Abstract
The present study investigated the clinical significance of Snail, a zinc‐finger transcription factor, in the development and progression of gastric cancer. To elucidate the relationship between Snail expression and dedifferentiation status with cancer stem cell phenotype in gastric cancer cells, we used western blot analysis, RT‐PCR, quantitative real‐time PCR and flow cytometry. Immunohistochemistry staining and evaluation of Snail expression in 10 human normal gastric samples versus 103 clinicopathologically characterized gastric cancer tissues followed by statistical analyses were applied to evaluate the prognostic value of Snail expression for progression and patient survival of gastric carcinomas. The results showed that functional Snail expression interlinks dedifferentiation status with cancer stem cell phenotype in gastric cancer cells. In addition, expression levels of Snail in gastric cancer tissues were significantly associated with tumor cell differentiation, local tumor growth, lymph node status, distant metastasis and tumor stage. The overall survival rate of gastric cancer patients with high Snail expression was significantly lower than for those patients with low Snail expression. Multivariate Cox regression analyses showed that Snail expression is an independent prognostic predictor for patient survival of gastric carcinomas. Thus, our data suggest that Snail expression could be a reliable independent prognostic factor to predict gastric carcinoma progression, which might open a new avenue for potential clinical intervention with functional Snail expression in gastric cancer patients. (Cancer Sci 2012; 103: 1296–1303)
Despite a marked decline in the incidence of gastric carcinoma in many industrialized nations, gastric cancer remains the second most frequent cause of cancer‐related deaths worldwide, accounting for a total of 989 000 new cases and 738 000 deaths in 2008, over 70% of which occurred in developing countries.1, 2, 3 More than 90% of gastric cancers have been reported to be gastric adenocarcinomas, which can be subdivided into two categories, including an intestinal type characterized by histologically differentiated cohesive neoplastic cells forming gland‐like tubular structures with an expanding growth pattern, and a diffuse type, where histologically undifferentiated tumor cells lose cell adhesion/cohesion and thicken the gastric wall through a diffuse infiltrative growth pattern.1, 4, 5 Hereditary diffuse gastric carcinoma accounts for approximately 1–3% of gastric cancer patients, and in 30% of these patients, a germline mutation in one allele of the E‐cadherin gene (CDH1) has been identified.2, 6 Sporadic gastric cancer of the intestinal type develops through prolonged sequential precancerous stages from chronic gastritis, atrophy, intestinal metaplasia to dysplasia initiated frequently by Helicobacter pylori infection.2, 7, 8, 9, 10 Although the intestinal type tends to predominate in geographic regions with a high incidence of gastric carcinoma (e.g. Japan, Korea and China) and is less likely to be found in areas where the frequency is declining (e.g. North America, Africa, South Asia and Oceania), the incidence of diffuse lesions is similar in most populations throughout the world.1, 2, 11 The decline in the incidence of gastric cancer during this century appears to be largely attributable to a decrease in the number of intestinal‐type lesions.1
Although the carcinogenic pathway of intestinal‐type gastric carcinomas, which are mainly associated with Helicobacter pylori infection, subsequent inflammation and tissue regeneration and genetic changes underlying diffuse‐type gastric carcinoma, including CDH1 mutation, has been identified in previous studies, the molecular oncogenic mechanisms demarcating intestinal‐type and diffuse‐type gastric cancer remain poorly understood. Defects in E‐cadherin function are specifically associated with diffuse‐type gastric cancer, which possibly develops through a shorter, unidentified sequence of events from gastric epithelial cells.5 As patients with a germline mutation in CDH1 exclusively develop diffuse‐type gastric carcinomas, E‐cadherin might be specifically involved in gastric epithelial‐cell differentiation.5 However, inappropriate activation of intestine‐specific CDX2 is one of the dominant determinants linked with the induction of intestinal metaplasia occurring in intestinal‐type lesions derived from gastric mucosa cells.5 During carcinogenic events in intestinal metaplasia, gastric mucosa cells deviate from the normal pathway of gastric differentiation to an intestinal phenotype, which is thought to be a crucial precancerous change associated with intestinal‐type gastric cancer.2, 5 Aberrant transcriptional mechanism orchestrating gut differentiation might contribute to the occurrence of intestinal‐type and diffuse‐type gastric cancer.
The Snail superfamily of zinc‐finger transcription factors is best known for the induction of a phenotype change called epithelial‐to‐mesenchymal transition (EMT).12 Snail‐induced EMT converts epithelial cells into mesenchymal cells with migratory properties that contribute to the formation of many tissues during embryonic development and to the acquisition of invasive properties in epithelial tumors,13, 14 which is partly due to the direct repression of CDH1 transcription through binding with the E‐box containing the CAGGTG core motif located within its promoter both during development and tumor progression.12, 13 SNAI1 genes could also protect cells from the death induced either by the loss of survival factors or by direct apoptotic stimuli, which emerge concomitant with or without the induction of EMT during cell movement.13 Therefore, SNAI1 genes have been proposed to act primary as survival factors and inducers of cell movement.13 As the loss of E‐cadherin expression in epithelial tumors is considered to be a marker of a poor clinical outcome, potent E‐cadherin repressors are regarded as potential prognostic markers and therapeutic targets for invasive cancers. Although initial studies showed that an increase in SNAI1 mRNA transcripts is associated with CDH1 downregulation in diffuse‐type gastric tumors,15 which is not confirmed in an immunohistochemical analysis of a larger series,16 the clinical significance of Snail expression in gastric cancer development and progression remains poorly understood.
In the present study, we seek to determine the clinical implications of Snail expression in gastric carcinoma progression. Our investigation reveals that Snail upregulation, which interlinks the dedifferentiation process with cancer stem cell properties of gastric cancer cells, is as a promising marker for determining the prognosis of gastric cancer patients and a potential molecular target for gastric carcinoma therapy.
Materials and Methods
Cell culture
Four human gastric cancer cell lines, including N87 (well differentiated), SGC7901 (moderate differentiated), AGS (poorly differentiated and HGC27 (undifferentiated), were obtained directly from Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cell lines have been characterized at the bank by DNA fingerprinting analysis using short tandem repeat markers. All cell lines were placed under cryostage after they were obtained from the bank and used within 6 months of thawing fresh vials. Three gastric cancer cell lines (N87, SGC7901 and AGS) were routinely maintained in RPMI‐1640 (Sigma–Aldrich, St. Louis, MO, USA) and HGC27 was cultured in DMEM (Sigma–Aldrich) supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified 5% CO2 incubator.
Human samples
Ten human normal gastric samples were obtained from partial gastrectomy of adjacent gastric ulcer tissues. Human gastric cancer tissue samples were collected at the time of surgical resection from 103 patients with gastric adenocarcinoma who had not had any chemotherapy or radiation therapy before surgery. The samples had been formalin‐fixed and paraffin‐embedded. Gastric adenocarcinoma had been clinically and histopathologically diagnosed from 2000 to 2005 at Zhongshan Hospital of Fudan University. Routine chemotherapy had been given to the patients with advanced‐stage disease after operation, but no radiation treatment was carried out in any of the patients included in our study. Signed informed consent was obtained from all patients and the study was approved by the Clinical Research Ethics Committee of Zhongshan Hospital of Fudan University.
Western blot
Protein extraction from cultured cells and western blot analysis were carried out as previously described.17 Primary antibodies used included those against Snail (Cell Signaling Technology, Danvers, MA, USA), E‐cadherin and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
RT‐PCR and quantitative real‐time PCR
Total RNA isolated from cultured cells was extracted using TRIzol reagent (Invitrogen). RT‐PCR and quantitative real‐time PCR (qPCR) were performed as previously described.18 The PCR primer sets used here for the respective genes were designed as follows: Snail 5′‐CACCTCCAGACCCACTCAGAT‐3′ (sense) and 5′‐CCTGAGTGGGGTGGGAGCTTCC‐3′ (antisense); E‐cadherin 5′‐CAAGCTATCCTTGCACCTCAG‐3′ (sense) and 5′‐GCATCAGAGAACTCCTATCTTG‐3′ (antisense). GAPDH was used as an internal control and amplified with forward primer, 5′‐GGCTGAGAACGGGAAGCTTGTCAT‐3′, and reverse primer, 5′‐CAGCCTTCTCCATGGTGGTGAAGA‐3′.
Flow cytometry
Flow cytometry analysis for cancer stem cell phenotype of gastric cancer cells was performed with anti‐CD24‐FITC, anti‐CD44‐PE, anti‐CD90‐FITC, anti‐EpCAM‐PE (BD Biosciences, San Jose, CA, USA) and anti‐CD133‐PE (Miltenyi Biotec, Bergisch Gladbach, Germany), as described previously.19 All flow cytometry data were acquired on a BD FACSCalibur (BD Biosciences) and analyzed by FlowJo software (Tree Star, San Carlos, CA, USA).
Immunohistochemistry analysis and evaluation
A total of 10 human normal gastric samples and 103 human gastric cancer sections were H&E stained and immunohistochemically analyzed as described previously.20 Primary anti‐Snail antibody (Abcam, Cambridge, MA, USA) was used for immunohistochemistry analysis and the intensity of immunohistochemistry staining in the tumor tissues was scored independently by two pathologists using a semiquantitative immunoreactive score according to Remmele and Stegner.21
Statistical analysis
Experimental data were presented as mean ± SEM of at least three independent replicates through analyzing with GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). The correlation between Snail expression and clinicopathologic features was assessed using the chi‐squared or Fisher's exact test with SPSS version 17.0 statistical software package (SPSS Inc., Chicago, IL, USA). Survival was calculated from the date of surgery to the date of death or last follow‐up. Survival curves were estimated using the Kaplan–Meier method, and the log‐rank test was used to compute differences between the curves. Independent prognostic factors were analyzed using the Cox multivariate proportional hazards regression model in a stepwise manner. Differences were considered significant at values of P < 0.05.
Results
Snail expression intertwines dedifferentiation status with cancer stem cell phenotype in gastric cancer cells
Although aberrant transcriptional control of gut differentiation contributes to precancerous intestinal metaplasia of the gastric mucosa prior to intestinal‐type gastric cancer,5 and transcription repressor Snail is one of the most crucial contributing factors to mesoderm differentiation and metazoan development,14 the fundamental role of Snail expression in gut differentiation and carcinogenesis remains unknown. To elucidate the involvement of SNAI gene in gastric cancer cell differentiation, western blot analysis for Snail and its target gene E‐cadherin protein expression was performed in four human gastric cancer cell lines, including the well differentiated N87, moderate differentiated SGC7901, poorly differentiated AGS and undifferentiated HGC27. As shown in Figure 1(A,B), gradually increased Snail protein and decreased E‐cadherin protein expression was found accompanied with the dedifferentiation process in gastric cancer cells. Further RT‐PCR (Fig. 1C) and qPCR (Fig. 1D) analysis for Snail and E‐cadherin mRNA transcription levels revealed that suppressed Snail transcription and instigated E‐cadherin expression occurred in well differentiated intestinal‐type gastric cancer cells (N87) compared with poorly differentiated gastric cancer cells (SGC7901, AGS and HGC27). There are some contradictions between the protein and mRNA expression levels of Snail and E‐cadherin, which appears to point out a post‐transcriptional regulation. The proteins of Snail and E‐cadherin are unstable; the phosphorylation of serine/tyrosine residues and potential modification of adjacent residues implicated in the protein stability of Snail and E‐cadherin may account for the contradictions. The abovementioned results indicate that Snail functional expression might be positively correlated with the dedifferentiation process in gastric cancer cells.
Figure 1.
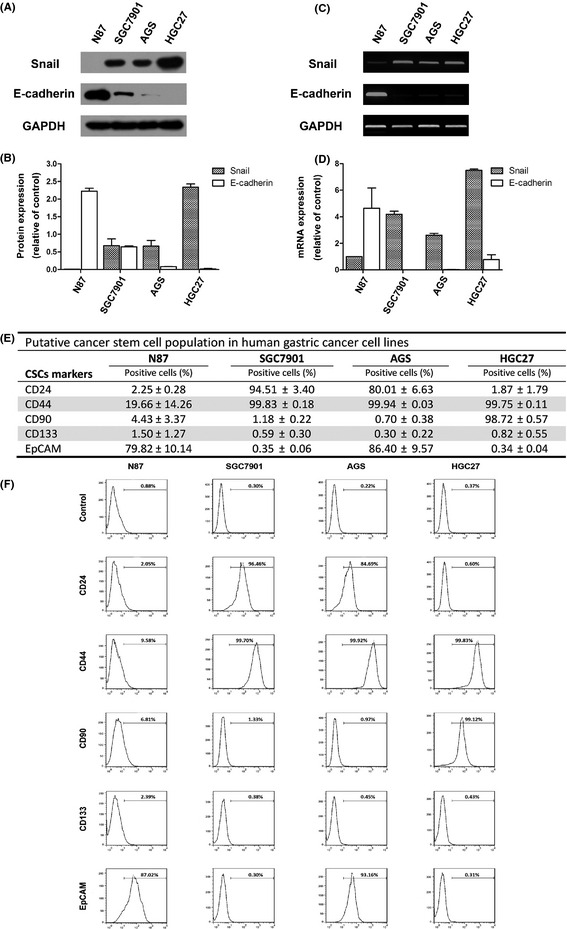
Functional Snail expression and putative cancer stem cell surface markers in four human gastric cancer cell lines. (A) Western blot analysis for Snail and E‐cadherin protein levels in four human gastric cancer cell lines (N87, SGC7901, AGS and HGC27). (B) Densitometry quantification of Snail and E‐cadherin protein levels shown in (A). RT‐PCR (C) and quantitative real‐time PCR (D) analysis for Snail and E‐cadherin mRNA levels in abovementioned four human gastric cancer cell lines. GAPDH was used for internal control for protein and mRNA level analysis. (E) Flow cytometry analysis for putative cancer stem cell surface markers (CD24, CD44, CD90, CD133 and EpCAM) on human gastric cancer cell lines. (F) Representative flow cytometry histograms for detecting CD24, CD44, CD90, CD133 and EpCAM positive cells on human gastric cancer cell lines.
Cancer stem cells are a subpopulation of tumor cells that selectively possess tumor initiation and self‐renewal capacity and the potential to develop into bulk populations of nontumorigenic cancer cell progeny through differentiation.22, 23 Although the possible origins of the gastric cancer stem cells from organ‐specific gastric epithelial stem cells versus bone‐marrow derived cells remain controversial, some combination of intrinsic and niche‐derived cues likely converts gastric cells with proliferative potential into cells with aberrant, metaplastic differentiation patterns that lead to dysplasia and carcinoma.24, 25 Flow cytometry analysis was performed to evaluate potential cancer stem cell surface markers CD24, CD44, CD90, CD133 and EpCAM (epithelial cellular adhesion molecule) expression in the aforementioned gastric cancer cells. As shown in Figure 1(E,F), CD44+ cell population was significantly enriched in the poorly differentiated human gastric cancer cells with high Snail expression (SGC7901, AGS and HGC27) compared with the well differentiated human gastric cancer cells with low Snail expression (N87). Another potential cancer stem cell marker, CD90, was only enriched in the undifferentiated HGC27 cells with the highest Snail expression compared with differentiated gastric cancer cells with lower Snail expression (N87, SGC7901 and AGS). However, in this study, CD24, CD133 and EpCAM showed no positive correlation with Snail expression or dedifferentiation status of gastric cancer cells, excluding their potential significance in gastric cancer stem cell phenotype. In summary, Snail expression might interlink dedifferentiation status with cancer stem cell properties (CD44+ and CD90+) in gastric cancer cells.
Snail expression involves clinical progression of human gastric cancer patients
To ascertain whether Snail protein elevation is linked to clinical progression of gastric cancer, immunohistochemical staining with Snail antibody was carried out for the following samples: 10 paraffin‐embedded, archived noncancerous human gastric tissues and 103 paraffin‐embedded, archived gastric cancer tissue samples. Immunohistochemical results indicating Snail expression in 103 gastric cancer patients are summarized in Table 1. No Snail staining was detected in the 10 noncancerous human gastric epithelial tissues, whereas low or high Snail protein was detected, respectively, in 65 (63.11%) and 38 (36.89%) of the 103 human gastric cancer cases. As shown in Figure 2, Snail protein increased gradually, accompanied with gastric cancer progression from noncancerous gastric tissue (Fig. 2A), tubular adenocarcinoma (Fig. 2B), moderate differentiated adenocarcinoma (Fig. 2C) and poorly differentiated adenocarcinoma (Fig. 2D). Moreover, increased nuclear Snail staining versus weak cytoplasmic Snail expression occurred in the gastric dedifferentiation process during clinical gastric cancer progression. Taken together, these observations suggest that elevated functional Snail expression is associated with clinical development of gastric cancer.
Table 1.
Relation between snail expression and clinicopathologic factors in 103 patients with gastric cancer
| Factor | Patients | Snail expression | P * | ||
|---|---|---|---|---|---|
| Number | % | Low | High | ||
| All patients | 103 | 100 | 65 | 38 | |
| Age (years)† | 0.739 | ||||
| ≤60 | 52 | 50.49 | 32 | 20 | |
| >60 | 51 | 49.51 | 33 | 18 | |
| Gender | 0.402 | ||||
| Female | 38 | 36.89 | 22 | 16 | |
| Male | 65 | 63.11 | 43 | 22 | |
| Localization | 0.252 | ||||
| Proximal | 10 | 9.71 | 8 | 2 | |
| Middle | 51 | 49.51 | 34 | 17 | |
| Distal | 42 | 40.78 | 23 | 19 | |
| Differentiation | 0.005 | ||||
| Well | 7 | 6.80 | 6 | 1 | |
| Moderate | 36 | 34.95 | 29 | 7 | |
| Poorly | 60 | 58.25 | 30 | 30 | |
| Lauren classification | 0.258 | ||||
| Intestinal type | |||||
| High | 7 | 6.80 | 6 | 1 | |
| Medium | 36 | 34.95 | 29 | 7 | 0.748a |
| Low | 34 | 33.01 | 16 | 18 | 0.003b |
| Diffuse type | |||||
| Undifferentiated | 26 | 25.24 | 14 | 12 | 0.602c |
| T classification | <0.001 | ||||
| Tis + T1 + T2 | 42 | 40.78 | 37 | 5 | |
| T3 + T4 | 61 | 59.22 | 28 | 33 | |
| N classification | <0.001 | ||||
| N0 | 44 | 42.72 | 38 | 6 | |
| N1 + 2 + 3 | 59 | 57.28 | 27 | 32 | |
| Distant metastasis | 0.022 | ||||
| No | 100 | 97.09 | 65 | 35 | |
| Yes | 3 | 2.91 | 0 | 3 | |
| TNM stage | <0.001 | ||||
| 0 + I + II | 55 | 53.40 | 47 | 8 | |
| III + IV | 48 | 46.60 | 18 | 30 | |
| Number of resected lymph nodes | 0.272 | ||||
| <25 | 39 | 37.86 | 22 | 17 | |
| ≥25 | 64 | 62.14 | 43 | 21 | |
| Tumor size (cm)† | 0.055 | ||||
| <3 | 44 | 42.72 | 32 | 11 | |
| ≥3 | 59 | 57.28 | 33 | 26 | |
| Kiel stage | <0.001 | ||||
| 0 + ΙI + ΙΙII + ΙΙΙΑIIIA | 51 | 49.51 | 45 | 6 | |
| IIIBΙΙΙΒ + IV | 52 | 50.49 | 20 | 32 | |
Kiel proposal of stage grouping: Stage 0 (TisN0M0); Stage I (T1a‐bN0M0); Stage II (T2‐4aN0M0); Stage IIIA (T1a‐3N1M0); Stage IIIB (T4bN0M0, T4a‐bN1M0, TxN2M0, T1‐3N3M0); Stage IV (T4a‐bN3M0‐1).
*P < 0.05 was considered statistically significant.†Split at median.
P‐value was calculated between high and medium differentiated.
P‐value was calculated between medium and low differentiated.
P‐value was calculated between low differentiated and undifferentiated. Kiel stage, Kiel proposal of stage grouping.
Figure 2.
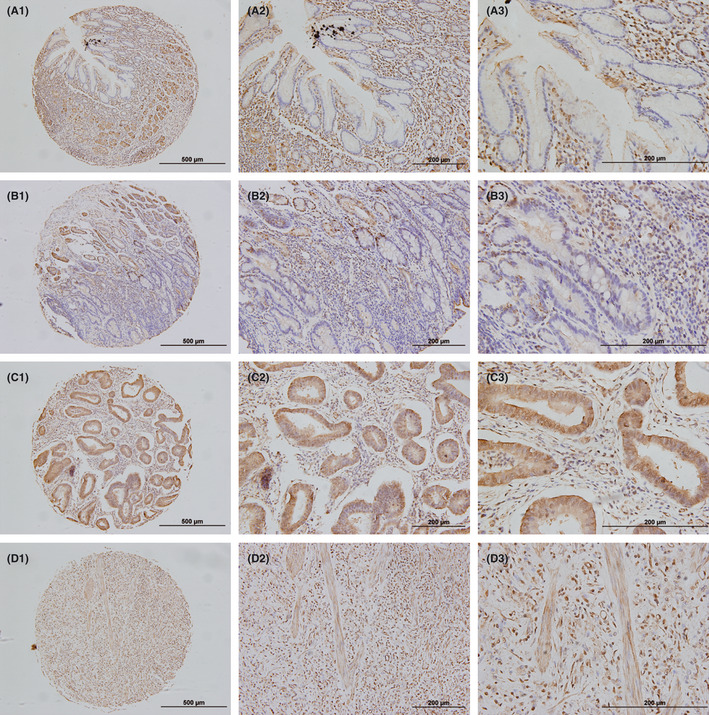
Immunohistochemical Snail staining in human noncancerous gastric tissues and gastric cancer lesions. (A) 1–3 represent negative immunohistochemical Snail staining in human noncancerous gastric epithelial tissues with ×100, ×200 and ×400 magnifications, respectively. (B) 1–3 represent negative or weakly immunohistochemical Snail staining in human gastric tubular adenocarcinoma with ×100, ×200 and ×400 magnifications, respectively. (C) 1–3 represent moderately immunohistochemical Snail staining in human gastric differentiated adenocarcinoma with ×100, ×200 and ×400 magnifications, respectively. (D) 1–3 represent highly immunohistochemical Snail staining in human gastric poorly differentiated adenocarcinoma with ×100, ×200 and ×400 magnifications, respectively.
Correlation between Snail overexpression with clinicopathologic features in gastric cancer patients
Immunohistochemical staining of Snail levels was statistically analyzed to determine their relationship with clinicopathologic features of the 103 gastric cancer patients. As shown in Table 1, Snail overexpression strongly correlated with the tumor cell differentiation (P = 0.005), local tumor growth (T classification; P < 0.001), lymph node status (N classification; P < 0.001), distant metastasis (P = 0.022) and tumor TNM stage (P < 0.001) of gastric cancer patients, whereas it was not associated with age, gender, tumor localization, Lauren classification, number of resected lymph nodes, and tumor size. Because gastric differentiation deviation occurs in intestinal and diffuse types of gastric cancer which is defined by Lauren classification,5 correlation between immunohistochemical Snail staining and differentiation fractionation in Lauren classification were analyzed in 103 gastric cancer patients. As shown in Table 1, although the relationship between Snail expression and Lauren classification is not significant, the different Snail expression could significantly stratify medium and low differentiated groups in intestinal‐type gastric cancer tissues (P = 0.003). Overall, these data indicate that Snail overexpression correlates with clinical gastric cancer progression, especially the dedifferentiation process, local tumor growth, lymph node infiltration, distant metastasis and tumor TNM stage.
Snail expression is an independent prognostic predictor for patient survival of gastric cancer
To further determine the prognostic value of Snail expression, univariate and multivariate analyses were performed in 103 gastric cancer patients. As shown in Table 2, univariate analysis revealed that Lauren classification (P = 0.010), T classification (P < 0.001), N classification (P < 0.001), distant metastasis (P < 0.001), TNM stage (P < 0.001) and Snail expression (P < 0.001) all significantly influenced survival of gastric cancer patients. Moreover, Kaplan–Meier survival analysis indicated that the overall survival rate of gastric cancer patients with high Snail expression was significantly lower than for those patients with low Snail expression (P < 0.001) (Fig. 3). More importantly, Cox multivariate regression analysis suggested that Snail expression (P = 0.039), as well as Lauren classification (P = 0.018) and TNM stage (P < 0.001) are independent prognostic factors in gastric cancer patients (Table 2). In order to provide an exact distinction of node‐positive and node‐negative patients, Warneke et al.26 propose a revised staging system, termed Kiel proposal of stage grouping of gastric cancer on the basis of the TNM classification. As shown in Table 1, Snail expression was also significantly correlated with Kiel stage (P < 0.001). Furthermore, Cox multivariate regression analysis showed that Lauren classification (P = 0.032), Kiel stage (P = 0.001) and Snail expression (P = 0.039) were independent prognostic factors (Table 3). Taken together, our data suggest that Snail might represent a novel and potentially useful independent marker for the prognosis of patients with gastric carcinoma.
Table 2.
Univariate and multivariate analyses of factors for overall survival in 103 patients with gastric cancer
| Factor | Patients | Overall survival | ||||
|---|---|---|---|---|---|---|
| Univariate P * | Multivariate | |||||
| Number | % | HR | 95%CI | P * | ||
| Age (years)† | ||||||
| ≤60 | 52 | 50.49 | 0.083 | NA | ||
| >60 | 51 | 49.51 | ||||
| Gender | ||||||
| Female | 38 | 36.89 | 0.946 | NA | ||
| Male | 65 | 63.11 | ||||
| Localization | ||||||
| Proximal | 10 | 9.71 | 0.318 | NA | ||
| Middle | 51 | 49.51 | ||||
| Distal | 42 | 40.78 | ||||
| Differentiation | ||||||
| Well | 7 | 6.80 | 0.201 | NA | ||
| Moderate | 36 | 34.95 | ||||
| Poorly | 60 | 58.25 | ||||
| Lauren classification | ||||||
| Intestinal type | 77 | 74.76 | 0.010 | 2.470 | 1.166–5.230 | 0.018 |
| Diffuse type | 26 | 25.24 | ||||
| T classification | ||||||
| Tis + T1 + T2 | 42 | 40.78 | <0.001 | NA | ||
| T3 + T4 | 61 | 59.22 | ||||
| N classification | ||||||
| N0 | 44 | 42.72 | <0.001 | NA | ||
| N1 + 2 + 3 | 59 | 57.28 | ||||
| Distant metastasis | ||||||
| No | 100 | 97.09 | <0.001 | NA | ||
| Yes | 3 | 2.91 | ||||
| TNM stage | ||||||
| 0 + I + II | 55 | 53.40 | <0.001 | 10.386 | 2.966–39.367 | <0.001 |
| III + IV | 48 | 46.60 | ||||
| Number of resected lymph nodes | ||||||
| <25 | 39 | 37.86 | 0.193 | NA | ||
| ≥25 | 64 | 62.14 | ||||
| Tumor size (cm)† | ||||||
| <3 | 44 | 42.72 | 0.058 | NA | ||
| ≥3 | 59 | 57.28 | ||||
| Snail expression | ||||||
| Low | 65 | 63.11 | <0.001 | 2.460 | 1.045–5.790 | 0.039 |
| High | 38 | 36.89 | ||||
*P < 0.05 was considered statistically significant. †Split at median. HR, hazard ratio; 95% CI, 95% confidence interval; NA: not adopted.
Figure 3.
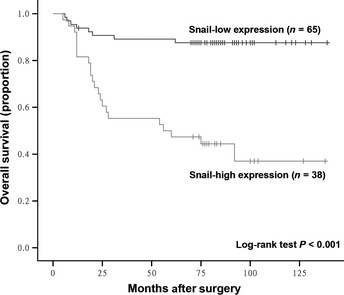
Comparison of overall survival in gastric cancer patients with high and low expression levels of Snail. Kaplan–Meier survival analysis was performed using 65 gastric cancer patients with low Snail expression and 38 gastric cancer patients with high Snail expression. P‐value was calculated by log‐rank test.
Table 3.
Univariate and multivariate analyses with inclusion of Kiel stage for overall survival in 103 patients with gastric cancer
| Factor | Patients | Overall survival | ||||
|---|---|---|---|---|---|---|
| Univariate P * | Multivariate | |||||
| Number | % | HR | 95%CI | P * | ||
| Lauren classification | ||||||
| Intestinal type | 77 | 74.76 | 0.010 | 2.260 | 1.072–4.763 | 0.032 |
| Diffuse type | 26 | 25.24 | ||||
| Kiel stage | ||||||
| 0 + I + II + IIIA | 51 | 49.51 | <0.001 | 8.019 | 2.248–28.596 | 0.001 |
| IIIB + IV | 52 | 50.49 | ||||
| Snail expression | ||||||
| Low | 65 | 63.11 | <0.001 | 2.502 | 1.048–5.973 | 0.039 |
| High | 38 | 36.89 | ||||
*P < 0.05 was considered statistically significant. HR, hazard ratio; 95% CI, 95% confidence interval; Kiel stage, Kiel proposal of stage grouping.
Discussion
Gastric cancer can be divided into two distinct histological groups: the histologically differentiated intestinal‐type carcinomas derived from gastric mucosa cells and the histologically undifferentiated diffuse‐type carcinomas originated from gastric epithelial cells.4, 5 Inappropriate activation of specific developmental pathways is likely to be involved in the development of intestinal metaplasia and intestinal‐type gastric carcinomas, indicating that gastric epithelial cells deviate from their normal differentiation pathways towards an intestinal phenotype. Helicobacter pylori infection frequently induces a transition from normal mucosa to chronic superficial gastritis, followed by atrophic gastritis, intestinal metaplasia, adenoma and intestinal‐type gastric carcinoma through perturbing transcriptional control of gut differentiation.5 During this malignant transformation, abnormal activation of intestinal‐specific CDX2 transcription most likely contributes to the induction of intestinal metaplasia, followed less frequently by adenoma–carcinoma sequence attributable to APC/β‐catenin mutations.5 Other genetic changes, such as methylation of MLH1 promoter, TP53 alteration, microsatellite instability and transforming growth factor‐β (TGF‐β) type II receptor (TGF‐βRII) gene mutations, have been associated with a small subset of intestinal‐type gastric carcinomas.5 Moreover, somatic mutations and loss and methylation of CDH1 gene are frequently observed in diffuse‐type gastric cancer cells,27, 28 and germline mutations of CDH1 have been identified in patients with hereditary diffuse gastric carcinomas,29 indicating that E‐cadherin might be specifically involved in gastric epithelial cell differentiation. As a potent E‐cadherin repressor, zinc‐finger transcription factor Snail attracts our research interest for its potential clinical implications in gastric cancer progression.
Ryu et al.30 indicate that the expression level of EMT‐related molecules (Snail‐1, ZEB‐1, E‐cadherin, vimentin and β‐catenin) and a stem cell marker (CD44) show significant association for determining prognosis for gastric cancer. The combination of EMT and stem cell‐like phenotypes is significantly correlated with the disease‐free survival, but not the overall survival. Uchikado et al.31 demonstrate that evaluation of the coexpression of E‐cadherin and Slug in patients with preserved E‐cadherin expression would be useful for predicting malignant properties of gastric cancer. However, E‐cadherin expression and slug expression are not independent prognostic factors. In the present study, we have demonstrated that the overall survival rate of gastric cancer patients with high Snail expression is significantly lower than for those patients with low Snail expression, and Snail serves as an independent prognostic predictor for progression and patient survival of gastric cancer.
Epithelial‐to‐mesenchymal transition is a crucial characteristic of metastatic cancer cells during tumor development. As a pivotal transcriptional factor governing EMT, Snail‐induced EMT of cancer cells has been found to accelerate cancer metastasis through immunosuppression.32 In the present study, our data suggest that Snail overexpression strongly correlates with EMT features and the progression of gastric cancer, including the tumor cell differentiation, local tumor growth, lymph node status, distant metastasis and tumor TNM stage of gastric cancer patients. Our results indicate that Snail expression is a determination factor for gastric wall invasion, lymph node metastasis and distant metastasis, which confirms previous conclusions that Snail‐induced EMT contributes to the development of malignant tumors.13 In addition, the present study reveals that CD44 and CD90 might be useful markers for determining the gastric cancer stem cell phenotype with dedifferentiation status of human gastric cancer cells. Snail expression might interlink dedifferentiation status with cancer stem cell properties in gastric cancer cells. However, clarifying the mechanism of underlying Snail expression and the gastric cancer stem cell properties await further investigation.
A series of genes and signaling molecules, including FGF, Wnt, bone morphogenetic protein, TGF‐β and parathyroid‐hormone‐related peptide receptor, have been implicated in the activation of Snail gene in several processes that subsequently lead to the conversion of epithelial cells into mesenchymal cells.12 Besides its primary contributions to acquisition of migratory properties, Snail, acting as a survival factor and inducer of cell movement during development and pathology, could decrease proliferation and promote resistance to cell death.13 The functional role of Snail protein in gut differentiation, especially precancerous intestinal metaplasia in gastric carcinogenesis, merits further study. Abnormal activation of the intestinal‐specific transcription factor CDX2 is most likely involved in the induction of intestinal metaplasia of gastric through activating transcription of intestinal‐specific proteins, such as MUC2, sucrose/isomaltase and carbonic anhydrase I, and a cyclin‐dependent kinase inhibitor p21.5 Followed by precancerous intestinal metaplasia accompanied by CDX2 expression and intestinal differentiation, gastric cancer cells inevitably become less differentiated during progression, resulting in the downregulation of CDX2 with methylation of its gene promoter.5 Because Snail protein acts primarily as an E‐cadherin repressor through methylating its gene promoter,14 and our present data indicate functional Snail expression during the dedifferentiation process of gastric cancer cells, we might propose that Snail protein functions as a transcriptional repressor for intestinal specific CDX2 gene transcription during gastric cancer progression. However, this awaits further investigation. Moreover, the molecular mechanism underlying Snail expression during gastric carcinogenesis due to intrinsic or microenvironmental stimuli, as well as target genes of Snail protein involved in gastric carcinoma progression remain to be elucidated in future.
The present study reveals the functional role of Snail protein in the convergent interaction of the dedifferentiation process and cancer stem cell phenotype in gastric cancer cells, as well as the clinical significance of Snail overexpression in gastric carcinoma progression. The pathological activation of Snail expression in gastric cancer progression revealed herein indicates the novel prognostic value and potential therapeutic implications of Snail overexpression in gastric carcinoma patients.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgments
This work was supported by grants from the Key Project of Science and Technology Commission of Shanghai Municipality (09DZ1950101), the State Key Project Specialized for Infectious Diseases of China (2012ZX10002‐008, 2012ZX10002‐012), the National Basic Research Program of China 973 Program (2010CB912104, 2011CB910604, 2012CB8221004) and the National Natural Science Fund (30900266, 30930025, 31000348, 31000600, 31010103906, 31170766).
References
- 1. Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med 1995; 333: 32–41. [DOI] [PubMed] [Google Scholar]
- 2. Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet 2009; 374: 477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 4. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 5. Yuasa Y. Control of gut differentiation and intestinal‐type gastric carcinogenesis. Nat Rev Cancer 2003; 3: 592–600. [DOI] [PubMed] [Google Scholar]
- 6. Guilford PJ, Hopkins JB, Grady WM et al E‐cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat 1999; 14: 249–55. [DOI] [PubMed] [Google Scholar]
- 7. Forman D, Newell DG, Fullerton F et al Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ 1991; 302: 1302–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez‐Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med 1991; 325: 1132–6. [DOI] [PubMed] [Google Scholar]
- 9. Parsonnet J, Friedman GD, Vandersteen DP et al Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med 1991; 325: 1127–31. [DOI] [PubMed] [Google Scholar]
- 10. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992; 52: 6735–40. [PubMed] [Google Scholar]
- 11. Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med 2008; 47: 1077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieto MA. The snail superfamily of zinc‐finger transcription factors. Nat Rev Mol Cell Biol 2002; 3: 155–66. [DOI] [PubMed] [Google Scholar]
- 13. Barrallo‐Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 2005; 132: 3151–61. [DOI] [PubMed] [Google Scholar]
- 14. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 2007; 7: 415–28. [DOI] [PubMed] [Google Scholar]
- 15. Rosivatz E, Becker I, Specht K et al Differential expression of the epithelial‐mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol 2002; 161: 1881–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosivatz E, Becker KF, Kremmer E et al Expression and nuclear localization of Snail, an E‐cadherin repressor, in adenocarcinomas of the upper gastrointestinal tract. Virchows Arch 2006; 448: 277–87. [DOI] [PubMed] [Google Scholar]
- 17. Xu J, Yun X, Jiang J et al Hepatitis B virus X protein blunts senescence‐like growth arrest of human hepatocellular carcinoma by reducing Notch1 cleavage. Hepatology 2010; 52: 142–54. [DOI] [PubMed] [Google Scholar]
- 18. Zhang W, Xu W, Xiong S. Blockade of Notch1 signaling alleviates murine lupus via blunting macrophage activation and M2b polarization. J Immunol 2010; 184: 6465–78. [DOI] [PubMed] [Google Scholar]
- 19. Zhang W, Xu W, Xiong S. Macrophage differentiation and polarization via phosphatidylinositol 3‐kinase/Akt‐ERK signaling pathway conferred by serum amyloid P component. J Immunol 2011; 187: 1764–77. [DOI] [PubMed] [Google Scholar]
- 20. Liu H, Xu J, Zhou L et al Hepatitis B virus large surface antigen promotes liver carcinogenesis by activating the Src/PI3K/Akt pathway. Cancer Res 2011; 71: 7547–57. [DOI] [PubMed] [Google Scholar]
- 21. Boulay JL, Dennefeld C, Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. Nature 1987; 330: 395–8. [DOI] [PubMed] [Google Scholar]
- 22. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 2006; 355: 1253–61. [DOI] [PubMed] [Google Scholar]
- 23. Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest 2010; 120: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol 2008; 26: 2876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mills JC, Shivdasani RA. Gastric epithelial stem cells. Gastroenterology 2011; 140: 412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warneke VS, Behrens HM, Hartmann JT et al Cohort study based on the seventh edition of the TNM classification for gastric cancer: proposal of a new staging system. J Clin Oncol 2011; 29: 2364–71. [DOI] [PubMed] [Google Scholar]
- 27. Oda T, Kanai Y, Oyama T et al E‐cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA 1994; 91: 1858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S. Silencing of the E‐cadherin invasion‐suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci USA 1995; 92: 7416–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guilford P, Hopkins J, Harraway J et al E‐cadherin germline mutations in familial gastric cancer. Nature 1998; 392: 402–5. [DOI] [PubMed] [Google Scholar]
- 30. Ryu HS, Park doJ, Kim HH, Kim WH, Lee HS. Combination of epithelial‐mesenchymal transition and cancer stem cell‐like phenotypes has independent prognostic value in gastric cancer. Hum Pathol 2012; 43: 520–8. [DOI] [PubMed] [Google Scholar]
- 31. Uchikado Y, Okumura H, Ishigami S et al Increased Slug and decreased E‐cadherin expression is related to poor prognosis in patients with gastric cancer. Gastric Cancer 2011; 14: 41–9. [DOI] [PubMed] [Google Scholar]
- 32. Kudo‐Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail‐induced EMT of cancer cells. Cancer Cell 2009; 15: 195–206. [DOI] [PubMed] [Google Scholar]


