Abstract
Des‐gamma‐carboxy prothrombin (DCP) is a useful tumor marker for hepatocellular carcinoma (HCC), but its utility is limited in patients taking vitamin K antagonists. We evaluated the NX‐DCP ratio, a newly developed method to measure serum DCP, for its ability to identify DCP elevation induced by HCC in this patient subpopulation. Conventional DCP measurements and the NX‐DCP ratio were compared in patients with and without HCC, all of whom were taking the vitamin K antagonist warfarin. We found no differences in conventional DCP measurements between patients with and without HCC due to warfarin treatment. In contrast, the NX‐DCP ratio was significantly higher in patients with HCC; the NX‐DCP ratio in all patients without HCC was <1.50. When the cut‐off was fixed at 1.50, sensitivity and specificity for HCC diagnosis were 60.0% and 100.0%, respectively, which are comparable to those of conventional DCP measurements in patients not taking warfarin. The novel NX‐DCP ratio identifies patients on warfarin with elevated DCP due to HCC and is useful as a tumor marker for HCC in this patient subpopulation. (Cancer Sci 2012; 103: 921–925)
Prothrombin, or coagulation factor II, is a 71 600 Da protein that consists of three regions: fragment 1; fragment 2; and prothrombin. Fragment 1 consists of 156 amino acids, including 41 amino acids forming an N‐terminal gamma‐glutamic acid (Gla)‐containing domain. Prothrombin is first synthesized in the liver as a precursor with 10 glutamic acid (Glu) residues, which are then modified to Gla residues by gamma‐glutamylcarboxylase in the presence of vitamin K, O2, and CO2 before it is released into the bloodstream.
However, in the absence of vitamin K or in the presence of vitamin K antagonists, gamma‐carboxylation is impaired, and prothrombin with the remaining Glu residues, which is inactive with respect to coagulation, is released into the bloodstream.1 Prothrombin in this form is called des‐gamma‐carboxy prothrombin (DCP) or protein induced by vitamin K absence/antagonist‐II (PIVKA‐II). As the number of Glu residues unconverted to Gla varies, DCP is present as a mixture of prothrombin with various numbers of Glu residues, ranging from 1 to 10. In addition, because the Gla residue can bind to calcium, it is known that the 3‐D protein structure of DCP will be different in the presence of calcium, and depends on the number of Glu residues.2
Des‐gamma‐carboxy prothrombin is frequently found in the blood of patients with hepatocellular carcinoma (HCC). Because DCP is elevated in many patients with HCC but not in patients with chronic hepatitis or cirrhosis without HCC,3 it has been routinely used as a tumor marker of HCC in clinical settings.4, 5, 6 However, serum DCP levels are also increased in the absence of HCC when there is a shortage of vitamin K or in the presence of vitamin K antagonists.7 The value of DCP as a marker of HCC, therefore, is significantly reduced in patients who are taking vitamin K antagonists such as warfarin.
Previous studies reported differences in the number of Glu residues in DCP between patients with HCC and patients taking vitamin K antagonists.8, 9 Conventionally, DCP is measured using a mAb produced by the cell line MU‐3 (Picolumi PIVKA‐II; EIDIA, Tokyo, Japan), which reportedly reacts predominantly with DCP with 9–10 Glu residues. MU‐3 had lower affinity for DCP with one to five Glu residues.10 However, measuring DCP with this antibody alone can not differentiate between HCC‐induced and vitamin K antagonist‐associated elevations of DCP, making it difficult to evaluate whether rises in DCP are caused by HCC in patients taking vitamin K antagonists.
In the present study, we attempted to identify HCC‐induced DCP in patients with HCC taking the vitamin K antagonist warfarin through the use of two mAbs against DCP, P‐11 and P‐16 (Sekisui Medical, Tokyo, Japan), which have a reactivity profile different from MU‐3. We found clinical utility in DCP as a marker for HCC in patients taking warfarin when measured with the combination of MU‐3, P‐11, and P‐16.
Materials and Methods
Preparation of electrochemiluminescence immunoassay (ECLIA) reagents with P‐11 and P‐16
Magnetic beads coated with P‐16 mAb (Sekisui Medical) were prepared as follows: 1 mL of 30 mg/mL magnetic bead suspension (Dynabeads M‐450 Epoxy; Life Technologies, Carlsbad, CA, USA) was placed into a test tube and the magnetic beads were trapped by a magnet to separate the supernatant. After the supernatant was discarded, 1 mL P‐16 mAb (0.5 mg/mL in 0.15 mol/L PBS, pH 7.8) was added to the magnetic beads and stirred at 25°C for 18 h. After washing the magnetic beads, 2 mL of 1% BSA in 0.15 mol/L PBS (pH 7.8) were added and stirred at 25°C for 18 h to block the beads. These beads were diluted to 1 mg/mL using the bead dilution reagent (0.05 mol/L Tris buffer (pH 7.5), 0.15 mol/L NaCl, 0.01% Tween 20, 0.1% NaN3, 10% normal rabbit serum, and 0.1% mouse serum) when in use.
Ruthenium (Ru)‐conjugated P‐11 mAb was prepared by the following procedure: 68 μL Ru‐complex compounds (10 mg Ru (II) Tris (bipyridyl)‐NHS ester in 1 mL DMSO) was added to 1 mL P‐11 mAb (1 mg/mL in 0.15 mol/L PBS, pH 7.8) (Sekisui Medical) for conjugation, and stirred at 25°C for 30 min. Then, 50 μL of 2 mol/L glycine was added to terminate the conjugation reaction, and Ru‐conjugated P‐11 mAb was isolated by collecting the Ru‐bound protein fraction using Sephadex G‐25 (previously equilibrated with 10 mmol/L PBS, pH 6.0). The Ru‐conjugated P‐11 mAb was then diluted to 1 μg/mL using Ru dilution reagent (0.015 mol/L HEPES buffering solution [pH 7.8], 0.15 mol/L NaCl, 0.013 mol/L CaCl2, 0.1% Tween 20, 0.1% NaN3, 5% normal rabbit serum, and 0.1% mouse serum) when in use.
Measurement of conventional DCP (with MU‐3 antibody), NX‐DCP (with P‐11 and P‐16 antibodies), and NX‐DCP ratio
Conventional DCP, which is measured using MU‐3 antibody and is currently used in clinical settings, was measured with ECLIA using the Picolumi III automated analyzer (EIDIA). NX‐DCP was measured by ECLIA. Briefly, 25 μL magnetic beads coated with P‐16 mAb (1 mg/mL) and 150 μL Ru‐conjugated P‐11 mAb (1 μg/mL) were added to samples at 30°C for 9 min to obtain the value of NX‐DCP. The NX‐DCP ratio was calculated by dividing the value of DCP measured using the conventional Picolumi method by the value of NX‐DCP.
Reactivity of MU‐3, P‐11, and P‐16 mAbs based on the time allowed for decarboxylation from prothrombin
We prepared DCP with varying numbers of Glu residues by applying different time intervals for decarboxylation from prothrombin (Enzyme Research Laboratories, Swansea, UK), according to the method of Bajah et al.2 Specifically, 0.78 mL ammonium bicarbonate solution (0.1 mol/L, pH 8.0) was applied to 4.6 mg/mL prothrombin solution overnight for dialysis against an ammonium bicarbonate solution at 4°C. Then 0.1 mol/L EDTA*2Na was applied to the solution after dialysis until a final concentration of 10 mmol/L was achieved, and the solution was allowed to stand at room temperature for 30 min. This solution was dialyzed again against an ammonium bicarbonate solution for 2 h at 4°C then aliquoted into six heat‐resistant vials with a screw cap and lyophilized. The vials were then filled with nitrogen gas and heated to 110°C for 0, 30 min, 1, 2, 6, or 23 h to create six different samples. We coated microplates with 100 μL of each sample at 0.1 μg/mL, and tested reactivity of the MU‐3, P‐11, and P‐16 mAbs in the presence of 4 mmol/L calcium chloride. The experiments were repeated three times and the average value was calculated.
Patients
A total of 338 patients were diagnosed with primary, non‐recurrent HCC between January 2006 and December 2009 at Ogaki Municipal Hospital (Ogaki, Japan). Of these, 14 patients had been taking warfarin when HCC was diagnosed. Six patients at Osaka Red Cross Hospital (Osaka, Japan) who were diagnosed as having primary HCC during the same period and had been taking warfarin at the time of diagnosis were also enrolled in the study. We analyzed the stored serum samples from these 20 patients, obtained at the time of HCC diagnosis. The diagnosis of HCC was made by histological examination or appropriate imaging characteristics using criteria of the guidelines by the American Association for the Study of Liver Diseases.11 Tumor stage on imaging findings was assessed according to the TNM classification of the Liver Cancer Study Group of Japan.12
Control samples were obtained from 56 patients with chronic liver disease without HCC who were followed up at Ogaki Municipal Hospital. Samples were collected during routine HCC surveillance during the same period. These patients had been taking warfarin when serum samples were collected and provided informed consent for their stored serum samples to be used for research. The diagnosis of chronic liver disease was made with histological examination in 45 patients, including 10 with cirrhosis. The remaining 11 patients were diagnosed with cirrhosis based on imaging findings and biochemical tests. To ensure that controls did not have HCC, these patients were followed for 3 years after serum sampling by ultrasonography, CT, or MRI to ensure that none had developed HCC.
The protocol for the clinical part of this study was approved by the institutional review board of Ogaki Municipal Hospital and carried out in compliance with the Helsinki Declaration. Written informed consent was obtained from all study patients for the use of clinical and laboratory data and stored serum samples.
Statistical analyses
Differences in percentages between groups were analyzed using the χ2‐test. Differences in mean quantitative values were analyzed by the Mann–Whitney U‐test. Changes in the NX‐DCP ratio with increases in HCC stage were analyzed with the Jonckheere–Terpstra test. Data analyses were carried out using JMP statistical software, version 6.0 (Macintosh version; SAS Institute, Cary, NC, USA). All P‐values were derived from two‐tailed tests, with P < 0.05 considered to indicate statistical significance.
Results
Reactivity of MU‐3, P‐11, and P‐16 antibodies with DCP based on time allowed for decarboxylation from prothrombin
Figure 1 shows the reactivity of the MU‐3, P‐11, and P‐16 antibodies according to the time allowed for decarboxylation from prothrombin. MU‐3 did not react with prothrombin (0 min) and its reactivity increased as the heating time (time allowed for decarboxylation) increased, with maximum reactivity to the sample after 6 h of heating. In contrast, P‐11 and P‐16 showed maximum reactivity to the 1‐h sample and reactivity decreased as the heating time (time allowed for decarboxylation) increased.
Figure 1.
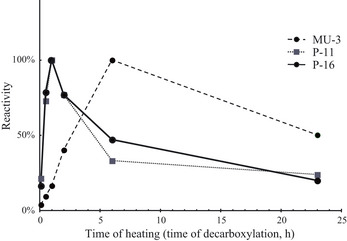
Reactivity of MU‐3, P‐11, and P‐16 antibodies according to the time allowed for decarboxylation from prothrombin. MU‐3 did not react with prothrombin (0 min) and its reactivity increased as the heating time (time allowed for decarboxylation) increased, with maximum reactivity to the sample after 6 h of heating. Both P‐11 and P‐16 showed maximum reactivity to the 1‐h sample and reactivity decreased as the heating time increased.
Patient characteristics and levels of conventional DCP, NX‐DCP, and NX‐DCP ratio
Warfarin was used to treat atrial fibrillation in 48 patients, a history of mitral or aortic valve replacement in 13 patients, and a history of cerebral infarction in 15 patients. Table 1 summarizes the characteristics of the patients with and without HCC. There were no differences in patient age, sex, serum albumin, serum total bilirubin, platelet count, or prothrombin levels.
Table 1.
Background characteristics of study patients with and without hepatocellular carcinoma (HCC) (n = 76)
| Patients with HCC (n = 20) | Patients without HCC (n = 56) | P‐value | |
|---|---|---|---|
| Mean age ± SD, years (range) | 72.4 ± 8.0 (46–83) | 70.0 ± 9.8 (46–86) | 0.3211 |
| Sex, female/male | 6 (30.0)/14 (70.0) | 19 (33.9)/37 (66.1) | 0.9651 |
| Albumin, g/dL (mean ± SD) | 3.82 ± 0.42 | 3.97 ± 0.51 | 0.2276 |
| Total bilirubin, mg/dL (mean ± SD) | 1.02 ± 0.65 | 0.82 ± 0.52 | 0.1288 |
| Platelets (×103/μL) | 158 ± 75 | 160 ± 49 | 0.4754 |
| INR | 1.75 ± 0.58 | 1.76 ± 0.58 | 0.7816 |
| Mean tumor size ± SD, cm (range) | 3.35 ± 1.84 (1.1–8.4) | – | – |
| Number of tumors, single/multiple | 15 (75.0)/5 (25.0) | – | – |
| Tumor stage, I/II/IIIa | 3 (15.0)/11 (55.0)/6 (30.0) | – | – |
According to the TNM classification of the Liver Cancer Study Group of Japan. Unless otherwise indicated, values in parentheses indicate percentages. INR, International normalized ratio.
Figure 2 compares conventional DCP levels, NX‐DCP levels, and NX‐DCP ratios between patients with and without HCC. No differences were found in conventional DCP levels between patients with and without HCC (median, 2600.5 mAU/mL and range, 1060–96920 mAU/mL in patients with HCC versus median, 20550.5 mAU/mL and range, 1355–71783 mAU/mL in patients without HCC; P = 0.7952). In contrast, NX‐DCP levels in patients with HCC (median, 34135.0 mAU/mL; range, 260–67581 mAU/mL) were significantly lower than in patients without HCC (median, 40708.0 mAU/mL; range, 5026–60443 mAU/mL; P = 0.0291). As a result, the NX‐DCP ratio was significantly higher in patients with HCC (median, 1.92; range, 0.35–10.32) than in patients without HCC (median, 0.49; range, 0.12–1.33; P < 0.0001).
Figure 2.
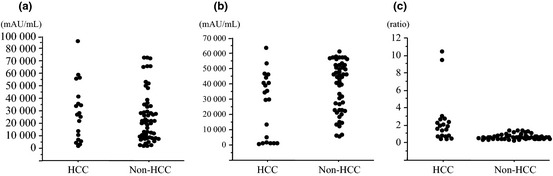
Serum levels of conventional des‐gamma‐carboxy prothrombin (DCP), NX‐DCP, and the NX‐DCP ratio in patients with and without hepatocellular carcinoma (HCC) taking warfarin. (a) Serum levels of conventional DCP. No differences were found between two groups (P = 0.7952). (b) Serum levels of NX‐DCP were significantly higher in patients without HCC compared to those with HCC (P = 0.0291). (c) The NX‐DCP ratio was significantly higher in patients with HCC than in those without HCC, consequently (P < 0.0001).
Sensitivity, specificity, and positive and negative predictive values of NX‐DCP ratio for diagnosis of HCC
Figure 3(a) shows the receiver operating characteristic (ROC) curve of the NX‐DCP ratio for the diagnosis of HCC. The area under the ROC curve was 0.8928. The highest Youden index was 0.68 when the cut‐off was fixed as 0.65 and the highest accuracy was 89.5% when the cut‐off was fixed as 1.50, based on the sensitivity and specificity analysis (Fig. 3b). When the cut‐off was fixed as 0.65, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were 95.0%, 73.2%, 55.9%, 97.6%, and 78.9%, respectively. When the cut‐off was fixed as 1.50, sensitivity, specificity, PPV, NPV, and accuracy were 60.0%, 100.0%, 100.0%, 87.5%, and 89.5%, respectively.
Figure 3.
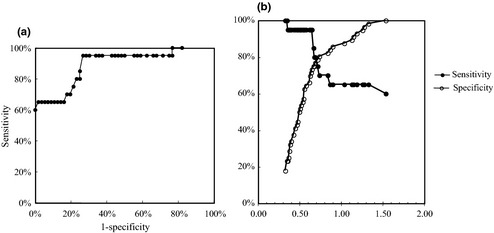
Receiver operating characteristic (ROC) analysis and the determination of cut‐off level of the NX‐DCP ratio for the diagnosis of hepatocellular carcinoma. (a) The area under the ROC curve was 0.8928. (b) The highest Youden index was 0.68 when the cut‐off was fixed as 0.65 and the highest accuracy was 89.5% when the cut‐off was fixed as 1.50.
Serum alpha‐fetoprotein (AFP) and Lens culinaris agglutinin‐reactive fraction of AFP (AFP‐L3) levels in patients with HCC
Serum levels of AFP and AFP‐L3 were measured13 in patients with HCC in the same serum for the measurement of NX‐DCP ratio (AFP‐L3 was not measured in five patients). The median (range) values were 18.4 ng/mL (0.8–68 470 ng/mL) for AFP and 3.6% (0–45.2%) for AFP‐L3. When the cut‐off levels of AFP and AFP‐L3 were fixed as 20 ng/mL and 5%, respectively, according to previous reports,14, 15, 16 10 of 20 patients (50.0%) showed elevation of AFP and seven of 15 patients (46.7%) showed elevation of AFP‐L3. These two tumor markers were not increased in six of 15 patients (40.0%).
NX‐DCP ratio and progression of HCC
Figure 4 shows the NX‐DCP ratio in patients according to HCC stage. Despite the small number of patients, there was a gradual increase in the NX‐DCP ratio as the stage increased (P = 0.0315).
Figure 4.
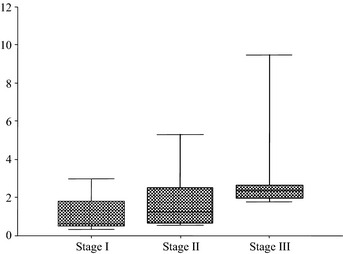
The NX‐DCP ratio according to hepatocellular carcinoma (HCC) stage in 20 patients with HCC taking warfarin (box plot). There was a gradual increase in the NX‐DCP ratio as the HCC stage increased (P = 0.0315).
Discussion
Hepatocellular carcinoma is the sixth most common cancer and the third most common cause of cancer‐related death worldwide.17, 18 In Japan, HCC is currently the third most common cause of death from cancer in men and the fifth in women.19 The incidence of HCC is also increasing in the US.20, 21 Improvements of tumor markers specific for HCC contribute to early detection of HCC. Three markers for HCC are currently used clinically, AFP, AFP‐L3, and DCP. The utility of each of these tumor markers for detection and diagnosis of HCC, for evaluation of tumor progression, and for determination of patient prognosis has been reported.4, 22, 23, 24 Elevation of DCP is often observed in HCC patients without elevation of AFP or AFP‐L3, and is useful as a complement to these other two markers in the diagnosis of HCC. In addition, elevation of DCP is reportedly associated with a high rate of portal vein invasion and poor prognosis.25 Elevation of DCP is also associated with better outcomes when hepatectomy, rather than radiofrequency ablation, is carried out in patients treated with curative intent.26, 27
However, DCP loses its value as a tumor marker of HCC in patients taking warfarin.7 Due to the marked decrease in vitamin K level caused by warfarin intake, DCP levels significantly increase in individuals taking warfarin, even in the absence of HCC. Therefore, DCP has no clinical utility as a tumor marker for HCC in this patient subpopulation.
The present study evaluated the reactivity of new antibodies against DCP, antibodies P‐11 and P‐16, based on the number of Glu residues. The number of Glu residues increases as the time allowed for decarboxylation from prothrombin increases.2 Our results showed P‐11 and P‐16 have higher reactivity with DCP with fewer Glu residues than MU‐3, the antibody that is conventionally used for the measurement of DCP. The NX‐DCP level that is measured by P‐11 and P‐16 antibodies, therefore, represents predominantly DCP caused by reduced vitamin K availability. Consequently, the elevation of the NX‐DCP ratio calculated in the equation: conventional DCP/NX‐DCP, reflects more specifically the elevation of DCP by HCC.
There were no differences in the conventional measurements of DCP between patients with and without HCC who are taking warfarin. The NX‐DCP ratio was significantly lower in patients without HCC than in patients with HCC. The NX‐DCP ratio varied in patients with HCC, as was conventional DCP in patients not taking warfarin, because the production of DCP by HCC is variable. In contrast, in all patients without HCC, the NX‐DCP ratio was low despite high conventional DCP levels in the same patients; no patients had an elevated NX‐DCP ratio. The results indicate that the NX‐DCP ratio could pinpoint the elevation of DCP caused by HCC, thereby restoring the value of DCP as a marker for HCC in patients taking warfarin.
When the cut‐off level was fixed at 1.5 on the basis of maximal accuracy, the sensitivity, specificity, PPV, and NPV were comparable to those of conventional DCP in the general population with normal vitamin K levels, as previously reported.28 The NX‐DCP ratio, therefore, seems to be useful as a marker for HCC in patients taking warfarin.
The elevation of other tumor markers for HCC, AFP and AFP‐L3, were observed in only half of the patients with HCC taking warfarin. In addition, both AFP and AFP‐L3 were negative in 40% of patients with HCC. Des‐gamma‐carboxy prothrombin is a complimentary marker of AFP/AFP‐L3 for HCC. The elevation of DCP without the elevation of AFP and AFP‐L3 was observed in 16.1% of patients (cut‐off, 20 ng/mL for AFP, 10% for AFP‐L3, and 40 mAU/mL for DCP)29 and 24.8% of patients (cut‐off, 400 ng/mL for AFP, 15% for AFP‐L3, and 100 mAU/mL for DCP).30 The measurement of the NX‐DCP ratio, therefore, will be important for the detection and diagnosis of HCC even when AFP or AFP‐L3 is measured simultaneously.
There are several limitations to this study. The most important limitation was the small number of study patients, especially patients with HCC. The number of patients with HCC taking warfarin is low, so it was difficult to increase the number of study patients. Consequently, it was difficult to evaluate the value of the NX‐DCP ratio in indicating progression of HCC, including tumor stage progression and portal vein invasion, and in predicting patient outcome. Further studies will be necessary to establish the value of the NX‐DCP ratio as a tumor marker for HCC in patients taking warfarin. In addition, the value of the NX‐DCP ratio was evaluated only in patients who were taking the vitamin K antagonist warfarin: its value was not evaluated in HCC patients in whom vitamin K is reduced or absent through other mechanisms such as heavy alcohol intake or nutritional deficiency. The value of the NX‐DCP ratio as a marker for HCC should be confirmed for these subpopulations in the future.
In conclusion, the novel NX‐DCP ratio identified elevation of DCP due to HCC in patients taking the vitamin K antagonist warfarin. Thus, by using this ratio, DCP can be used as a marker for HCC even in patients taking warfarin.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgments
We thank Jun Nishimura and Masato Uehara (EIDIA, Tokyo, Japan) for their technical assistance.
References
- 1. Blanchard RA, Furie BC, Jorgensen M, Kruger SF, Furie B. Acquired vitamin K‐dependent carboxylation deficiency in liver disease. N Engl J Med 1981; 305: 242–8. [DOI] [PubMed] [Google Scholar]
- 2. Bajaj SP, Price PA, Russell WA. Decarboxylation of gamma‐caboxyglutamic acid residues in human prothrombin. J Biol Chem 1982; 257: 3726–31. [PubMed] [Google Scholar]
- 3. Kuromatsu R, Tanaka M, Shimauchi Y et al Usefulness of ED036 kit for measuring serum PIVKA‐II levels in small hepatocellular carcinoma. J Gastroenterol 1997; 32: 507–12. [DOI] [PubMed] [Google Scholar]
- 4. Liebman HA, Furie BC, Tong MJ et al Des‐gamma‐carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med 1984; 310: 1427–31. [DOI] [PubMed] [Google Scholar]
- 5. Fujiyama S, Morishita T, Hashiguchi O, Sato T. Plasma abnormal prothrombin (des‐gamma‐carboxy prothrombin) as a marker of hepatocellular carcinoma. Cancer 1988; 61: 1621–8. [DOI] [PubMed] [Google Scholar]
- 6. Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des‐gamma‐carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer 1998; 82: 1643–8. [DOI] [PubMed] [Google Scholar]
- 7. Deyashiki Y, Nishioka Y, Takahashi K, Kosaka Y, Suzuki K. Evaluation of des‐gamma‐carboxy prothrombin as a marker protein of hepatocellular carcinoma. Cancer 1989; 64: 2546–51. [DOI] [PubMed] [Google Scholar]
- 8. Liebman HA. Isolation and characterization of a hepatoma‐associated abnormal (des‐gamma‐carboxy) prothrombin. Cancer Res 1989; 49: 6493–7. [PubMed] [Google Scholar]
- 9. Uehara S, Gotoh K, Handa H, Tomita H, Senshuu M. Distribution of the heterogeneity of des‐gamma‐carboxyprothrombin in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2005; 20: 1545–52. [DOI] [PubMed] [Google Scholar]
- 10. Naraki T, Kohno N, Saito H et al Gamma‐carboxyglutamic acid content of hepatocellular carcinoma‐associated des‐gamma‐carboxy prothrombin. Biochem Biophys Acta 2002; 1586: 287–98. [DOI] [PubMed] [Google Scholar]
- 11. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208–36. [DOI] [PubMed] [Google Scholar]
- 12. Liver Cancer Study Group of Japan . The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, English edn. Tokyo: Kanehara, 2003. [Google Scholar]
- 13. Kagebayashi C, Yamaguchi I, Akinaga A et al Automated immunoassay system for AFP‐L3% using on‐chip electrokinetic reaction and separation by affinity electrophoresis. Anal Biochem 2009; 388: 306–11. [DOI] [PubMed] [Google Scholar]
- 14. Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha‐fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology 1994; 19: 61–6. [PubMed] [Google Scholar]
- 15. Koda M, Murawaki Y, Mitsuda A et al Predictive factors for intrahepatic recurrence after percutaneous ethanol injection therapy for small hepatocellular carcinoma. Cancer 2000; 88: 529–37. [PubMed] [Google Scholar]
- 16. Toyoda H, Kumada T, Tada T et al Clinical utility of highly sensitive Lens culinaris agglutinin‐reactive alpha‐fetoprotein in hepatocellular carcinoma patients with alpha‐fetoprotein < 20 ng/mL. Cancer Sci 2011; 102: 1025–31. [DOI] [PubMed] [Google Scholar]
- 17. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2002; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 18. Befeler AS, DiBisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology 2002; 122: 1609–19. [DOI] [PubMed] [Google Scholar]
- 19. Umemura T, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. Hepatol Res 2007; 37: S95–100. [DOI] [PubMed] [Google Scholar]
- 20. El‐Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999; 340: 745–50. [DOI] [PubMed] [Google Scholar]
- 21. El‐Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 2003; 139: 817–23. [DOI] [PubMed] [Google Scholar]
- 22. Tsukuma H, Hiyama T, Tanaka S et al Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med 1993; 328: 1797–801. [DOI] [PubMed] [Google Scholar]
- 23. Taketa K, Sekiya C, Namiki N et al Lectin‐reactive profiles of alpha‐fetoprotein characterizing hepatocellular carcinoma and related conditions. Gastroenterology 1990; 99: 508–18. [DOI] [PubMed] [Google Scholar]
- 24. Fujiyama S, Tanaka M, Maeda S, Ashihara H, Hirata R, Tomita K. Tumor markers in early diagnosis, follow‐up and management of patients with hepatocellular carcinoma. Oncology 2002; 62: S57–63. [DOI] [PubMed] [Google Scholar]
- 25. Koike Y, Shiratori Y, Sato S et al Des‐gamma‐carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: a prospective analysis of 227 patients. Cancer 2001; 91: 561–9. [DOI] [PubMed] [Google Scholar]
- 26. Toyoda H, Kumada T, Kaneoka Y et al Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol 2008; 49: 223–32. [DOI] [PubMed] [Google Scholar]
- 27. Kobayashi M, Ikeda K, Kawamura Y et al High serum des‐gamma‐carboxy prothrombin level predicts poor prognosis after radiofrequency ablation of hepatocellular carcinoma. Cancer 2009; 115: 571–80. [DOI] [PubMed] [Google Scholar]
- 28. Nomura F, Ishijima M, Kuwa K, Tanaka N, Nakai T, Ohnishi K. Serum des‐gamma‐carboxy prothrombin levels determined by a new generation of sensitive immunoassay in patients with small‐sized hepatocellular carcinoma. Am J Gastroenterol 1999; 94: 650–4. [DOI] [PubMed] [Google Scholar]
- 29. Toyoda H, Kumada T, Kiriyama S et al Prognostic significance of simultaneous measurement of three tumor markers in patients with hepatocellular carcinoma. Clin Gastroenterol Hepatol 2006; 4: 111–7. [DOI] [PubMed] [Google Scholar]
- 30. Toyoda H, Kumada T, Osaki Y et al Staging hepatocellular carcinoma by a novel scoring system (BALAD score) based on serum markers. Clin Gastroenterol Hepatol 2006; 4: 1528–36. [DOI] [PubMed] [Google Scholar]


