:1154–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ara N, Atique M, Ahmed S, Bukhari SGA. Frequency of p53 gene mutation and protein expression in oral squamous cell carcinoma. J Coll Physicians Surg Pakistan 2014; 24:749–753. [DOI] [PubMed] [Google Scholar]
- 22.Huang MF, Chang YC, Liao PS, Huang TH, Tsay CH, Chou MY. Loss of heterozygosity of p53 gene of oral cancer detected by exfoliative cytology. Oral Oncol 1999; 35:296–301. [DOI] [PubMed] [Google Scholar]
- 23.Yang L, Wang Y, Guo L, Wang L, Chen W, Shi B. The Expression and Correlation of iNOS and p53 in Oral Squamous Cell Carcinoma. Biomed Res Int 2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang XH, Ding L, Fu Y, et al. P53-positive expression in dysplastic surgical margins is a predictor of tumor recurrence in patients with early oral squamous cell carcinoma. Cancer Manag Res 2019; 11:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cierpikowski P, Lis-nawara A, Gajdzis P, Bar J. PDGFRα/HER2 and PDGFRα/p53 Co-expression in Oral Squamous Cell Carcinoma. Anticancer Res 2018; 38:795–802. [DOI] [PubMed] [Google Scholar]
- 26.Arora R, Bharti V, Gaur P, Aggarwal S, Mittal M, Das SN. Operculina turpethum extract inhibits growth and proliferation by inhibiting NF-κB, COX-2 and cyclin D1 and induces apoptosis by up regulating P53 in oral cancer cells. Arch Oral Biol 2017; 80:1–9. [DOI] [PubMed] [Google Scholar]
- 27.Lin C-W, Wan L, Huang S-H, et al. Cis-3-O-p- hydroxycinnamoyl Ursolic Acid Induced ROS-Dependent p53-Mediated Mitochondrial Apoptosis in Oral Cancer Cells. Biomol Ther (Seoul) 2018; 27:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artavanis-Tsakona S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal transduction in development. Science (80- ) 1999; 284:770–776. [DOI] [PubMed] [Google Scholar]
- 29.Weng AP, Adolfo *, Ferrando A, et al. Activating Mutations of NOTCH1 in Human T Cell Acute Lymphoblastic Leukemia Downloaded from; 2004. [DOI] [PubMed] [Google Scholar]
- 30.Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila Notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991; 66:649–661. [DOI] [PubMed] [Google Scholar]
- 31.Stransky N, Egloff AM, Tward AD, et al. The Mutational Landscape of Head Squamous Cell Carcinoma. Science (80- ) 2014; 333:1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakamoto K Notch signaling in oral squamous neoplasia. Pathol Int 2016; 66:609–617. [DOI] [PubMed] [Google Scholar]
- 33.Pickering CR, Zhang J, Yoo SY, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov 2013; 3:770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song X, Xia R, Li J, et al. Common and complex Notch1 mutations in chinese oral squamous cell carcinoma. Clin Cancer Res 2014; 20:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vettore AL, Ramnarayanan K, Poore G, et al. Mutational landscapes of tongue carcinoma reveal recurrent mutations in genes of therapeutic and prognostic relevance. Genome Med 2015; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida R, Nagata M, Nakayama H, et al. The pathological significance of Notch1 in oral squamous cell carcinoma. Lab Investig 2013; 93:1068–1081. [DOI] [PubMed] [Google Scholar]
- 37.Osathanon T, Nowwarote N, Pavasant P. Expression and influence of Notch signaling in oral squamous cell carcinoma. J Oral Sci 2016; 58:283–294. [DOI] [PubMed] [Google Scholar]
- 38.Fan H, Paiboonrungruan C, Zhang X, et al. Nrf2 regulates cellular behaviors and Notch signaling in oral squamous cell carcinoma cells. Biochem Biophys Res Commun 2017; 493:833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeburg PH, Ullrich A, Mayes EL V., et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 2004; 309:418–425. [DOI] [PubMed] [Google Scholar]
- 40.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: Linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer 2006; 94:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 2006; 24:2666–2672. [DOI] [PubMed] [Google Scholar]
- 42.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer 2005; 5:341–354. [DOI] [PubMed] [Google Scholar]
- 43.Rogers SJ, Harrington KJ, Rhys-Evans P, O-Charoenrat P, Eccles SA. Biological significance of c-erbB family oncogenes in head and neck cancer. Cancer Metastasis Rev 2005; 24:47–69. [DOI] [PubMed] [Google Scholar]
- 44.Grandis JR, Melhem MF, Barnes EL, Tweardy DJ. Quantitative immunohistochemical analysis of transforming growth factor- α and epidermal growth factor receptor in patients with squamous cell carcinoma of the head and neck. Cancer 1996; 78:1284–1292. [DOI] [PubMed] [Google Scholar]
- 45.Chen IH, Chang JT, Liao CT, Wang HM, Hsieh LL, Cheng AJ. Prognostic significance of EGFR and Her-2 in oral cavity cancer in betel quid prevalent area. Br J Cancer 2003; 89:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia W, Lau YK, Zhang HZ, et al. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res 1999; 5:4164–4174. [PubMed] [Google Scholar]
- 47.Huang SF, Cheng S De, Chien HT, et al. Relationship between epidermal growth factor receptor gene copy number and protein expression in oral cavity squamous cell carcinoma. Oral Oncol 2012; 48:67–72. [DOI] [PubMed] [Google Scholar]
- 48.Ulanovski D, Stern Y, Roizman P, Shpitzer T, Popovtzer A, Feinmesser R. Expression of EGFR and Cerb-B2 as prognostic factors in cancer of the tongue. Oral Oncol 2004; 40:532–537. [DOI] [PubMed] [Google Scholar]
- 49.Ono Y, Nakanishi Y, Ino Y, et al. Clinicopathologic significance of laminin-5 gamma2 chain expression in squamous cell carcinoma of the tongue - immunohistochemical analysis of 67 lesions. Int J Oral Maxillofac Surg 2003; 29:397–397. [PubMed] [Google Scholar]
- 50.Lo HW. Nuclear mode of the EGFR signaling network: biology, prognostic value, and therapeutic implications. Discov Med 2010; 10:44–51. [PMC free article] [PubMed] [Google Scholar]
- 51.Mahipal A, Kothari N, Gupta S. Epidermal growth factor receptor inhibitors: Coming of age. Cancer Control 2014; 21:74–79. [DOI] [PubMed] [Google Scholar]
- 52.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJW, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005; 7:301–311. [DOI] [PubMed] [Google Scholar]
- 53.Loeffler-Ragg J, Schwentner I, Sprinzl GM, Zwierzina H. EGFR inhibition as a therapy for head and neck squamous cell carcinoma. Expert Opin Investig Drugs 2008; 17:1517–1531. [DOI] [PubMed] [Google Scholar]
- 54.Bourhis J, Rivera F, Mesia R, et al. Phase I/II study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol 2006; 24:2866–2872. [DOI] [PubMed] [Google Scholar]
- 55.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008; 359:1116–27. [DOI] [PubMed] [Google Scholar]
- 56.Karamouzis M V, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. J Am Med Assoc 2007; 298:70–82. [DOI] [PubMed] [Google Scholar]
- 57.Stewart JSW, Cohen EEW, Licitra L, et al. Phase III study of gefitinib 250 compared with intravenous methotrexate for recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2009; 27:1864–1871. [DOI] [PubMed] [Google Scholar]
- 58.Gregoire V, Hamoir M, Chen C, et al. Gefitinib plus cisplatin and radiotherapy in previously untreated head and neck squamous cell carcinoma: A phase II, randomized, double-blind, placebo-controlled study. Radiother Oncol 2011; 100:62–69. [DOI] [PubMed] [Google Scholar]
- 59.Harrington KJ, Temam S, D’Cruz A, et al. Final analysis: A randomized, blinded, placebo (P)-controlled phase III study of adjuvant postoperative lapatinib (L) with concurrent chemotherapy and radiation therapy (CH-RT) in high-risk patients with squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol 2019; 32:6005–6005. [Google Scholar]
- 60.De Souza JA, Davis DW, Zhang Y, et al. A phase II study of lapatinib in recurrent/metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res 2012; 18:2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martins RG, Parvathaneni U, Bauman JE, et al. Cisplatin and radiotherapy with or without erlotinib in locally advanced squamous cell carcinoma of the head and neck: A randomized phase ii trial. J Clin Oncol 2013; 31:1415–1421. [DOI] [PubMed] [Google Scholar]
- 62.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer 2006; 6:184–192. [DOI] [PubMed] [Google Scholar]
- 63.Matsuo FS, Andrade MF, Loyola AM, et al. Pathologic significance of AKT, mTOR, and GSK3β proteins in oral squamous cell carcinoma-affected patients. Virchows Arch 2018; 472:983–997. [DOI] [PubMed] [Google Scholar]
- 64.Martins F, de Sousa SCOM, dos Santos E, Woo S Bin, Gallottini M. PI3K–AKT–mTOR pathway proteins are differently expressed in oral carcinogenesis. J Oral Pathol Med 2016; 45:746–752. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Liu J, Fu X, Yang A. Identification of Key Genes and Pathways in Tongue Squamous Cell Carcinoma Using Bioinformatics Analysis. Med Sci Monit 2017; 23:5924–5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng C-H, Liao C-T, Ng K-P, et al. Somatic copy number alterations detected by ultra-deep targeted sequencing predict prognosis in oral cavity squamous cell carcinoma. Oncotarget 2015; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang H, Deng X, Zhang J, et al. Elevated Expression of Zinc Finger Protein 703 Promotes Cell Proliferation and Metastasis through PI3K/AKT/GSK-3β Signalling in Oral Squamous Cell Carcinoma. Cell Physiol Biochem 2017; 44:920–934. [DOI] [PubMed] [Google Scholar]
- 68.Yang H, Wen L, Wen M, et al. FoxM1 promotes epithelial-mesenchymal transition, invasion, and migration of tongue squamous cell carcinoma cells through a c-met/akt-dependent positive feedback loop. Anticancer Drugs 2018; 29:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Sun JD, Yan L jian, Zhao XP. PDGF-D/PDGFRβ promotes tongue squamous carcinoma cell (TSCC) progression via activating p38/AKT/ERK/EMT signal pathway. Biochem Biophys Res Commun 2016; 478:845–851. [DOI] [PubMed] [Google Scholar]
- 70.Ito K, Ota A, Ono T, et al. Inhibition of Nox1 induces apoptosis by attenuating the AKT signaling pathway in oral squamous cell carcinoma cell lines. Oncol Rep 2016; 36:2991–2998. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X, Liu N, Ma D, et al. Receptor for activated C kinase 1 (RACK1) promotes the progression of OSCC via the AKT/mTOR pathway. Int J Oncol 2016; 49:539–548. [DOI] [PubMed] [Google Scholar]
- 72.Jiang X, Wang J, Chen X, et al. Elevated autocrine chemokine ligand 18 expression promotes oral cancer cell growth and invasion via Akt activation. Oncotarget 2016; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li P, Xiao LY, Tan H. Muc-1 promotes migration and invasion of oral squamous cell carcinoma cells via PI3K-Akt signaling. Int J Clin Exp Pathol 2015; 8:10365–10374. [PMC free article] [PubMed] [Google Scholar]
- 74.Smolensky D, Rathore K, Bourn J, Cekanova M. Inhibition of the PI3K/AKT Pathway Sensitizes Oral Squamous Cell Carcinoma Cells to Anthracycline-Based Chemotherapy In Vitro. J Cell Biochem 2017; 118:2615–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu C-M, Lin P-M, Lin H-C, et al. NVP-BEZ235 Attenuated Cell Proliferation and Migration in the Squamous Cell Carcinoma of Oral Cavities and p70S6K Inhibition Mimics its Effect. Int J Mol Sci 2018; 19:3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lam TG, Jeong YS, Kim SA, Ahn SG. New metformin derivative HL156A prevents oral cancer progression by inhibiting the insulin-like growth factor/AKT/mammalian target of rapamycin pathways. Cancer Sci 2018; 109:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Day TA, Shirai K, O’Brien PE, et al. Inhibition of mTOR signaling and clinical activity of rapamycin in head and neck cancer in a window of opportunity trial. Clin Cancer Res 2019; 25:1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lyu J, Song H, Tian Z, Miao Y, Ren G, Guo W. Predictive value of pAKT/PTEN expression in oral squamous cell carcinoma treated with cetuximab-based chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 121:67–72. [DOI] [PubMed] [Google Scholar]
- 79.Razin A, Riggs AD. DNA Methylation and gene function. Science (80- ) 1980; 210:604–610. [DOI] [PubMed] [Google Scholar]
- 80.Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun 1983; 111:47–54. [DOI] [PubMed] [Google Scholar]
- 81.Andrew P F, Bert V. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature 1983; 301:89–92. [DOI] [PubMed] [Google Scholar]
- 82.D’Souza W, Saranath D. Clinical implications of epigenetic regulation in oral cancer. Oral Oncol 2015; 51:1061–1068. [DOI] [PubMed] [Google Scholar]
- 83.Shaw RJ, Liloglou T, Rogers SN, et al. Promoter methylation of P16, RARβ, E-cadherin, cyclin A1 and cytoglobin in oral cancer: Quantitative evaluation using pyrosequencing. Br J Cancer 2006; 94:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schussel J, Zhou XC, Zhang Z, et al. EDNRB and DCC salivary rinse hypermethylation has a similar performance as expert clinical examination in discrimination of oral cancer/dysplasia versus benign lesions. Clin Cancer Res 2013; 19:3268–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Viswanathan M, Tsuchida N, Shanmugam G. Promoter hypermethylation profile of tumor-associated genes p16, p15, hMLH1, MGMT and E-cadherin in oral squamous cell carcinoma. Int J Cancer 2003; 105:41–46. [DOI] [PubMed] [Google Scholar]
- 86.Hasegawa M, Nelson HH, Peters E, Ringstrom E, Posner M, Kelsey KT. Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene 2002; 21:4231–4236. [DOI] [PubMed] [Google Scholar]
- 87.Ogi K, Toyota M, Ohe-Toyota M, et al. Aberrant methylation of multiple genes and clinicopathological features in oral squamous cell carcinoma. Clin Cancer Res 2002; 8:3164–3171. [PubMed] [Google Scholar]
- 88.Allameh A, Moazeni-Roodi A, Harirchi I, et al. Promoter DNA Methylation and mRNA Expression Level of p16 Gene in Oral Squamous Cell Carcinoma: Correlation with Clinicopathological Characteristics. Pathol Oncol Res 2018. [DOI] [PubMed] [Google Scholar]
- 89.Strzelczyk JK, Krakowczyk L, Owczarek AJ. Aberrant DNA methylation of the p16, APC, MGMT, TIMP3 and CDH1 gene promoters in tumours and the surgical margins of patients with oral cavity cancer. J Cancer 2018; 9:1896–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet 2011; 88:450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin RK, Hsieh YS, Lin P, et al. The tobacco-specific carcinogen NNK induces DNA methyltransferase 1 accumulation and tumor suppressor gene hypermethylation in mice and lung cancer patients. J Clin Invest 2010; 120:521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takeshima M, Saitoh M, Kusano K, et al. High frequency of hypermethylation of p14, p15 and p16 in oral pre-cancerous lesions associated with betel-quid chewing in Sri Lanka. J Oral Pathol Med 2008; 37:475–479. [DOI] [PubMed] [Google Scholar]
- 93.Saatci C, Caglayan AO, Ozkul Y, Tahiri S, Turhan AB, Dundar M. Detection of p16 promotor hypermethylation in “Maras powder” and tobacco users. Cancer Epidemiol 2009; 33:47–50. [DOI] [PubMed] [Google Scholar]
- 94.Kresty LA, Mallery SR, Knobloch TJ, et al. Alterations of p16 INK4a and p14 ARF in Patients with Severe Oral Epithelial Dysplasia 1; 2002. [PubMed] [Google Scholar]
- 95.Kulkarni V, Saranath D. Concurrent hypermethylation of multiple regulatory genes in chewing tobacco associated oral squamous cell carcinomas and adjacent normal tissues. Oral Oncol 2004; 40:145–153. [DOI] [PubMed] [Google Scholar]
- 96.Sogabe Y, Suzuki H, Toyota M, et al. Epigenetic inactivation of SFRP genes in oral squamous cell carcinoma. Int J Oncol 2008; 32:1253–1261. [DOI] [PubMed] [Google Scholar]
- 97.Ishida E, Nakamura M, Ikuta M, et al. Promotor hypermethylation of p14ARF is a key alteration for progression of oral squamous cell carcinoma. Oral Oncol 2005; 41:614–622. [DOI] [PubMed] [Google Scholar]
- 98.Imai T, Toyota M, Suzuki H, et al. Epigenetic inactivation of RASSF2 in oral squamous cell carcinoma. Cancer Sci 2008; 99:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kordi-Tamandani DM, Ladies MAR, Hashemi M, Moazeni-Roodi A-K, Krishna S, Torkamanzehi A. Analysis of p15 INK4b and p16 INK4a Gene Methylation in Patients with Oral Squamous Cell Carcinoma. Biochem Genet 2012; 50:448–453. [DOI] [PubMed] [Google Scholar]
- 100.Huang KH, Huang SF, Chen IH, Liao CT, Wang HM, Hsieh LL. Methylation of RASSF1A, RASSF2A, and HIN-1 is associated with poor outcome after radiotherapy, but not surgery, in oral squamous cell carcinoma. Clin Cancer Res 2009; 15:4174–4180. [DOI] [PubMed] [Google Scholar]
- 101.De Freitas Cordeiro-Silva M, Lima Oliveira ZF, De Podestá JRV, Gouvea SA, Von Zeidler SV, Louro ID. Methylation analysis of cancer-related genes in non-neoplastic cells from patients with oral squamous cell carcinoma. Mol Biol Rep 2011; 38:5435–5441. [DOI] [PubMed] [Google Scholar]
- 102.Bhatia V, Goel MM, Makker A, et al. Promoter region hypermethylation and mRNA expression of MGMT and p16 genes in tissue and blood samples of human premalignant oral lesions and oral squamous cell carcinoma. Biomed Res Int 2014; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaw RJ, Hobkirk AJ, Nikolaidis G, et al. Molecular staging of surgical margins in oral squamous cell carcinoma using promoter methylation of p16INK4A, cytoglobin, E-cadherin, and TMEFF2. Ann Surg Oncol 2013; 20:2796–2802. [DOI] [PubMed] [Google Scholar]
- 104.González-Ramírez I, García-Cuellar C, Sánchez-Pérez Y, Granados-García M. DNA methylation in oral squamous cell carcinoma: Molecular mechanisms and clinical implications. Oral Dis 2011; 17:771–778. [DOI] [PubMed] [Google Scholar]
- 105.Wiklund ED, Gao S, Hulf T, et al. MicroRNA alterations and associated aberrant DNA methylation patterns across multiple sample types in oral squamous cell carcinoma. PLoS One 2011; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaminskas E, Farrell AT, Wang Y-C, Sridhara R, Pazdur R. FDA Drug Approval Summary: Azacitidine (5-azacytidine, Vidaza TM ) for Injectable Suspension Oncologist ® FDA Commentary LEARNING OBJECTIVES.; 2005. [DOI] [PubMed]
- 107.Chang KW, Chu TH, Gong NR, et al. miR-370 modulates insulin receptor substrate-1 expression and inhibits the tumor phenotypes of oral carcinoma. Oral Dis 2013; 19:611–619. [DOI] [PubMed] [Google Scholar]
- 108.Jayaprakash C, Radhakrishnan R, Ray S, Satyamoorthy K. Promoter methylation of MGMT in oral carcinoma: A population-based study and meta-analysis. Arch Oral Biol 2017; 80:197–208. [DOI] [PubMed] [Google Scholar]
- 109.Tian X, Sun B, Chen C, et al. Circulating tumor DNA 5-hydroxymethylcytosine as a novel diagnostic biomarker for esophageal cancer. Cell Research. 2018:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Almangush A, Heikkinen I, Mäkitie AA, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: A systematic review and meta-analysis. Br J Cancer 2017; 117:856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao SF, Yang XD, Lu MX, et al. Prognostic significance of VEGF immunohistochemical expression in oral cancer: A meta-analysis of the literature. Tumor Biol 2013; 34:3165–3171. [DOI] [PubMed] [Google Scholar]
- 112.Bozec A, Sudaka A, Fischel JL, Brunstein MC, Etienne-Grimaldi MC, Milano G. Combined effects of bevacizumab with erlotinib and irradiation: A preclinical study on a head and neck cancer orthotopic model. Br J Cancer 2008; 99:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ganjibakhsh M, Monshizadeh R, Nasimian A, et al. Anti-angiogenic efficacy of aflibercept and bevacizumab in primary oral squamous cell carcinoma cells. J Oral Pathol Med 2018; 47:575–582. [DOI] [PubMed] [Google Scholar]
- 114.Liu PF, Kang BH, Wu YM, et al. Vimentin is a potential prognostic factor for tongue squamous cell carcinoma among five epithelial-mesenchymal transition-related proteins. PLoS One 2017; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Angadi P V, Patil P V, Angadi V, et al. Immunoexpression of Epithelial Mesenchymal Transition Proteins E-Cadherin, β-Catenin, and N-Cadherin in Oral Squamous Cell Carcinoma. Int J Surg Pathol 2016; 24:696–703. [DOI] [PubMed] [Google Scholar]
- 116.Bu J-Q, Chen F. TGF-beta1 promotes cells invasion and migration by inducing epithelial mesenchymal transformation in oral squamous cell carcinoma. Eur Rev Med Pharmacol Sci 2017; 21:2137–2144. [PubMed] [Google Scholar]
- 117.Cirillo N, Hassona Y, Celentano A, et al. Cancer-associated fibroblasts regulate keratinocyte cell-cell adhesion via TGF-β-dependent pathways in genotype-specific oral cancer. Carcinogenesis 2017; 38:76–85. [DOI] [PubMed] [Google Scholar]
- 118.Hoogsteen IJ, Marres HAM, Bussink J, Van Der Kogel AJ, Kaanders JHAM. Tumor microenvironment in head and neck squamous cell carcinomas: Predictive value and clinical relevance of hypoxic markers. A review. Head Neck 2007; 29:591–604. [DOI] [PubMed] [Google Scholar]
- 119.Santos M dos, Mercante AM da C, Louro ID, et al. HIF1-Alpha Expression Predicts Survival of Patients with Squamous Cell Carcinoma of the Oral Cavity. PLoS One 2012; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaneko T, Dehari H, Sasaki T, et al. Hypoxia-induced epithelial-mesenchymal transition is regulated by phosphorylation of GSK3-β via PI3 K/Akt signaling in oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 122:719–730. [DOI] [PubMed] [Google Scholar]
- 121.Li Z, Liu J, Li L, et al. Epithelial mesenchymal transition induced by the CXCL9/CXCR3 axis through AKT activation promotes invasion and metastasis in tongue squamous cell carcinoma. Oncol Rep 2018; 39:1356–1368. [DOI] [PubMed] [Google Scholar]
- 122.Weaver AN, Burch MB, Cooper TS, et al. Notch Signaling Activation Is Associated with Patient Mortality and Increased FGF1-Mediated Invasion in Squamous Cell Carcinoma of the Oral Cavity. Mol Cancer Res 2016; 14:883–891. [DOI] [PubMed] [Google Scholar]
- 123.Kayamori K, Katsube KI, Sakamoto K, et al. NOTCH3 Is Induced in Cancer-Associated Fibroblasts and Promotes Angiogenesis in Oral Squamous Cell Carcinoma. PLoS One 2016; 11:e0154112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ishida T, Hijioka H, Kume K, Miyawaki A, Nakamura N. Notch signaling induces EMT in OSCC cell lines in a hypoxic environment. Oncol Lett 2013; 6:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Z, Li H, Fan S, Lin H, Lian W. STAT3-induced upregulation of long noncoding RNA HNF1A-AS1 promotes the progression of oral squamous cell carcinoma via activating Notch signaling pathway. Cancer Biol Ther 2019; 20:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li B, Chen M, Lu M, Xin-Xiang J, Meng-Xiong P, Jun-Wu M. Glutaredoxin 3 promotes migration and invasion via the Notch signalling pathway in oral squamous cell carcinoma. Free Radic Res 2018; 52:390–401. [DOI] [PubMed] [Google Scholar]
- 127.Laxmidevi LB, Angadi P V., Pillai RK, Chandreshekar C. Aberrant β-catenin expression in the histologic differentiation of oral squamous cell carcinoma and verrucous carcinoma: an immunohistochemical study. J Oral Sci 2010; 52:633–640. [DOI] [PubMed] [Google Scholar]
- 128.González-Moles MA, Ruiz-Ávila I, Gil-Montoya JA, Plaza-Campillo J, Scully C. β-Catenin in oral cancer: An update on current knowledge. Oral Oncol 2014; 50:818–824. [DOI] [PubMed] [Google Scholar]
- 129.Temam S, Kawaguchi H, El-Naggar AK, et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J Clin Oncol 2007; 25:2164–2170. [DOI] [PubMed] [Google Scholar]
- 130.Chung CH, Ely K, McGavran L, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol 2006; 24:4170–4176. [DOI] [PubMed] [Google Scholar]
- 131.Fujiwara T, Eguchi T, Sogawa C, et al. Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody cetuximab. Oral Oncol 2018; 86:251–257. [DOI] [PubMed] [Google Scholar]
- 132.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012; 1:1223–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev 2005; 24:95–105. [DOI] [PubMed] [Google Scholar]
- 134.Jie HB, Gildener-Leapman N, Li J, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br J Cancer 2013; 109:2629–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25 highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007; 13:6301–6311. [DOI] [PubMed] [Google Scholar]
- 136.Saussez S, Duray A, Demoulin S, Hubert P, Delvenne P. Immune suppression in head and neck cancers: A review. Clin Dev Immunol 2010; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Moskovitz JM, Ferris RL. Tumor Immunology and Immunotherapy for Head and Neck Squamous Cell Carcinoma. J Dent Res 2018; 97:622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019; 393:156–167. [DOI] [PubMed] [Google Scholar]
- 140.Burtness B, Harrington KJ, Greil R, et al. LBA8_PR KEYNOTE-048: Phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Ann Oncol 2018; 29:2018. [Google Scholar]
- 141.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016; 375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schwab KS, Kristiansen G, Schild HH, Held SEA, Heine A, Brossart P. Successful Treatment of Refractory Squamous Cell Cancer of the Head and Neck with Nivolumab and Ipilimumab. Case Rep Oncol 2018; 11:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carbognin L, Pilotto S, Milella M, et al. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One 2015; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferris RL, Blumenschein G, Harrington K, et al. Abstract CT021: Tumor-associated immune cell PD-L1 expression and peripheral immune profiling: Analyses from CheckMate 141. In: Proceedings: AACR Annual Meeting.; 2017. [Google Scholar]
- 145.Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: Results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol 2016; 34:3838–3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017; 377:2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yarchoan M, Albacker LA, Hopkins AC, et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (80- ) 2017; 357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015; 372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee CH, Yelensky R, Jooss K, Chan TA. Update on Tumor Neoantigens and Their Utility: Why It Is Good to Be Different. Trends Immunol 2018; 39:536–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hellmann MD, Nathanson T, Rizvi H, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018; 33:843–852.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2018; 33:853–861.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oliva M, Spreafico A, Taberna M, et al. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann Oncol 2019; 30:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol 2016; 17:956–965. [DOI] [PubMed] [Google Scholar]
- 155.Seiwert TY. Biomarkers Predictive of Response to Pembrolizumab in head and neck cancer (HNSCC). In: American Association for Cancer Research Annual Meeting; April 14–18, 2018; Chicago.; 2018:Abstract LB-339. [Google Scholar]