Abstract
Objectives
To present our experience with the long-term preventive effect of immunotherapy with Uro-Vaxom® on recurrent urinary tract infections (UTI) in adult patients.
Materials and Methods
Retrospective analysis of 79 patients with recurrent UTI treated with Uro-Vaxom. Recurrent UTIs were defined as ≥ 2 infections in 6 months or ≥ 3 in 12 months. Patients received a 6 mg Uro-Vaxom capsule daily for 90 days followed by discontinuation for 3 months and then administration for the first 10 days of subsequent months 7, 8 and 9 as a ‘booster’ regime. The primary outcome measure was the number of UTIs encountered in the 12 months pre-treatment compared to 12 months post-treatment.
Results
There was a significant decrease in the mean number of UTIs in the year following initiation of Uro-Vaxom® compared to the year preceding administration 3.14 versus 1.53 (p < 0.05) respectively.
Conclusion
Uro-Vaxom represents a safe and effective treatment option for prophylaxis of recurrent UTIs. In the UK, Uro-Vaxom is currently unlicensed. This study adds to a growing body of evidence in favor of non-antibiotic immune-prophylaxis for recurrent UTI.
Key Words: Uro-Vaxom, Immunotherapy, Escherichia coli, Recurrent urinary tract infection
Introduction
Urinary tract infections (UTIs) are the most common bacterial infection encountered by health care professionals, estimated to affect 150 million people each year worldwide [1]. The cost implication is truly significant with data from NHS England in 2013/2014 reporting expenditure of over £400 million treating 184,000 unplanned hospital admissions as a result of UTIs not being adequately treated in the primary care setting [2]. The lifetime incidence of UTI in women is 40% [3]; by contrast only 0.1% of men under 50 will experience UTI with incidence rising with age [4].
Recurrent UTIs are defined as ≥ 2 infections in 6 months or ≥ 3 in 12 months [5]. Long-term low dose prophylactic antibiotics have long been the mainstay of treatment and prevention of recurrent UTIs. There is no doubt that this is a successful strategy; one meta-analysis of 10 randomized controlled trials concluded a risk reduction of almost 80% in women aged over 18 treated with prophylactic antibiotics for recurrent UTI [6]. The disadvantage is that prolonged bacterial exposure to long-term antibiotics increases bacterial resistance. Data on hospital acquired UTIs from the worldwide surveillance study Global Prevalence of Infections in Urology highlights disturbingly high rates of Escherichia coli resistance to commonly used antibiotics; 25% to piperacillin/ tazobactam, 45% to ciprofloxacin and 30% to gentamicin [7]. The ECO-SENS study looked specifically at antibiotic resistance in community acquired UTIs between 1999-2000 and 2007-2008. It found a steady increase in the resistance rates of Escherichia coli to ciprofloxacin (1.1-3.9%) and trimethoprim (13.3-16.7%) across the two study periods [8].
In an era where media-termed ‘superbugs’ are becoming a reality and mounting antibiotic resistance is far-outstripping the production of novel antibiotics, antibiotic stewardship is becoming critical. Non-antibiotic treatment strategies are becoming an increasingly attractive alternative. Uro-Vaxom® (OM-89, OM Pharma), an immuno-modulater, has been shown in several randomized placebo controlled trials to reduce the frequency of UTI recurrence [9]. Uro-Vaxom contains a lyophilized mix of membrane proteins from 18 different strains of Escherichia coli. It increases both humoral and cellular immune responses to UTI by stimulating macrophages, lymphocytes and increasing the levels of circulating endogenous IgA/IgG antibodies [10,11]. Uro-Vaxom is currently licensed in over 30 countries worldwide but despite its long history, first approved in Switzerland in 1988, and its endorsement in the latest European Association of Urology guidelines, Uro-Vaxom is not currently licensed in the UK.
We present, to our knowledge, the first retrospective study in the United Kingdom examining recurrent UTI treatment with prophylactic Uro-Vaxom immunotherapy.
Materials and Methods
The study was undertaken as a registered audit and we obtained approval from the regional health board committee since Uro-Vaxom is not currently licensed in the UK. Recurrent UTIs were defined as ≥ 2 UTIs within 6 months or ≥ 3 within 12 months. UTI was defined as the presence of with ≥ 103 c.f.u bacteria/ml with concomitant symptoms or the presence of a positive urine bacterial culture with concomitant symptoms. All selected patients had previously undergone cystoscopic or radiological investigations and patients with vesicoureteral reflux, obstructive uropathy, urinary lithiasis, renal impairment (serum creatinine > 120 mmol/l) and urologic procedures that induced UTI were excluded. The study protocol was approved by the institutional review board in our hospital.
At the start of the trial, all patients who were in acute recurrence were treated with antibiotics. After confirming that their urine was sterile, patients with a clinical diagnosis of recurrent UTIs, were treated with Uro-Vaxom, they received one 6 mg capsule daily for 90 days followed by discontinuation for 3 months and then administration for the first 10 days of subsequent months 7, 8 and 9 as a ‘booster’ regime.
The time period for the study was September 2012 to March 2015. Patients were identified from pharmacy records for prescriptions of Uro-Vaxom. Retrospective analysis of medical notes and microbiology results were used to assess response to treatment and patient satisfaction. The primary efficacy measure was the number of UTIs 1 year prior to Uro-Vaxom administration compared to the number of UTIs 1 year post administration. Secondary efficacy measures included patient satisfaction and adverse medication side effects. Further data was gathered on the most common uropathogens; average time to first follow-up and previous trial of antibiotic prophylaxis. For statistical analysis GraphPad QuickCalcs software (GraphPad Software, California, USA) was used to perform a paired t-test comparison of before and after treatment for the study group. Significance was set at a p-value of < 0.05.
Results
A total of 79 patients were included in the study; 75 females (95%) and 4 males (5%). The mean age was 56 ranging 19-90 years. There was a significant decrease in the mean number of UTIs in the year following initiation of Uro-Vaxom as compared to the year preceding administration 3.14 versus 1.53 (p < 0.05) respectively.
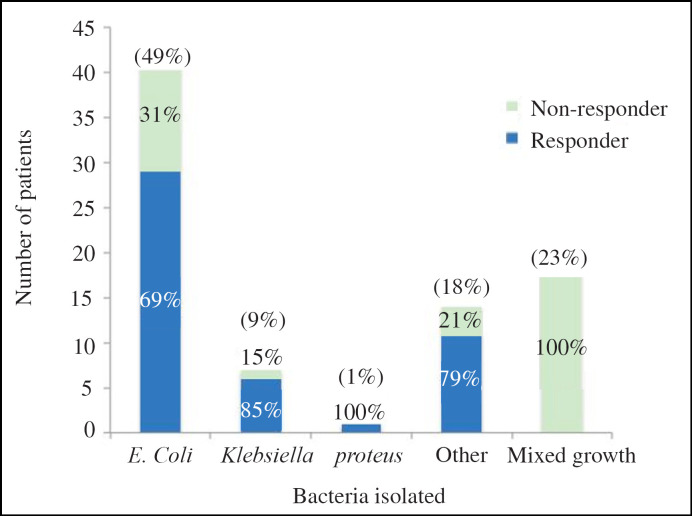
The most common causative bacteria for UTI cultured in mid-stream urines was Escherichia coli in 49%. Klebsiella accounted for 9% and proteus 1% of UTIs. In 23% of the recorded UTIs mixed growth was cultured and in the remaining 18% a variety of different bacteria were noted including enterococcus, citrobacter, pseudomonas and coliforms. Figure 1 plots the differing bacteriology in this study and the percentage of patients responding to Uro-Vaxom for each different bacteria isolated. Responders were defined as those patients with a reduction in number of UTI after commencing Uro-Vaxom. In the 23% of patients with mixed growth cultured, 0% responded to Uro-Vaxom. Total 63% of patients with Escherichia coli in their mid-stream urine cultures responded to Uro-Vaxom. Overall response rate to Uro-Vaxom regardless of bacteria cultured was 59.4%. Urinary pathogens cultured whilst on Uro-Vaxom were no different from previously grown pathogens. It is also important to note that there was no significant difference in response rate between patients growing different pathogens (p < 0.76).
Fig. 1.
Bacteria cultured in mid-stream urines and percentage of responders versus non-responders for each bacteria isolated.
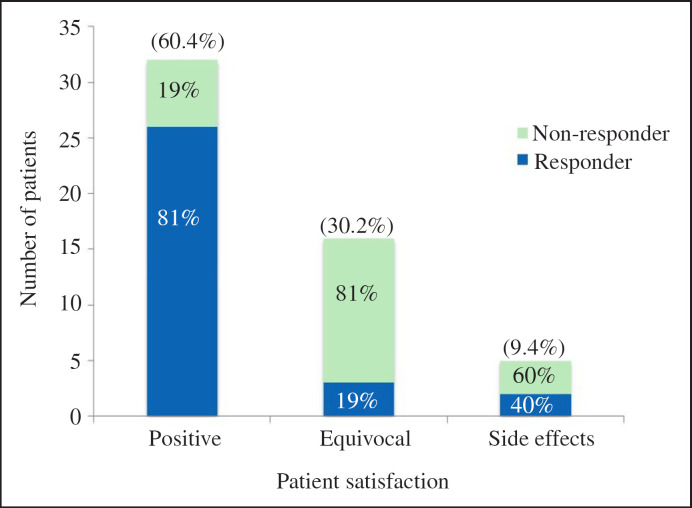
Analysis of the evaluable patients on patient satisfaction following Uro-Vaxom found 32/53 (60.4%) reported a positive satisfaction rating; 16/53 (30.2%) reported equivocal change in their symptoms and 5/53 (9.4%) of patients noted side effects. Figure 2 demonstrates the overall patient satisfaction with a percentage breakdown of responder versus non-responder for each satisfaction category. Of the 60.4% of patients that reported a positive satisfaction, 81% were responders to Uro-Vaxom. In the group of patient reporting equivocal satisfaction, 81% were non-responders. There were 5 out of 53 patients (9.4%) reported adverse effects, 40% of these were responders. Those side effects reported included urethral symptoms, rash, gastrointestinal discomfort and increased blood pressure. There were no reports of anaphylaxis. The median follow-up period was 100 days (1-279 days).
Fig. 2.
Patient satisfaction and percentage of non-responders versus responders for each satisfaction category.
Discussion
Recurrent UTIs are a common problem encountered by healthcare professionals worldwide. The emergence of antibiotic resistance is a serious concern in the treatment and prophylaxis of recurrent UTI and has serious consequences for specific patients groups and the wider population. There are numerous non-antibiotic treatments for recurrent UTIs including cranberry products, intravaginal lactobacillus suppositories, D-Mannose and ascorbic acid. The evidence for these non-antibiotic prophylactic options has been mixed and many questions still remain regarding their efficacy [12].
Uro-Vaxom represents an effective alternative to antibiotic therapy in recurrent UTI prevention. Our study described shows a significant decrease in the mean number of UTIs in the 12 months following administration of Uro-Vaxom compared to the 12 months prior to treatment (3.14 vs. 1.53, p ≤ 0.05). Similar results have been demonstrated elsewhere. A meta-analysis [13] of 5 double-blind randomized clinical trials; Tammen et al. [14], Schulman et al. [15], Bauer et al. [16], Frey et al. [5] and Magasi et al. [17] concluded that Uro-Vaxom was efficacious in reducing dysuria, bacteriuria and UTI at 3-6 month follow-up. The meta-analysis accepted that all studies were concordant and showed a clear benefit of Uro-Vaxom, however there was criticism for the lack of long-term follow-up data and inter-study heterogeneity in basic definitions of bacteriuria and UTI. Our study demonstrates a significant reduction in the number of UTIs in the 12 months following Uro-Vaxom administration and thus provides data over a longer follow-up period. In a similar population Bauer et al. [18] likewise found a significant reduction in UTI recurrence at 12 month follow-up following Uro-Vaxom as compared to placebo (0.84 vs. 1.28; p < 0.003). There is scant data on longer-term follow-up and the role of maintenance with Uro-Vaxom beyond 12 months.
In the described study 32/53 (60.4%) of the evaluable patients reported a positive satisfaction rating in regards to Uro-Vaxom treatment. Of these 32 patients, 81% were responders to Uro-Vaxom with a decrease in number of UTI following treatment. Interestingly, 6/32 (19%) of the positive reporting participants did not have a reduction in their number of UTIs post Uro-Vaxom but still felt positively towards their treatment and this may be explained by a reduction in the severity of UTI-related symptoms. The effect of Uro-Vaxom on symptoms was not directly assessed in this study but has been assessed elsewhere. Kim et al. [9] reported a reduction in the frequency, urgency and dysuria symptoms in those patients with continued UTI despite Uro-Vaxom treatment. The beneficial effect of Uro-Vaxom may extend beyond simply reducing the number of UTI recurrences and include reducing the severity of UTI-associated symptoms. In 5/53 (9.4%) patients reported side effects, which were mild and ranged from gastrointestinal upset to rash. There were no reported incidences of anaphylaxis. Schulmann et al. [14] similarly reported good tolerance with 2% of the study population experiencing side effects.
The most commonly cultured bacteria in mid-stream urine samples was Escherichia coli (53%) followed by Klebsiella (9%). This is comparable to a previous microbiological survey of uropathogens finding Escherichia coli and Klebsiella were responsible for 43.5 and 13.3% of UTIs, respectively [19]. Uro-Vaxom has been described as an immune-modulator, priming the body's natural defence to all bacteria not specific to just Escherichia coli. This is demonstrated by the good response rates regardless of the bacteria cultured (Fig. 1).
Immunotherapy is not a new concept to urology; for almost 45 years intravesical Bacillus Calmette-Guerin has successfully been used to treat superficial bladder cancer by activating the immune response in the bladder urothelium [20]. Animal experiments in mice have shown following ingestion of Uro-Vaxom mice exhibited increased levels of immunoglobulins A and G and increased lymphocyte and macrophage activity both in the serum and specifically within the urogenital tract [16]. Thus it has been postulated that Uro-Vaxom reduces UTI recurrence principally by stimulating the immune system and not by direct action on bacteria thereby reducing the chance of bacterial resistance.
The literature suggests Uro-Vaxom is the most investigated and widely used immuno-modulater agent however other forms of immune-prophylaxis does exist. The vaginal vaccine UroVac contains 10 heat-killed uro-pathogenic bacterial species. It induces immunoglobulin A and G in the urogenital tract, thereby reducing possible colonization of the vagina and bladder with uropatho-gens [21]. A phase II trial randomized 54 women with recurrent UTI to receive either placebo, primary immunization with UroVac, or UroVac plus booster immunizations. Time until recurrence was significantly longer for women in the booster immunization group but not in the primary immunization or placebo group (p < 0.02). There were no reinfections during the 6-month trial period in 55.6% of the booster immunization group, 22.2% of the primary immunization group, and 22.2% of the placebo group (p = 0.06) [22]. However, No long-term follow-up data is available.
Uromune is a sublingual vaccine which stimulates mucosal and systemic immune responses in the genitourinary tract. This vaccine has been recently shown in a UK prospective study to increase time to first UTI recurrence in 77 females in a follow-up period of 12 months and 78% experienced no recurrent infection in the 12 month study period. The data suggests that Uromune may be effective in reducing the burden of UTI disease in women with recurrent infections [23]. Lorenzo-Gomez et al. [24] retrospectively compared the risk reduction of developing UTI recurrence between 3 months of Uro-mune prophylaxis and 6 months of antibiotic prophylaxis over a 1-year follow-up period. A shorter time to first recurrence in the antibiotic group, as well as a 90.28% (95% CI: 87.18-93.38) absolute risk reduction when using Uromune was reported. Indeed, a scientifically rigorous phase III trial will be required to demonstrate efficacy and safety prior to routine use.
There are limited phase III trials which exist for par-enteral immune-modulater agents however in similar smaller phase II trials e.g. StorVac and Solco-Urovac have shown to be effective especially when administered with a booster cycle of the same agent [25].
Our study is limited by its retrospective nature and lack of randomization. The study is an uncontrolled observational study and thus may be subject to selection bias and confounding factors. Despite this our study offers a significant follow-up period, which is rarely seen in other studies assessing Uro-Vaxom and represents the first report of this treatment in the UK.
Conclusion
Uro-Vaxom represents a safe and effective treatment option for prophylaxis of recurrent UTIs. However, despite Uro-Vaxom's widespread use in Europe and current recommendation in European Association of Urology guidelines on urological infections, it is currently unlicensed in the UK, limiting its use to hospital prescription by a urology specialist or costly private purchase by patients. This study adds to a growing body of evidence in favor of non-antibiotic immune-prophylaxis for recurrent UTI.
References
- 1.Stamm WE, Norrby SR. Urinary tract infections: disease panorama and challenges. J Infect Dis. 2001;183((suppl 1)):S1–S4. doi: 10.1086/318850. [DOI] [PubMed] [Google Scholar]
- 2.Medical technology Group Admissions of failure - the truth about unplanned NHS admissions in England 2105. Available from: http://www.mtg.org.uk/wp-content/up-loads/2016/07/Admissions-of-Failure-2015.pdf. [Google Scholar]
- 3.Tan CW, Chlebicki MP. Urinary tract infections in adults. Singapore Med J. 2016;57:485–490. doi: 10.11622/smedj.2016153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002;113((Suppl 1A)):5S–13S. doi: 10.1016/s0002-9343(02)01054-9. [DOI] [PubMed] [Google Scholar]
- 5.Frey C, Obolensky W, Wyss H. Treatment of recurrent urinary tract infections: efficacy of an orally administered biological response modifier. Urol Int. 1986;41:444–446. doi: 10.1159/000281253. [DOI] [PubMed] [Google Scholar]
- 6.Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;3:CD001209. doi: 10.1002/14651858.CD001209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tandogdu Z, Cek M, Wagenlehner F, Naber K, Tenke P, van Ostrum E, Johansen TB. Resistance patterns of nosocomial urinary tract infections in urology departments: 8-year results of the global prevalence of infections in urology study. World J Urol. 2014;32:791–801. doi: 10.1007/s00345-013-1154-8. [DOI] [PubMed] [Google Scholar]
- 8.Kahlmeter G, Poulsen HO. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO· SENS study revisited. Int J Antimicrob Agents. 2012;39:45–51. doi: 10.1016/j.ijantimicag.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Beerepoot MA, Geerlings SE, van Haarst EP, van Charante NM, ter Riet G. Nonantibiotic prophylaxis for recurrent urinary tract infections: a systematic review and meta-analysis of randomized controlled trials. J Urol. 2013;190:1981–1989. doi: 10.1016/j.juro.2013.04.142. [DOI] [PubMed] [Google Scholar]
- 10.Kim KS, Kim JY, Jeong IG, Paick JS, Son H, Lim DJ, Shim HB, Park WH, Jung HC, Choo MS. A prospective multi-center trial of Escherichia coli extract for the prophylactic treatment of patients with chronically recurrent cystitis. J Korean Med Sci. 2010;25:435–439. doi: 10.3346/jkms.2010.25.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meredith M, Chiavaroli C, Bauer HW. Immunotherapy for recurrent urinary tract infections: effects of an Escherichia coli extract. Curr Urol. 2009;3:1–8. [Google Scholar]
- 12.Beerepoot M, Geerlings S. Non-antibiotic prophylaxis of urinary tract infections. Pathogens. 2016;5:36. doi: 10.3390/pathogens5020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taha Neto KA, Nogueira Castilho L, Reis LO. Oral vaccine (OM-89) in the recurrent urinary tract infection prophylaxis: A realistic systematic review with meta-analysis. Actas Urol Esp. 2016;40:203–208. doi: 10.1016/j.acuro.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Tammen H. Immunobiotherapy with Uro-Vaxom in recurrent urinary tract infection. Br J Urol. 1990;65:6–9. doi: 10.1111/j.1464-410x.1990.tb14649.x. [DOI] [PubMed] [Google Scholar]
- 15.Schulman CC, Corbusier A, Michiels H, Taenzer HJ. Oral immunotherapy of recurrent urinary tract infections: a double-blind placebo-controlled multicenter study. J Urol. 1993;150:917–922. doi: 10.1016/s0022-5347(17)35648-3. [DOI] [PubMed] [Google Scholar]
- 16.Huber M, Krauter K, Winkelmann G, Bauer HW, Rahlfs VW, Lauener PA, Blessmann GS, Bessler WG. Immunostimulation by bacterial components: II. Efficacy studies and meta-analysis of the bacterial extract OM-89. Int J Immunopharmacol. 2000;22:1103–1111. doi: 10.1016/s0192-0561(00)00070-9. [DOI] [PubMed] [Google Scholar]
- 17.Magasi P, Pánovics J, Illés A, Nagy M. Uro-Vaxom and the management of recurrent urinary tract infection in adults: a randomized multicenter double-blind trial. Eur Urol. 1994;26:137–140. doi: 10.1159/000475363. [DOI] [PubMed] [Google Scholar]
- 18.Bauer HW, Alloussi S, Egger G, Blumlein HM, Cozma G, Schulman CC. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol. 2005;47:542–548. doi: 10.1016/j.eururo.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Behzadi P, Behzadi E, Yazdanbod H, Aghapour R, Akbari Cheshmeh M, Salehian Omran D. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica (Buchar) 2010;5:111–115. [PMC free article] [PubMed] [Google Scholar]
- 20.Morales A, Eidinger D, Bruce AW. Intracav-itary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 21.Uehling DT, Hopkins WJ, Elkahwaji JE, Schmidt DM, Leverson GE. Phase 2 clinical trial of a vaginal mucosal vaccine for urinary tract infections. J Urol. 2003;170:867–869. doi: 10.1097/01.ju.0000075094.54767.6e. [DOI] [PubMed] [Google Scholar]
- 22.Hopkins WJ, Elkahwaji J, Beierle LM, Le-verson GE, Uehling DT. Vaginal mucosal vaccine for recurrent urinary tract infections in women: results of a phase 2 clinical trial. J Urol. 2007;177:1349–1353. doi: 10.1016/j.juro.2006.11.093. [DOI] [PubMed] [Google Scholar]
- 23.Yang B, Foley S. First experience in the UK of treating women with recurrent urinary tract infections with the bacterial vaccine Uromune®. BJU Int. 2018;121:289–292. doi: 10.1111/bju.14067. [DOI] [PubMed] [Google Scholar]
- 24.Lorenzo-Gomez MF, Padilla-Fernandez B, Garcia-Cenador MB, Virseda-Rodríguez ÁJ, Martín-García I, Sánchez-Escudero A, Vicente-Arroyo MJ, Mirón-Canelo JA. Comparison of sublingual therapeutic vaccine with antibiotics for the prophylaxis of recurrent urinary tract infections. Front Cell Infect Microbiol. 2015;3(5):50. doi: 10.3389/fcimb.2015.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riedasch G, Moehring K. Immunotherapy in women with recurrent urinary tract infections. Therapiewoche. 1986;6:896–900. [Google Scholar]